Abstract
Cigarette smoking has been noted to impair wound healing in tissues such as skin, bone, and gut. This study was designed to examine whether nicotine adversely affects postinfarction cardiac wound healing and remodeling in an experimental model of myocardial infarction. For this purpose, two groups of rats were studied. The control group received a simple bandage, and the nicotine group had a section (1.75 mg/day) of a nicotine patch attached on their backs. After a 7-day treatment period, an anterior wall infarction was induced. A bandage-free 7-day healing period followed, after which hearts were isolated for mechanical tests. Nicotine-treated rats developed significantly enlarged left ventricles with thin, infarcted walls and a right-ward shift in the passive pressure-volume relationship. Pressure-strain analysis also indicated possible changes in the material properties of the wound for nicotine-treated rats. In conclusion, nicotine has significant adverse effects on postinfarction healing and left ventricular remodeling. These observations have important clinical implications because of the enhanced risk for development of heart failure.
Keywords: wound healing, scarring, heart failure, cigarette smoke
CIGARETTE SMOKING is one of the most important risk factors for coronary artery disease and myocardial infarction (MI) (9). Subsequent to MI, rapid and efficient healing and scarring must occur for the patient to avoid cardiac rupture. In the absence of cardiac rupture, impaired cardiac wound healing can lead to enhanced infarct expansion and thus adversely modified cardiac remodeling (6). These events increase the chances of development of ventricular dysfunction and, ultimately, heart failure.
In 1977, Mosely and Finseth (3) first reported the impaired healing of a hand wound in a cigarette smoker. Similar observations have been made over the last 20 years in many tissues including skin, bone, mouth, peptic ulcers, and others (8). The relationship between impaired tissue wound healing and cigarette smoking has not been examined in prospective clinical studies. The extent to which impaired wound healing is noted to occur is such that smokers are commonly advised to stop smoking before elective surgery or when recovering from trauma, surgery, or disease (8). Cigarette smoke generates >4,000 toxins (9). However, recent research has focused attention on the actions of nicotine on wound healing (8).
Two properties of nicotine appear to be relevant to the issue of impaired wound healing. The first property relates to the capacity of nicotine to impair vascular function. Nicotine-impaired blood flow and oxygenation in smokers appears to contribute significantly to a higher degree of failure in plastic surgery procedures (3, 4). The second property of nicotine relates to its capacity to inhibit the proliferation of macrophages and fibroblasts (7, 10, 11). Scar tissue development depends on fibroblast proliferation, migration, and deposition of extracellular matrix proteins (2). Thus high plasma levels of nicotine could reduce fibroblast proliferation and lead to impaired wound healing.
Surprisingly, quantitative assessments of impaired wound healing in smokers have not been sought in clinical or experimental studies in organs such as the heart. The corroboration of such findings in the infarcted healing human heart will have important clinical implications related to the evaluation and treatment of patients who smoke. Thus the objectives of the current study were to explore under controlled experimental conditions the capacity of nicotine to adversely modify global ventricular remodeling, as well as local (wound) ventricular mechanics in the infarcted rat heart.
MATERIALS AND METHODS
Nicotine treatment
Male Sprague-Dawley rats weighing ~250 g had hair removed from their backs via chemical depilation. The control group (n = 6) received a section of a bandage (Band-Aid) attached on their backs and changed daily for a period of 7 days before infarction. The nicotine-treated group (n = 6) had a section of a nicotine patch (Nicoderm) attached on their back underneath a larger piece of bandage. The aim was to approximate nicotine levels to a serum concentration of 45 ng/ml. This concentration is comparable to blood levels found in heavy-use human smokers (30 ng/ml) (12). The section of the patches used in this study provided a dose of 1.75 mg/day. This dose was selected following published data that indicate that such a dosage regime generates average blood concentrations of 43 ng/ml in rats of ~225-g weight (12). Patches were changed daily for 7 days. All procedures were approved by the Institutional Animal Care and Use Committee and conform to published NIH guidelines for animal research.
Surgical preparation
Animals were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) intramuscularly, intubated, and ventilated with room air. A left thoracotomy was performed, the pericardium was opened, the heart was exposed, and the left anterior descending coronary artery was occluded. The chest was closed, and animals were allowed to recover for 7 days without patches.
Terminal study
The techniques for measuring passive mechanics in the rat heart have been previously described (1, 5). Rats were anesthetized and ventilated, and the heart was arrested with a modified hyperkalemic buffer solution. The isolated ventricles were vented, and a balloon was inserted into the left ventricle (LV) and secured to the mitral annulus. To measure surface strain in these hearts, a set of markers was painted on the surface of the infarcted area of the LV. The positions of the markers were recorded on videotape during inflation of the balloon to 25 mmHg. Simultaneous recordings of ventricular volume were taken via analog-to-digital conversion on a personal computer. Two-dimensional homogeneous passive myocardial strains were computed with respect to a cardiac coordinate system [circumferential (E11), longitudinal (E22) and in-plane shear strains (E12)] referred to the zero-pressure state. For each heart, strains were fit to polynomials as functions of volume from which pressure-strain data were obtained. Pressure-volume curves for each animal were fit to third-order polynomials and averaged at the prescribed pressure loads.
The LV were sectioned into five short-axis rings. The section that most clearly traversed the infarct area was selected for the following measurements: anterior-posterior internal and external LV diameter, septal-free wall internal and external LV diameter, LV anterior (infarct) wall thickness, and posterior (normal) wall thickness. All diameter and wall thickness measurements were made in triplicate with NIH Image software and averaged. This same ring section as well as the two adjacent rings were used to determine wound size by tracing the pale infarct zone and total tissue area and calculating the percent infarct area in each ring section.
Data analysis
For the purposes of surgical procedures, data acquisition, and analysis, the personnel involved were blinded as to the animal treatment received (i.e., sham treatment or nicotine). Statistical analysis was performed with either a Student’s t-test or repeated-measures ANOVA. Results were considered to be statistically significant at P ≤ 0.05. All data are shown as means ± SD.
RESULTS
Assessment of ventricular remodeling
Table 1 summarizes the results obtained from morphometric measurements of control and nicotine-treated animals. No differences were noted between the two groups in either heart or animal weight at the time of surgery or after infarction (Table 1). Significant increases were noted in the LV external and internal septal-free wall and anterior-posterior diameters for the nicotine-treated group. A significant shortening of the long axis of the heart in nicotine-treated animals was also noted (i.e., cardiac spheration). The infarcted anterior LV wall of nicotine-treated animals was also significantly thinner versus controls with no differences in noninfarcted posterior wall. Infarct size as derived from the morphometric assessment of wound size was comparable.
Table 1.
Summary of cardiac remodeling parameters from infarcted control and nicotine-treated animals
| Parameter | Control | Nicotine |
|---|---|---|
| Surgical wt, g | 291 ± 10 | 295 ± 8.5 (0.41) |
| 7 days post-MI wt, g | 298 ± 7.6 | 294 ± 13 (0.58) |
| Heart wt, g | 1.24 ± 0.07 | 1.20 ± 0.08 (0.4) |
| Int. LV diameter (AP), cm | 0.49 ± 0.07 | 0.64 ± 0.08 (0.005)* |
| Int. LV diameter (SF), cm | 0.38 ± 0.04 | 0.48 ± 0.05 (0.002)* |
| Ext. LV diameter (AP), cm | 1.10 ± 0.08 | 1.22 ± 0.06 (0.01)* |
| Ext. LV diameter (SF), cm | 0.89 ± 0.04 | 1.08 ± 0.09 (0.0007)* |
| Long axis, cm | 1.81 ± 0.06 | 1.72 ± 0.07 (0.037)* |
| Anterior wall thickness, cm | 0.15 ± 0.03 | 0.11 ± 0.02 (0.02)* |
| Posterior wall thickness, cm | 0.46 ± 0.08 | 0.44 ± 0.02 (0.46) |
| Infarct size, % | 23 ± 5 | 18 ± 5.5 (0.14) |
Values are means ± SD; n = 6 rats/group. MI, myocardial infarction; Int., interior; Ext., exterior; LV, left ventricular; AP, anterior-posterior; SF, septal-free wall. Statistical significance is noted in parenthesis;
P ≤ 0.05.
Ventricular mechanics
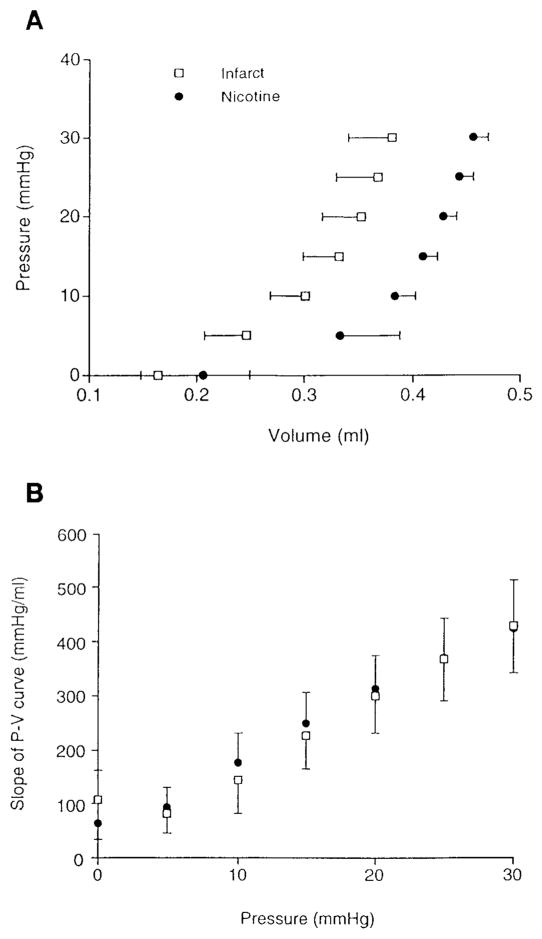
Figure 1A shows the average pressure-volume (P-V) curves (n = 5 in control group caused by incomplete intraventricular balloon unfolding in 1 animal) for the inflation portion of the loading cycle. Volumes include the empty balloon. There was a significant effect of nicotine treatment on the volume. Thus the P-V curve in the nicotine-treated hearts is shifted to the right with no apparent change in slope. The increase in unloaded volume corresponds to the increase seen in the internal diameters. The slopes at 5-mmHg pressure increments were not different by ANOVA (Fig. 1B), and this was also true when the absolute volumes were normalized to the unloaded, zero-pressure volume. Thus nicotine treatment did not appear to modify overall compliance of the ventricle, although the shift in unloaded volume together with comparable slopes could imply a decrease in overall tissue compliance.
Fig. 1.
A: average passive left ventricular (LV) pressure-volume (P-V) relationships for nicotine-treated (n = 6) and control (n = 5) infarcted hearts. Two-way ANOVA indicates significant effect of treatment on pressure (P < 0.011; i.e., right shifted). Interactive effect of treatment on P-V relationship was not significant. B: average slopes of LV P-V relationships for nicotine-treated (n = 6) and control (n = 5) infarcted hearts. Nicotine treatment did not cause a significant change in slope (i.e., compliance) of P-V relationship at any pressure.
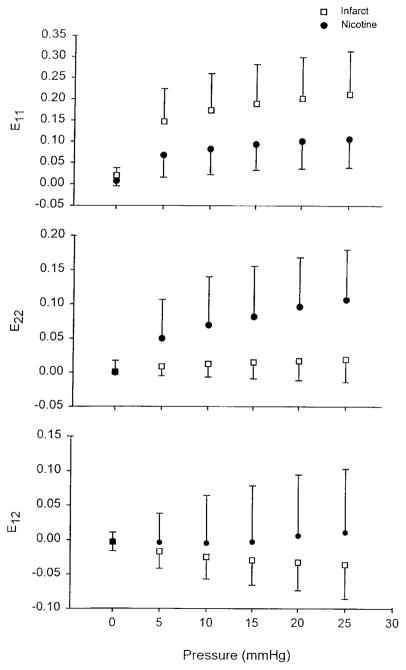
To examine differences in tissue mechanics in the infarcted area independent of LV geometry epicardial strains were computed as functions of pressure (Fig. 2). Positive strains indicate segment lengthening. ANOVA showed a significant effect of treatment on the strain-pressure relationship for both E11 and E22 within the infarct; E12 was not different. As can be observed, E11 tended to decrease with nicotine treatment, whereas E22 increased substantially.
Fig. 2.
Two-dimensional LV epicardial strains in infarcted area as functions of inflation pressure. There was a significant effect of treatment on pressure-strain relationship for both circumferential (E11; P = 0.012) and longitudinal (E22; P = 0.001) strains. Shear strains (E12) were not different. Thus function in infarcted wall appears to be altered by nicotine treatment.
DISCUSSION
After MI in surviving patients, a process of wound healing begins in the affected tissue. The extent and efficacy of postinfarction healing greatly influences the ensuing process of cardiac remodeling (6). Adversely affected cardiac remodeling parameters such as progressive cardiac enlargement are known to correlate closely with the development of heart failure or death. Although the association between cigarette smoking and delayed or impaired wound healing has been recognized in clinical fields such as surgery (4), there are no carefully controlled studies that examine the effects of cigarette smoking on postinfarction wound healing and ventricular remodeling. The purpose of the current study was to assess the effects of nicotine, an important derivative of cigarette smoke, on wound healing and remodeling after infarction in rats. Results from the current study indicate that the application of transdermal nicotine before MI in rats generates important and adverse modifications to various LV remodeling parameters 7 days after MI. Elevation of nicotine levels induced an enlargement of the LV cavity, thinning of the infarcted wall, and a more spherically shaped LV (shortened long axis). These geometric changes were accompanied by changes in the passive mechanics of the LV as evidenced by a right-shifted P-V relationship and a change in wound (i.e., developing scar) two-dimensional pressure strain characteristics, independent of wound size.
Thus results from this study provide initial evidence for the adverse effects of nicotine on postinfarction LV healing and remodeling. Future studies should examine the mechanism of action of nicotine on tissue wound healing and long-term consequences that follow these observations, in particular those that follow myocardial infarction. Ultimately large clinical studies will be needed to establish the consequences of MI in humans who are using tobacco or nicotine products. The establishment of impaired scarring in humans might also lead to reassessment of current accepted postinfarction therapy in patients who smoke.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grant HL-03160 to F. Villarreal.
References
- 1.Emery JL, Omens JH, McCulloch AD. Biaxial mechanics of the passively overstretched left ventricle. Am J Physiol. 1997;272(41):H2299–H2305. doi: 10.1152/ajpheart.1997.272.5.H2299. Heart Circ. Physiol. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. In: Fibronectins. Rich A, editor. New York: Springer; 1990. pp. 349–364. [Google Scholar]
- 3.Mosely LH, Finseth F. Cigarette smoking: impairment of digital blood flow and wound healing in the hand. Hand. 1977;9:97–101. doi: 10.1016/s0072-968x(77)80001-6. [DOI] [PubMed] [Google Scholar]
- 4.Nolan J, Jenkins RA, Kurihara K, Schultz RC. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg. 1985;29:544–549. doi: 10.1097/00006534-198504000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Omens JH, MacKenna DA, McCulloch AD. Two-dimensional strain and analysis of stress in the arrested rat left ventricle. J Biomech. 1993;26:665–676. doi: 10.1016/0021-9290(93)90030-i. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 7.Sherwin MA, Gastwirth CM. Detrimental effects of cigarette smoking on lower extremity wound healing. J Foot Surg. 1990;29:84–87. [PubMed] [Google Scholar]
- 8.Silverstein P. Smoking and wound healing. Am J Med. 1992;93:22S–24S. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]
- 9.Surgeon General. The Health Consequences of Smoking: Cardiovascular Disease. Rockville, MD: U. S. Department of Health and Human Services; 1983. [Google Scholar]
- 10.Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol. 1995;66:1056–1064. doi: 10.1902/jop.1995.66.12.1056. [DOI] [PubMed] [Google Scholar]
- 11.Tomek RJ, Rimas S, Eghbali M. Nicotine regulates collagen gene expression, collagenase activity and DNA synthesis in cultured cardiac fibroblasts. Mol Cell Biochem. 1994;136:97–103. doi: 10.1007/BF00926068. [DOI] [PubMed] [Google Scholar]
- 12.Witschi H, Lungard SM, Rajini P, Hendrickx AG, Last JA. Effects of exposure to nicotine and to sidestream smoke on pregnancy outcome in rats. Toxicol Lett. 1994;71:279–286. doi: 10.1016/0378-4274(94)90114-7. [DOI] [PubMed] [Google Scholar]