Abstract
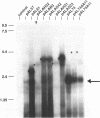
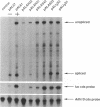
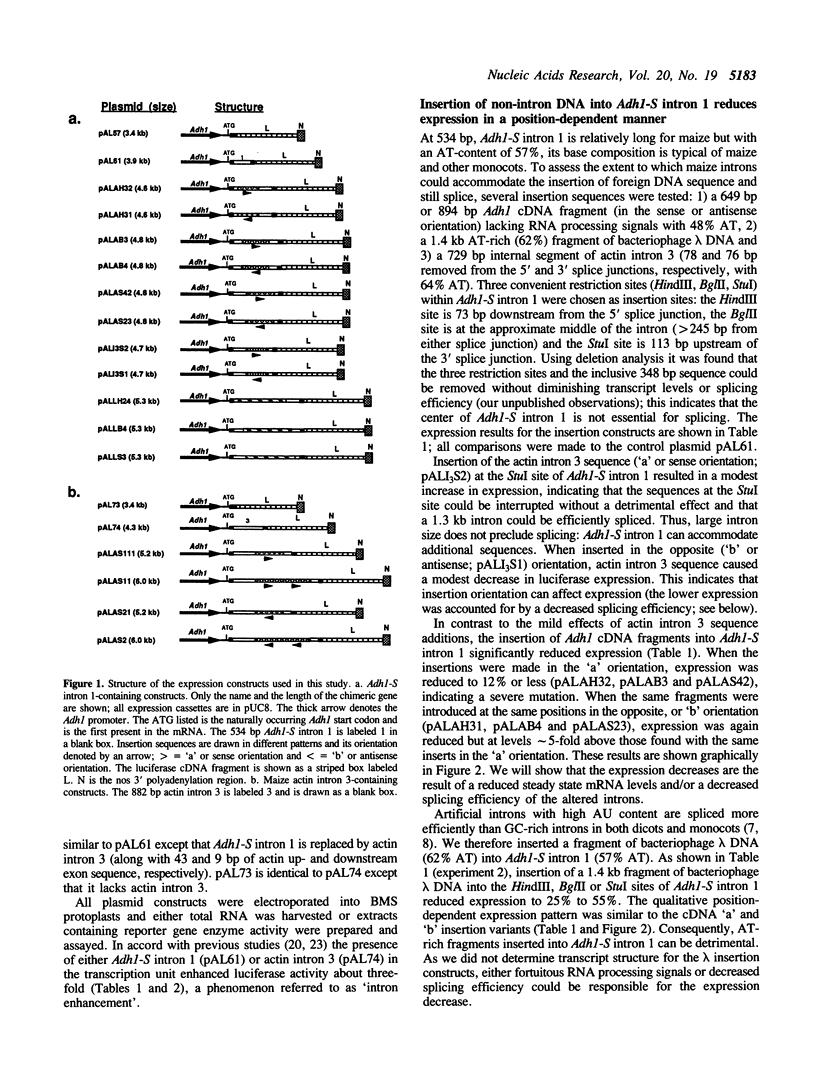
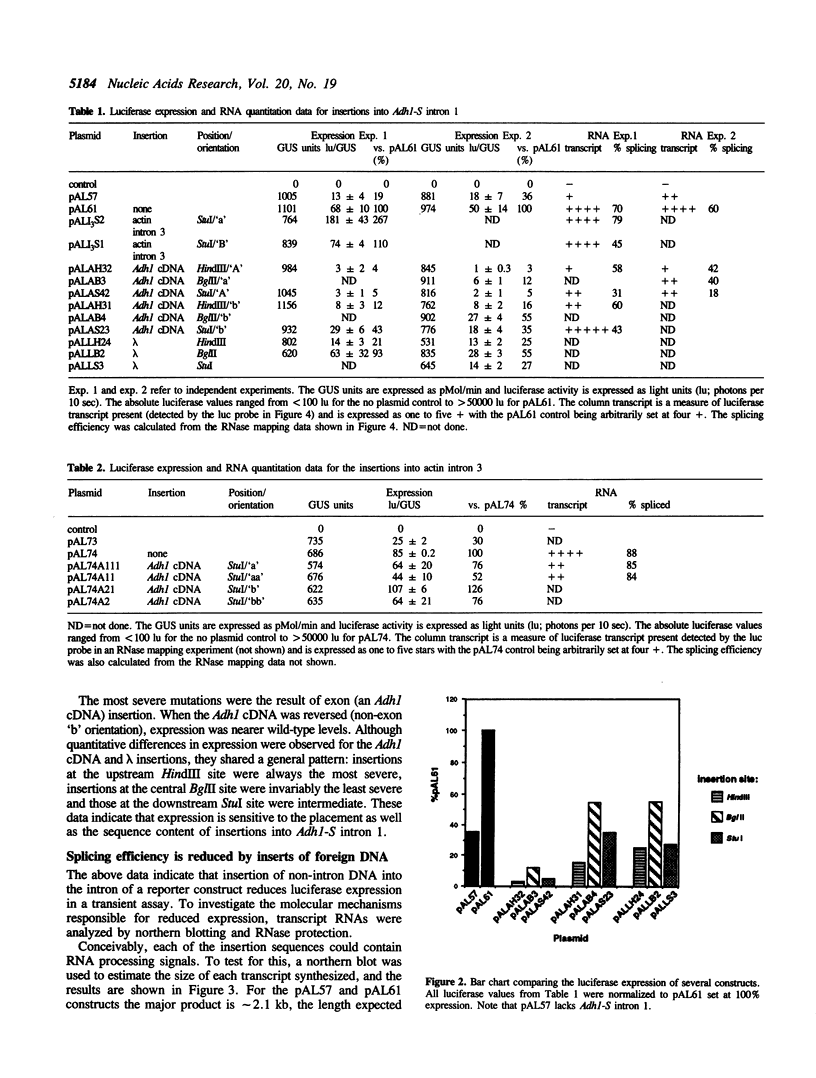
Transposable element (TE) insertion into or near plant introns can cause intron skipping and alternative splicing events, resulting in reduced expression. To explore the impact of inserted sequences on splicing, we added non-intron sequence to two maize introns and tested these chimeric introns in a maize transient expression assay. Non-intron sequence inserted into Adh1-S intron 1 and actin intron 3 decreased expression from the luciferase reporter gene; the insertion sites tested were not in intron regions thought to be essential for splicing. Alternatively spliced mRNAs were not observed in transcripts derived from the insertion variants. In contrast, addition of an internal segment of an intron to Adh1-S intron 1 resulted in normal splice site selection and efficient processing. Because the normal intron sequence (including the conserved splice junctions) was retained in all constructs, we hypothesize that added non-intron sequence can interfere with intron recognition and/or splicing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M., Piñol-Roma S., Staknis D., Dreyfuss G., Reed R. Differential binding of heterogeneous nuclear ribonucleoproteins to mRNA precursors prior to spliceosome assembly in vitro. Mol Cell Biol. 1992 Jul;12(7):3165–3175. doi: 10.1128/mcb.12.7.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. R., Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988 Oct;8(10):4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987 Dec;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Csank C., Taylor F. M., Martindale D. W. Nuclear pre-mRNA introns: analysis and comparison of intron sequences from Tetrahymena thermophila and other eukaryotes. Nucleic Acids Res. 1990 Sep 11;18(17):5133–5141. doi: 10.1093/nar/18.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot and dicot plants. EMBO J. 1991 Sep;10(9):2635–2644. doi: 10.1002/j.1460-2075.1991.tb07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989 Aug 11;58(3):473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Goodall G. J., Filipowicz W. The minimum functional length of pre-mRNA introns in monocots and dicots. Plant Mol Biol. 1990 May;14(5):727–733. doi: 10.1007/BF00016505. [DOI] [PubMed] [Google Scholar]
- Hanley B. A., Schuler M. A. Plant intron sequences: evidence for distinct groups of introns. Nucleic Acids Res. 1988 Jul 25;16(14B):7159–7176. doi: 10.1093/nar/16.14.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. D. A survey on intron and exon lengths. Nucleic Acids Res. 1988 Nov 11;16(21):9893–9908. doi: 10.1093/nar/16.21.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Gorman C. M. Intervening sequences increase efficiency of RNA 3' processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990 Feb 25;18(4):937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y., Schiefelbein J. W., Raboy V., Furtek D. B., Nelson O. E., Jr RNA splicing permits expression of a maize gene with a defective Suppressor-mutator transposable element insertion in an exon. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5863–5867. doi: 10.1073/pnas.84.16.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Luehrsen K. R., Walbot V. Insertion of Mu1 elements in the first intron of the Adh1-S gene of maize results in novel RNA processing events. Plant Cell. 1990 Dec;2(12):1225–1238. doi: 10.1105/tpc.2.12.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen K. R., Walbot V. Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol Gen Genet. 1991 Jan;225(1):81–93. doi: 10.1007/BF00282645. [DOI] [PubMed] [Google Scholar]
- Michaud S., Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991 Dec;5(12B):2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- Pautot V., Brzezinski R., Tepfer M. Expression of a mouse metallothionein gene in transgenic plant tissues. Gene. 1989 Apr 15;77(1):133–140. doi: 10.1016/0378-1119(89)90367-3. [DOI] [PubMed] [Google Scholar]
- Reed R. Protein composition of mammalian spliceosomes assembled in vitro. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8031–8035. doi: 10.1073/pnas.87.20.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Abelson J. Pre-mRNA splicing in yeast. Trends Genet. 1991 Mar;7(3):79–85. doi: 10.1016/0168-9525(91)90276-V. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Simon R, Starlinger P. Transposable element Ds2 of Zea mays influences polyadenylation and splice site selection. Mol Gen Genet. 1987 Aug;209(1):198–199. doi: 10.1007/BF00329859. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagona M. J., Purugganan M., Wessler S. R. Alternative splicing induced by insertion of retrotransposons into the maize waxy gene. Plant Cell. 1992 Jul;4(7):811–820. doi: 10.1105/tpc.4.7.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Baran G., Varagona M. The maize transposable element Ds is spliced from RNA. Science. 1987 Aug 21;237(4817):916–918. doi: 10.1126/science.3039661. [DOI] [PubMed] [Google Scholar]
- Wessler S. R. The maize transposable Ds1 element is alternatively spliced from exon sequences. Mol Cell Biol. 1991 Dec;11(12):6192–6196. doi: 10.1128/mcb.11.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Varagona M. J. Molecular basis of mutations at the waxy locus of maize: correlation with the fine structure genetic map. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4177–4181. doi: 10.1073/pnas.82.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Herrero J. J., Filipowicz W. Nuclear pre-mRNA processing in plants: distinct modes of 3'-splice-site selection in plants and animals. Mol Cell Biol. 1988 May;8(5):2042–2051. doi: 10.1128/mcb.8.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Yu X. M., Gelembiuk G. W., Wang C. Y., Ryu W. S., Mertz J. E. Expression from herpesvirus promoters does not relieve the intron requirement for cytoplasmic accumulation of human beta-globin mRNA. Nucleic Acids Res. 1991 Dec;19(25):7231–7234. doi: 10.1093/nar/19.25.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen V. L., Spritz R. A. Splicing of plant pre-mRNAs in animal systems and vice versa. Gene. 1987;56(2-3):253–265. doi: 10.1016/0378-1119(87)90142-9. [DOI] [PubMed] [Google Scholar]