Abstract
Hepatic β-adrenergic receptors (β-ARs) play a pivotal role in mobilization of reserves via gluconeogenesis and glycogenolysis to supply the animal with its energy needs during decreased nutrient availability. Using a unique nutrient-deprived baboon model, we have demonstrated for the first time that immunoreactive hepatic β1- and β2-AR subtypes are regionally distributed and localized on cells around the central lobular vein in 0.5 and 0.9 gestation (G) fetuses of ad libitum fed control (CTR) and maternal nutrient restricted (MNR) mothers. Furthermore, MNR decreased fetal liver immunoreactive β1-AR and increased immunoreactive β2-AR at 0.5G. However, at 0.9G, immunohistochemistry and Western blot analysis revealed a decrease in β1-AR and no change in β2-AR levels. Thus, MNR in a nonhuman primate species has effects on hepatic β1- and β2-ARs that are receptor- and gestation stage-specific and may represent compensatory systems whose effects would increase glucose availability in the presence of nutrient deprivation.
Keywords: β-adrenergic receptor, immunolocalization, gene expression, protein expression, baboon
Introduction
The fetus is completely dependent on its mother for nutrients. Decreased maternal, and hence fetal, nutrient supply leads to slowing of fetal and placental growth and compensatory changes in fetal metabolism.1 Several fetal and placental endocrine and metabolic responses to maternal nutrient reduction (MNR) have been intensively studied including changes in fetal liver development. Among the documented fetal hepatic responses to reduced nutrient availability in baboon pregnancy are altered functions of the insulin-like growth factor (IGF) axis including increased liver glycogen.2 Since the fetal liver plays a central role in basal as well as perturbed fetal metabolic function, we have begun a series of studies to evaluate both normal ontogeny and responses to MNR.2,3 Part of our rationale is the need to rectify the scarcity of information available on the ontogeny and physiological responses of fetal hepatic beta-adrenergic receptors (β-ARs) to challenges such as reduced maternal nutrition. A PubMed search at the beginning of 2009 using the search words “fetus” and “catecholamines” listed 2060 publications but not one on the liver in the first hundred references back to 2004. When “liver” was added to the search, the number of publications was reduced to 104. The first relevant paper was in 1996 and reported a study on developing receptors in neonatal rats.4 There were no references to studies on effects of decreased nutrient delivery to the fetus on the development of the adrenergic system within the fetal liver.
Catecholamines acting via β-ARs coupled to various effectors mediate adrenergic responsiveness in a wide variety of tissues.5–9 Agonist-activation of hepatic β-ARs (β1- and β2-AR subtypes) linked to adenylyl cyclase (AC) augment intracellular cyclic adenosine monophosphate (cAMP) levels accelerating liver glycogenolysis and gluconeogenesis pathways.5,8 An increase in β-AR numbers heightens hepatic activities of gluconeogenic enzymes and plasma glucose levels, suggesting functional importance of receptor numbers in gluconeogenesis.5 Furthermore, since β2-ARs regulate lipolysis in adipose tissue, they likely play a central role in energy management in late fetal life and the perinatal period.10
The goal of the present study was to determine the effects of a carefully controlled and monitored moderate degree of global MNR on the fetal baboon hepatic β-AR system. We have previously demonstrated that in baboons of similar phenotype, maternal consumption of 70% of the global ad libitum diet fed from 0.16 to 0.5 gestation (G) of pregnancy results in an 11% decrease in maternal body weight while not significantly altering fetal weight that fell about 10% and a marginal effect on fetal liver weight which fell 12% (0.10 > P > .05). However, this moderate level of MNR does alter the detailed histology of the kidney and expression of key genes11 as well as produce major changes in the hepatic and placental IGF systems.2,12 Importantly, fetal liver glycogen somewhat paradoxically increased2 and both messenger RNA (mRNA) and protein expression of the key hepatic gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK-1) increased.3 A similar rise in liver glycogen has been shown in a model of intrauterine growth restriction (IUGR) in the sheep.13 We therefore hypothesized that MNR would increase the abundance of β-AR in the fetal liver.
Materials and Methods
Animal Care and Maintenance
All procedures were approved by the Southwest Foundation for Biomedical Research (SFBR) and University of Texas Health Science Center at San Antonio (UTHSCSA) Institutional Animal Care and Use Committees and conducted in Association for Assessment and Accreditation of Laboratory Animal Care approved facilities. Details of housing structure and environmental enrichment provided have been published in detail previously.14
System for controlling and recording individual feeding
Once a day prior to feeding, all baboons were run into individual feeding cages. Baboons passed along the chute, over a weighing scale and into one of the individual feeding cages. Once in the individual cages, they were fed either between 0700 h and 0900 h or 1100 h and 1300 h as previously described.14,15 Food was provided as Purina Monkey Diet 5038, standard biscuits. Water was continuously available in the feeding cage through individual lixits and at several locations in the group housing.
Formation of stable grouping for the nutrient reduction study
Groups of 16 female baboons were initially housed with a vasectomized male to establish a stable social group.14,15 All female baboons were observed twice a day for well-being and 3 times a week for turgescence (sex skin swelling) and signs of vaginal bleeding to enable timing of pregnancy.16 At the end of a 30-day period of adaptation to the feeding system, a fertile male was introduced into each breeding cage. Cages were chosen at random to provide either control (CTR) mothers fed ad libitum or MNR mothers fed 70% of feed consumed by CTR on a weight-adjusted basis.14 Pregnancy was dated initially by following the changes in the swelling of the sex skin and confirmed at 30 days of gestation (30 dga) by ultrasonography. All baboons were fed ad libitum until 30 dga after which 15 control (CTR) baboons continued to feed ad libitum and feed of 12 MNR baboons was reduced.
Diet and food consumption
The Purina Monkey Diet 5038 is described by the vendor as “a complete life-cycle diet for all Old World Primates.” The biscuit contains stabilized vitamin C as well as all other required vitamins. Its basic composition is crude protein not less than 15%, crude fat not less than 5%, crude fiber not more than 6%, ash not more than 5%, and added minerals not more than 3%.14,15 At the start of the feeding period, each baboon was given 60 biscuits in the feeding tray. At the end of the 2-hour feeding period after the baboons had returned to the group cage, the biscuits remaining in the tray and on the floor of the cage and in the pan were counted. Food consumption of animals, their weights, and health status were recorded daily. The weight of each baboon was obtained as she crossed the electronic scale system (GSE 665; GSE Scale Systems, Michigan). A commercial software application designed to capture weight data was modified to permit the recording of 50 individual measurements over 3 seconds. If the standard deviation of the weight measurement was greater than 0.01 of the mean weight, the weight was automatically discarded and the weighing procedure begun again.
Cesarean Section, Fetal and Maternal Morphometry, and Blood Sampling
Maternal morphometric measurements were made prior to pregnancy to ensure homogeneity of weight and general morphometrics in the female baboons used in the present study. Cesarean sections were evenly spread throughout the year and performed at 90 dga (0.5G—Term 184 dga; CTR, n = 8 [3 males, 5 females]; MNR, n = 6 [3 males, 3 females]) or at 165 dga (0.9G; CTR, n = 7 [3 males, 4 females]; MNR, n = 6 [3 males, 3 females]) under isoflurane anesthesia (2%, 2 L/min) to obtain the fetus and placenta. Prior to exteriorizing the fetus from the uterine cavity, umbilical vein blood sampling was performed as described previously.15 Maternal fasting blood samples were drawn from the femoral vein on the morning before cesarean section directly into a 4-mL Vacutainer clot tubes containing Na-F (Becton Dickinson, Franklin Lakes, New Jersey).15 Clotted blood was centrifuged at 10 000g for 10 minutes and the serum removed within 1 hour of collection. Glucose was measured in maternal and fetal serum using a Beckman Synchron CX5CE analyzer (Beckman Coulter, Inc., Fullerton, California). Techniques used and postoperative maintenance have been previously described in detail.15 Analgesia was provided with Buprenorphine hydrochloride 0.015 mg.kg−1.d−1 during 3 postoperative days (Buprenex Injectable, Reckitt Benckiser Health care (UK) Ltd, Hull, England HU8 7DS). The placenta and fetus were submitted for morphometric measurements, complete pathologic evaluation and tissue sampling. Fetal livers were rapidly removed, cut into pieces, and either fixed in 10% buffered formalin or quick-frozen in liquid nitrogen before the fetus was euthanized by exsanguination while still under general anesthesia. Liver sections of the central lobe were used in the present study.
Quantitative Reverse Transcription−Polymerase Chain Reaction
Total RNA was isolated from frozen fetal (0.9G) liver (central lobe) pieces using TRI Reagent (Molecular Research Center, Ohio) according to the manufacturer’s instructions. The RNA samples were treated with DNAse I (RNAse-free) in the presence of 4.2 mmol/L MgCl2 in a standard thermocycler (37°C/30 min, 75°C/10 min, 4°C). RNA samples were reverse transcribed in the presence of 5X First strand buffer, 10 mmol/L DTT, random hexamer primers, and SuperScript RTIII. Complementary DNA (cDNA) synthesis was carried out in a thermocycler at 25°C/10 min, 42°C/50 min, 72°C/10 min, and 4°C. Real time reverse transcription−polymerase chain reaction (RT-PCR) assay was then performed by the ΔΔCt method17 using the TaqMan probe set for human β1-AR (Hs00265096_s1; Applied Biosystems, California) and β2-AR (Hs00240532_s1; Applied Biosystems) on an ABI 7900 Sequence Detection System. In each experiment, mRNA levels were normalized to 18S ribosomal RNA (rRNA; measured using TaqMan Gene Expression Assay for 18S, #Hs99999901_s1, from Applied Biosystems), which did not change under experimental conditions (data not shown).
Immunohistochemistry to Determine Location and Quantity of β1- and β2-AR Protein
Immunohistochemistry was done using standard avidin-biotin histochemical technique as previously described.2 Initial titrations were performed with 3 concentrations of primary antibody that bracketed the suggested dilution of the manufacturer. Final primary antibody concentration was adjusted to give the cleanest immunostaining achievable, for example, for a cytoplasmic protein, we would aim for the best signal with the least extracellular immunoreactivity and no nuclear immune product. Whenever possible, the primary antibody in question was “preabsorbed,” that is, incubated with excess of the antigen in question and then used in a staining run with the tissue of interest. Absence of immunostaining under these conditions assured that said antibody had only a single antigen in the tissue of interest; antibodies failing this test were never used. In the absence of availability of antigen for preabsorbtion, controls were run with normal serum from the species in which the antibody was generated in place of primary antibody to rule out nonspecific binding. Once the final dilution of the primary antibody was determined, all sections to be compared via counting program were immunostained in the same assay to assure identical conditions. In addition, sections known to contain antigen expression were included as a positive control in each run. The within-assay coefficient of variability for this assay was 21%. A standard curve was created at the final dilution plotting density versus time to assure linearity. Six pictures (2650 × 1920 pixels) were taken and analyzed with density windows of β1-AR = 0-170 and β2-AR = 0-185; γ 0.75) at the 2, 4, 6, 8, 10, and 12 O’clock section positions and analyzed with NIH Image J software for fraction (area immunostained ÷ area of the field of interest × 100%) and density (arbitrary density units).
Western Blot Analysis for β1- and β2-AR Protein
Frozen central lobe liver pieces were homogenized in lysis buffer (50 mmol/L NaCl, 1% NP-40, 50 mmol/L Tris-HCl, pH 7.4) containing protease and phosphatase inhibitors. Homogenates were rocked at 4°C for 30 minutes, followed by centrifugation at 10 000g for 2 minutes at 4°C. The supernatant proteins were estimated by BCA assay.18 Protein samples (40-70 µg) were added to 10 µL of 4X sample buffer (150 mmol/L Tris-HCl, pH 8.8, 1% SDS, 40% glycerol) and β-mercaptoethanol and then diluted with lysis buffer to a total volume of 40 µL. Samples were size-fractionated on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and electroblotted overnight onto 0.2 μm PVDF membranes. Membranes were immunoblotted overnight at 4°C with a primary antibody (1:250 dilution, β1-AR [# sc-568]; 1:250 dilution, β2-AR [#sc-9042]; 1:1000 dilution, β-actin [sc-47778]; Santa Cruz Biotechnology, California) followed by a secondary horseradish peroxidase-conjugated antibody [(1:10 000 dilution, anti-rabbit #111–036–046; 1:10 000 dilution, anti-mouse #115–036–071; Jackson ImmunoResearch Laboratories, Pennsylvania) for 1 hour at room temperature. Specific proteins were visualized using an enhanced chemiluminescence kit (Pierce, Illinois), and immunoblots were quantified with Scion Image analysis software (Scion Corporation, Maryland). β1-AR and β2-AR abundances were normalized against β-actin.
Data Analysis
Morphometric variables and immunohistochemical density of staining and fraction of field stained were compared between treatment groups at 0.5G and 0.9G using the nonpaired Student t-test. At 0.9G, fetal hepatic protein levels acquired by Western analysis and mRNA by RT-PCR were compared between CTR and MNR groups using Student t-test. The Statistical Analysis System (SAS) software program was used for all analyses and significance was accepted at P < .05.
Results
Morphometric Changes Resulting From Maternal Nutrient Reduction
Table 1 shows the major morphometric and organ weights in the mothers and fetuses. At both gestational ages, maternal weight was significantly reduced by the 30% level of nutrient reduction imposed. At 0.9G, placental weight and fetal body mass index (BMI) were significantly reduced by MNR while the decrease in weight of the fetal liver, spleen, and heart approached significance (0.05 < P < .10).
Table 1.
Maternal and Fetal Morphometric Data at 0.5G and 0.9G, in Control Ad Libitum Fed Pregnancies (CTR) and in the Presence of Maternal Nutrient Reduction (MNR) to 70% of the Food Eaten by CTR Mothers on a Weight-Adjusted Basis
| CTR 0.5G; N = 8 | MNR 0.5G; N = 6 | CTR 0.9G; N = 7 | MNR 0.9G; N = 6 | |
|---|---|---|---|---|
| Maternal weight pre-conception (kg) | 13.68 ± 0.51 | 13.02 ± 0.24 | 15 ± 0.82 | 14.93 ± 0.39 |
| Maternal weight at CS (kg) | 13.72 ± 0.41 | 12.16 ± 0.34 b | 16.63 ± 0.69 | 14.11 ± 0.77 b |
| Change in maternal weight (%) | 0.04 ± 0.28 | −0.86 ± 0.20 b | 11.43 ± 2.58 | −5.63 ± 3.89 b |
| Placental weight (g) | 70.36 ± 5.09 | 62.93 ± 1.48 | 177.29 ± 7.89 | 145.00 ± 7.23 b |
| Fetal weight (g) | 100.93 ± 3.37 | 95.43 ± 3.26 | 744.43 ± 31.59 | 668.5 ± 33.41 |
| Length (cm) | 17.94 ± 0.31 | 17.58 ± 0.44 | 36.36 ± 0.76 | 37.5 ± 1.02 |
| BMI (kg/m2) | 3.14 ± 0.09 | 3.1 ± 0.12 | 5.64 ± 0.2 | 4.77 ± 0.20 b |
| Fetal liver (g) | 3.27 ± 0.15 | 3.17 ± 0.20 | 20.48 ± 0.44 | 17.98 ± 1.36 c |
| Fetal spleen (g) | 0.59 ± 0.19 | 0.30 ± 0.04 | 1.43 ± 0.13 | 1.10 ± 0.03 c |
| Fetal kidney L (g) | 0.52 ± 0.11 | 0.44 ± 0.08 | 1.68 ± 0.15 | 1.82 ± 0.14 |
| Fetal kidney R (g) | 0.44 ± 0.07 | 0.40 ± 0.05 | 1.77 ± 0.10 | 1.74 ± 0.14 |
| Total fetal kidneys L + R (g) | 0.95 ± 0.15 | 0.83 ± 0.13 | 3.45 ± 0.24 | 3.56 ± 0.27 |
| Fetal lung (g) | 3.53 ± 0.11 | 3.38 ± 0.14 | 17.79 ± 0.86 | 17.94 ± 1.28 |
| Fetal heart (g) | 0.94 ± 0.27 | 0.62 ± 0.06 | 4.43 ± 0.37 | 3.52 ± 0.08 c |
| Fetal glucose (mg/dL) | 47.14 ± 4.35 | 39 ± 7.44 | 33.83 ± 4.71 | 41.67 ± 2.8 |
| Maternal glucose (mg/dL) | 74.38 ± 9.63 | 85.83 ± 10.63 | 61.86 ± 7.21 | 69.17 ± 5.52 |
| Fetal/maternal glucose | 0.66 ± 0.08 | 0.47 ± 0.07 | 0.63 ± 0.17 | 0.61 ± 0.03 |
Abbreviations: BMI, body mass index; L, left; R, right; CS, cesarean section.
a Data expressed as mean ± SEM.
b P < .05 MNR compared to CTR at same age.
c .05 < P < .10 MNR compared to CTR at same age.
Histological Localization and Quantification of Fetal Liver β1- and β2-AR Immunoreactivity
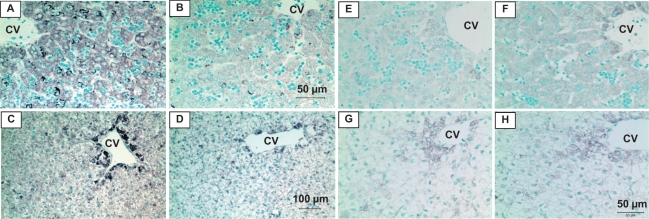
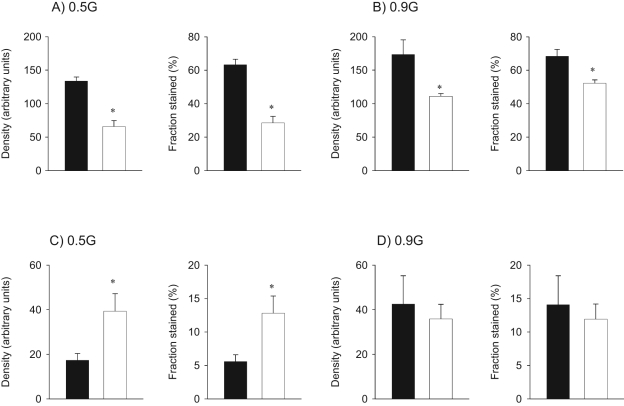
Both β1- and β2-AR immunoreactivity showed regional localization being concentrated around the central vein of the liver lobule (Figure 1 ). Maternal nutrient restricted decreased both density and fraction stained for hepatic β1-AR protein levels by 51% to 55% at 0.5G and by 24% to 36% at 0.9G when compared to fetuses of mothers fed ad libitum (Figure 2A and 2B). In contrast, MNR increased β2-AR immunoreactivity by 127% to 129% at 0.5G (Figure 2C) while there was no difference between fetuses of CTR and MNR at 0.9G (Figure 2D). No gender differences were observed in liver β1- and β2-AR immunoreactivity at 0.5G or 0.9G in the presence of MNR and hence the results were pooled.
Figure 1.
Immunoreactive β1-adrenergic receptors (AR; A-D) and β2-AR (E-H) in fetal baboon liver at 0.5 and 0.9 gestation (G). β1- and β2-AR immunoreactivity in liver of fetuses from mothers fed ad libitum (A, C and E, G) or nutrient-restricted (B, D and F, H) from 0.16G to 0.5G (A, B and E, F) or 0.9G (C, D and G, H). Central vein (CV). Panels A, B (50 µm); Panels C, D (100 µm); Panels E-H (50 µm).
Figure 2.
β1-adrenergic receptors (AR) and β2-AR immunoreactivity in fetal baboon liver. β1-AR (panels A and B) and β2-AR (panels C and D) immunoreactivity, expressed as density and fraction, at 0.5G and 0.9 gestation (G) in fetal baboon liver from mothers that were fed ad libitum (control [CTR], ▪) or 70% CTR diet (maternal nutrient restricted [MNR], □) from 0.16G to 0.5G (N = 8 CTR, 6 MNR) or 0.9G (N = 7 CTR, 6 MNR). Data expressed as mean ± SEM; *, P < .05 versus CTR.
Evaluation of β1- and β2-AR Protein by Western Blot Analysis
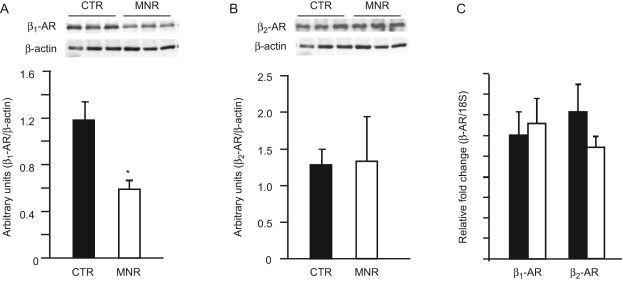
To confirm the findings obtained by immunohistochemistry at 0.9G, β-AR protein levels in fetal liver tissues of mothers on CTR versus MNR diet were measured by Western blot analysis. Several other analyses had been performed on the livers from fetuses at 0.5G and enough tissue was unavailable to perform Western blot analysis on liver at this age. As can be observed in Figure 3 , MNR reduced β1-AR protein by 51% (Figure 3A) while β2-AR protein levels remained unchanged (Figure 3B) confirming our immunohistochemical results at 0.9G.
Figure 3.
β1-adrenergic receptors (AR) and β2-AR protein and messenger RNA (mRNA) levels in fetal baboon liver at 0.9 gestation (G). β1-AR (panel A) and β2-AR (panel B) protein levels in fetal baboon liver at 0.9G from mothers fed ad libitum (control [CTR], ▪, N = 7), or 70% CTR diet (maternal nutrient restricted [MNR], □, N = 6) as measured by Western blotting. Inset shows representative immunoblots from 3 CTR and 3 MNR fetuses and protein levels of β-actin that were used as loading controls. Panel C: β1- and β2-AR mRNA levels as determined by quantitative reverse transcription−polymerase chain reaction (qRT-PCR) in liver homogenates from 0.9G fetuses of mothers on CTR or MNR diet. Data expressed as mean ± SEM; *, P < .05 compared to CTR.
Evaluation of β1- and β2-AR mRNA by Quantitative RT-PCR
Fetal liver β1- and β2-AR mRNA expression at 0.9G was determined by quantitative RT-PCR (qRT-PCR). As shown in Figure 3C, hepatic β1- and β2-AR mRNA expression remained unchanged at 0.9G between fetuses of CTR and MNR baboons.
Discussion
Beta-ARs transduce effects of catecholamines released by the adrenal glands and the sympathetic nervous system via adenylyl cyclase and other effectors7,9,19 to regulate a wide range of physiological functions, from cardiac performance to muscle tone, metabolism, and immune function.7,20,21 Catecholamines rise sharply at the time of birth and are involved in the body’s adaptation to several features of the new external environment including temperature and altered nutrient availability.22–24 No data are available for normal ontogeny and changes in β-AR abundance in response to MNR in primates. In the present study, we have shown for the first time that moderate global reduction in maternal nutrition has differential stage of gestation-specific effects on β1- and β2-AR subtypes in the fetal baboon liver.
Using molecular and functional approaches, three subtypes of β-ARs (β1-, β2-, and β3-ARs) have been characterized that can be distinguished based on their distinct tissue-specific expression patterns, pharmacological heterogeneity, ability to couple to different G-proteins, and desensitization patterns.21 Using receptor autoradiography, it was shown that the β1-AR is present in the fetal mouse heart and β2-AR in the liver and vasculature at E12.5, a stage of development considered equivalent to the second month of gestation in humans.25 In binding studies using β-AR agonist iodopindolol which does not distinguish between β1- and β2-ARs, it was observed that β-ARs are present in rat embryos as early as gestational day 12 and increase nearly 5-fold to levels comparable to that seen in mature tissues by gestational day 18. Further studies using β1- and β2-AR-specific ligands demonstrated that β-ARs are higher in fetal rat liver than in heart and brain, and predominantly of the β2-AR subtype.26 In the bovine from late fetal into adult life, mRNA for all 3 β-AR subtypes was observed in the liver, with β2-ARs being the dominant subtype.5 On the other hand, in human liver, the presence of β1- and β2-ARs but not β3-ARs has been documented.27 Taken together, these studies indicate that there are species-specific differences with respect to subtypes and age-dependency of hepatic β-AR expression.
Fetal human liver develops responsiveness to adrenergic stimuli at the 12th week of gestation.28 However, to our knowledge, the distribution and ontogenetic changes in β-ARs during fetal life has not been described in any primate species. As there is a high degree of evolutionary conservation between proteins in baboons and humans as well as the similarities between the 2 species in ontogeny, reproductive physiology, placentation, and maternal-fetal transfer,16,29,30 we conducted the present study to investigate the distribution and ontogenetic changes in β-ARs in fetal baboon liver in the normative and MNR situation. In the present study, we have demonstrated the presence of both β1- and β2-ARs but not β3-ARs (data not shown) in the fetal baboon liver. Immunoreactivity for both the receptors localized on cells around the central lobular vein. In 1 previous study in postnatal rats, liver β1-AR was regionally distributed around the central lobular vein just as we have observed in the fetal baboon.31 The reason for this localization is not clear but the partial pressure of oxygen (pO2) and nutrient concentrations are very different within the lobule in both normal fetal life and when the fetus is exposed to different challenges. Interestingly, fetal liver glycogen as well as phosphoenolpyruvate carboxykinase (PEPCK), the rate-limiting enzyme for gluconeogenesis, is distributed similarly around the central lobular vein.2,3 Numerous studies have suggested that one of the mechanisms by which catecholamines mobilize glucose from the liver in times of need is by stimulation of PEPCK via activation of β-ARs.5,32,33 It is therefore plausible that localization of β-ARs, glycogen, and PEPCK around the central lobular vein is to maintain an adequate glucose status during fetal development.
Adrenergic receptor numbers are regulated both in a homologous fashion by catecholamines that generally downregulate receptor numbers and hence sensitivity and a heterologous fashion by mechanisms not directly dependent on catecholamine binding to their receptors. Results of our immunohistochemical studies have demonstrated that MNR decreases β1-AR and increases β2-AR protein levels in 0.5G fetuses. Although regulation of hepatic β-ARs is not well understood, glucocorticoids (GCs) have been reported to regulate the expression of β-ARs in a number of tissues including human lymphocytes and granulocytes, and rat lung, liver, and smooth muscle cells.34–37 We have previously demonstrated that MNR during baboon pregnancy increases circulating levels of fetal cortisol, the major GC in baboons.3 In many tissues including rat liver, GC increase β2-AR mRNA levels acting as transcriptional activators operating via GC response elements (GREs) located in the β2-AR gene.34,36 On the other hand, GCs downregulate the expression of β1-ARs in rat liver.36 It is suggested that a GC repressor domain in the rat β1-AR gene37 may contribute to the transcriptional suppression of β1-ARs by GC.36 Although β1-AR protein levels decline at 0.5G and 0.9G in response to MNR, β2-AR protein levels that increase at 0.5G in MNR fetuses show no change in protein levels at 0.9G in response to MNR. Additionally, β1- and β2-AR mRNA levels show no change in response to MNR at 0.9G. The reason for this differential regulation of β1- and β2-AR at 0.9G is not clear. However, numerous in vivo and in vitro studies have indicated that the interplay between β-AR agonist-promoted destabilization and steroid-induced transcription may control β-AR mRNA levels.38 Although at present it is not known if catecholamine levels change at 0.9G compared to 0.5G in response to MNR, results of our study as well as those of others implicate a role for GC in regulating β-AR gene expression in fetuses of nutrient-restricted baboons.
In our previous studies, we have demonstrated that moderate MNR during baboon pregnancy paradoxically increases fetal liver glycogen.2 Although specific functions of β1- and β2-ARs have not been determined in fetal primates, the combined effects of β-ARs are directed toward the mobilization of reserves to supply the fetus with its energy needs. These include glycogenolysis, gluconeogenesis, lipolysis, and fatty acid oxidation. The final change in fetal liver carbohydrate metabolism will depend on the balance of current and past availability of nutrients and the turnover due to energy needs. Increased fetal liver glycogen in our model clearly shows the ability at this degree of fetal nutrient reduction to increase glycogen stores.
The importance of our studies lies in the fact that these are the first data available in a primate species and there appear to be species differences in β-AR responses in rodents compared with other species. It has previously been demonstrated that β-adrenergic control of liver glycogenolysis in rats at different stages of the life course follows a “U”-shaped curve with β-AR-mediated glycogenolysis being prominent in juvenile male rats, declining rapidly during subsequent development such that it is virtually abolished by the time of young adulthood, and then reemerging during postmaturational development.39,40 In mature male rats, therefore, catecholamine stimulation of hepatic glycogenolysis is attributed to α-adrenergic receptors and is thought to be independent of β-receptor-mediated processes. However, under physiological conditions in man, catecholamine-stimulated glycogenolysis is predominantly via β2-AR.32 These differences add value to data such as those presented in the current study from nonhuman primates. Our studies using a unique nutrient-deprived baboon model have demonstrated for the first time that immunoreactive hepatic β1- and β2-ARs are regionally distributed and localized on cells around the central lobular vein in 0.5 and 0.9G fetuses of CTR and MNR mothers. The observation of plentiful β-ARs in the fetal baboon liver strengthens the value of this model in the translation of experimental observations to human physiology. Furthermore, our investigations demonstrate that MNR decreases immunoreactive β1-ARs at both 0.5 and 0.9G and increases immunoreactive β2-ARs at 0.5G but not at 0.9G. Thus, MNR in a nonhuman primate species has effects on hepatic β1- and β2-ARs that are receptor- and gestation stage-specific and may represent compensatory systems whose effects would increase glucose availability in the presence of nutrient deprivation.
Acknowledgment
We would like to acknowledge contributions by Greg Langone for the immunohistochemistry procedures.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Grant support from NIH grant HD 21350, Department of Veterans Affairs VISN 17 Award (AK) and Grant-in-Aid American Heart Association Award (AK).
References
- 1. Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol (Lond). 2004;561(pt 2):355–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li C, Schlabritz-Loutsevitch NE, Hubbard GB, et al. Effects of maternal global nutrient restriction on fetal baboon hepatic IGF system genes and gene products. Endocrinology. 2009;150(10):4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588(pt 8):1349–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thai L, Galluzzo JM, McCook EC, Seidler FJ, Slotkin TA. Atypical regulation of hepatic adenylyl cyclase and adrenergic receptors during a critical developmental period: agonists evoke supersensitivity accompanied by failure of receptor down-regulation. Pediatr Res. 1996;39(4 pt 1):697–707 [DOI] [PubMed] [Google Scholar]
- 5. Carron J, Morel C, Hammon HM, Blum JW. Ontogenetic development of mRNA levels and binding sites of hepatic [beta]-adrenergic receptors in cattle. Domest Anim Endocrinol. 2005;28(3):320–330 [DOI] [PubMed] [Google Scholar]
- 6. Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metabolism. 1980;29(11 suppl 1):1155–1163 [DOI] [PubMed] [Google Scholar]
- 7. Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmelck PH, Hanoune J. The hepatic adrenergic receptors. Mol Cell Biochem. 1980;33(1-2):35–48 [DOI] [PubMed] [Google Scholar]
- 9. Xiao RP, Zhu W, Zheng M, et al. Subtype-specific [alpha]1- and [beta]-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27(6):330–337 [DOI] [PubMed] [Google Scholar]
- 10. Large V, Hellström L, Reynisdottir S, et al. Human beta-2 adrenoceptor gene polymorphisms are highly frequent in obesity and associate with altered adipocyte beta-2 adrenoceptor function. J Clin Invest. 1997;100(12):3005–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nijland MJ, Schlabritz-Loutsevitch N, Hubbard GB, Nathanielsz PW, Cox LA. Nonhuman primate fetal kidney transcriptome analysis indicates mTOR is a central nutrient responsive pathway. J Physiol. 2007;579(pt 3):643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li C, Levitz M, Hubbard GB, et al. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28(11-12):1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rozance PJ, Limesand SW, Barry JS, et al. Chronic late gestation hypoglycemia up-regulates hepatic PEPCK associated with increased PGC1{alpha} mRNA and pCREB in fetal sheep. Am J Physiol Endocrinol Metab. 2008;294:E365–E370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlabritz-Loutsevitch NE, Howell K, Rice K, et al. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33(3):117–126 [DOI] [PubMed] [Google Scholar]
- 15. Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, et al. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species). J Med Primatol. 2004;33(3):152–162 [DOI] [PubMed] [Google Scholar]
- 16. Hendrickx AG. Reproduction. Embryology of the Baboon. Chicago, IL: The University of Chicago Press; 1971:18–20 [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression using real-time quantitative PCR and the 2-(Delta Delta C(T)) Method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 18. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85 [DOI] [PubMed] [Google Scholar]
- 19. Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. PKA-mediated phosphorylation of the beta 1-adrenergic receptor promotes Gs/Gi switching. Cell Signal. 2004;16(12):1397–1403 [DOI] [PubMed] [Google Scholar]
- 20. Cagliani R, Fumagalli M, Pozzoli U, et al. Diverse evolutionary histories for β-adrenoreceptor genes in humans. Am J Hum Genet. 2009;85(1):64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rohrer DK, Chruscinski AJ, Schauble EH, Bernstein D, Kobilka BK. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem. 1999;274(24):16701–16708 [DOI] [PubMed] [Google Scholar]
- 22. Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev. 1994;6(2):141–150 [DOI] [PubMed] [Google Scholar]
- 23. Ream MA, Chandra R, Peavey M, et al. High oxygen prevents fetal lethality due to lack of catecholamines. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R942–R953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller RD, Degasparo M. The autonomic nervous system and perinatal metabolism. Ciba Found Symp. 1981;83:291–309 [DOI] [PubMed] [Google Scholar]
- 25. Chandra R, Portbury AL, Ray A, Ream M, Groelle M, Chikaraishi DM. Beta1-adrenergic receptors maintain fetal heart rate and survival. Biol Neonate. 2006;89(3):147–158 [DOI] [PubMed] [Google Scholar]
- 26. Slotkin TA, Lau C, Seidler FJ. Beta-adrenergic receptor overexpression in the fetal rat: distribution, receptor subtypes, and coupling to adenylate cyclase activity via G-proteins. Toxicol Appl Pharmacol. 1994;129(2):223–234 [DOI] [PubMed] [Google Scholar]
- 27. Krief S, Lönnqvist F, Raimbault S, et al. Tissue distribution of β3-adrenergic receptor mRNA in man. J Clin Invest. 1993;91(1):344–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andersson SM. Beta-adrenergic induction of tyrosine aminotransferase in organ culture of fetal rat and fetal human liver. Endocrinology. 1983;112(2):466–469 [DOI] [PubMed] [Google Scholar]
- 29. Pauerstein CJ, Eddy CA, Croxatto HD, Hess R, Siler-Khodr TM, Croxatto HB. Temporal relationships of estrogen, progesterone, and luteinizing hormone levels to ovulation in women and infrahuman primates. Am J Obstet Gynecol. 1978;130(8):876–886 [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto Y, Manyon AT, Osawa Y, Kirdani RY, Sandberg AA. Androgen metabolism in the baboon: a comparison with the human. J Steroid Biochem. 1978;9(8):751–759 [DOI] [PubMed] [Google Scholar]
- 31. Cardani R, Zavanella T. Immunohistochemical localization of β1-adrenergic receptors in the liver of male and female F344 rat. Histochem Cell Biol. 2001;116(5):441–445 [DOI] [PubMed] [Google Scholar]
- 32. Erraji-Benchekroun L, Couton D, Postic C, et al. Overexpression of {beta}2-adrenergic receptors in mouse liver alters the expression of gluconeogenic and glycolytic enzymes. Am J Physiol Endocrinol Metab. 2005;288(4):E715–E722 [DOI] [PubMed] [Google Scholar]
- 33. Asensio C, Jimenez M, Kühne F, Rohner-Jeanrenaud F, Muzzin P. The lack of beta-adrenoceptors results in enhanced insulin sensitivity in mice exhibiting increased adiposity and glucose intolerance. Diabetes. 2005;54(12):3490–3495 [DOI] [PubMed] [Google Scholar]
- 34. Hadcock JR, Wang HY, Malbon CC. Agonist-induced destabilization of beta-adrenergic receptor mRNA. Attenuation of glucocorticoid-induced up-regulation of beta-adrenergic receptors. J Biol Chem. 1989;264(33):19928–19933 [PubMed] [Google Scholar]
- 35. Jazayeri A, Meyer WJ., 3rd Glucocorticoid modulation of beta-adrenergic receptors of cultured rat arterial smooth muscle cells. Hypertension. 1988;12(4):393–398 [DOI] [PubMed] [Google Scholar]
- 36. Kiely J, Hadcock JR, Bahouth SW, Malbon CC. Glucocorticoids down-regulate beta 1-adrenergic-receptor expression by suppressing transcription of the receptor gene. Biochem J. 1994;302(pt 2):397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bahouth SW, Park EA, Beauchamp M, Cui X, Malbon CC. Identification of a glucocorticoid repressor domain in the rat beta 1-adrenergic receptor gene. Recept Signal Transduct. 1996;6(3-4):141–149 [PubMed] [Google Scholar]
- 38. Cornett LE, Hiller FC, Jacobi SE, Cao W, McGraw DW. Identification of a glucocorticoid response element in the rat beta 2-adrenergic receptor gene. Mol Pharmacol. 1998;54(6):1016–1023 [DOI] [PubMed] [Google Scholar]
- 39. Katz MS, Dax EM, Gregerman RI. Beta adrenergic regulation of rat liver glycogenolysis during aging. Exp Gerontol. 1993;28(4-5):329–340 [DOI] [PubMed] [Google Scholar]
- 40. Katz MS, McNair CL, Hymer TK, Boland SR. Emergence of beta adrenergic-responsive hepatic glycogenolysis in male rats during post-maturational aging. Biochem Biophys Res Commun. 1987;147(2):724–730 [DOI] [PubMed] [Google Scholar]