Abstract
c-Jun N-terminal kinase (JNK) pathway has been shown to be essential for cell cycle progression and mitosis. We previously showed that this pathway is activated in mitotic granulosa cells of follicles from transitional to antral stages. In this study, we, therefore, aimed to investigate whether this signaling pathway has any effect on in-vitro growth of murine preantral follicles and granulosa cell cycle control. Two structurally different pharmacologic JNK inhibitors, SP600125 and AS601245, were used in the experiments. First their inhibitory concentrations were determined in granulosa cells by Western blot analysis. Then preantral follicles isolated from immature and adult C57BL/6 mice were cultured in matrigel and standard culture plates for 6 days with these inhibitors. Spontaneously immortalized rat granulosa cells (SIGCs) were first synchronized at G1/S and G2/M stages of cell cycle and then treated with JNK inhibitors. Cell cycle progression was analyzed with Bromodeoxyuridine (BrdU) assay and flow cytometry analysis. Both inhibitors significantly inhibited phosphorylation of c-Jun in granulosa cells at 25, 50, and 100 μmol/L concentrations. Isolated preantral follicles cultured with these inhibitors exhibited arrested growth in culture in a dose-dependent manner. Cell cycle analyses showed that both inhibitors impair the progression of cell cycle at S phase and G2/M transition of granulosa cells. These results suggest that JNK pathway is essential for in vitro growth of preantral follicle growth and regulates both S phase and G2/M stages of cell cycle in granulosa cells.
Keywords: culture, preantral follicle, growth, matrigel, JNK, c-Jun, granulosa cell cycle
Introduction
Mitogen-activated protein kinases (MAPKs) are a family of protein kinases that phosphorylate specific serine and threonine residues of target protein substrates and integrate signals from a wide range of extracellular stimuli controlling cell proliferation, differentiation, survival, and apoptosis. The c-Jun NH2-terminal kinase (JNK) pathway is a member of MAPKs, which is activated primarily by cytokines and exposure to environmental stress. Specific stimuli trigger the activation of MAP3Ks, which then phosphorylate and activate the MAP2K isoforms MKK4 and MKK7, which in turn phosphorylate and activate JNK. A major target of the JNK signaling pathway is the activator protein 1 (AP-1) transcription factor, which is activated, in part, by the phosphorylation of c-Jun and related molecules.1 Both JNK and c-Jun have been shown to be essential for cell cycle progression and cell proliferation.2 We previously showed that phospho-c-Jun was exclusively expressed in mitotic granulosa cells of follicles from transitional to antral stages.3 SP600125 and AS601245 are among the most commonly used pharmacologic inhibitors of JNK signaling pathway. Both inhibitors are competetive inhibitors of JNK at adenosine triphosphate (ATP)-binding sites but their molecular structures are different.4
We have recently observed that JNK activity and phosphorylation of c-Jun become elevated during the G2/M transition of the cell cycle in immortalized fibroblasts and ovarian granulosa cells. Pharmacological inhibition of JNK causes a profound cell cycle arrest at the G2/M transition in both cell types.5
Spontaneously immortalized rat granulosa cells (SIGCs) were derived froman inbred strain of Berlin Duckrey (BD IV) rats and immortalized by serial passages. These cells exhibit an undifferentiated morphology, grow in culture in a monolayer fashion, and do not luteinize as the granulosa cells of preantral follicles.6 Therefore, this cell line could be used to dissect the mechanisms that control preantral follicle growth.
Matrigel is a solubulized basement membrane preparation extracted from Engelbreth-Holm-Swarm mouse sarcoma, a tumor rich in extracellular matrix proteins. At room temperature and 37°C, matrigel polymerizes to produce biologically active matrix material resembling the mammalian cellular basement membrane. Therefore, it provides a physiologically relevant 3-dimensional environment for a wide variety of studies varying from angiogenesis assays to in vitro cell differentiation, tumor growth, and transplantation.7 Our recent works showed that murine preantral follicles cultured in matrigel preserve their spherical structure, grow, and survive better compared to those cultured under standard conditions8 and that this model could be easily used to test the effects of different signaling pathways on in-vitro growth of preantral follicles.9,10
We have shown in this study that inhibiton of JNK pathway via its pharmacological inhibitors in granulosa cells caused a dose-dependent arrest in cycle progression of granulosa cells and follicle growth in vitro. We believe that these findings are novel and may have important implications for future. From the perspective of translational reproductive medicine and assisted reproduction, any explored signaling pathway in granulosa cells that has a direct effect on follicle growth is of paramount importance since early stages of follicle growth before gonadotropin-dependent antral stage are not completely understood.
Material and Methods
Animals and Follicle Isolations
Immature 14-day-old SV129/B6 and C57BL/6 mice were used in all experiments. The Institutional Animal Care and Use Committee approved the protocol. The ovaries were removed after euthanasia and minced into 2 or 3 pieces in pre-equilibrated and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered Dulbecco modified eagle medium-F12 (DMEM-F12) culture medium. Then the pieces were digested with collagenase, Dnase-I in DMEM-F12 supplemented with 5% bovine serum albumin (BSA) for 30 minutes at 37°C, as described previously.11 Preantral follicles were mechanically isolated using 28- to 30-gauge needles under stereomicroscope (Olympus SZX12, Olympus America Inc., Center Valley, PA, USA) and randomly assigned to different groups to be cultured for 6 days.
Culture Medium Formulation
All follicles were cultured in α-minimum essential media (α-MEM) with and without 10% fetal bovine serum (FBS) supplemented with 100 mIU/mL recombinant follicle-stimulating hormone (FSH), 3 mg/mL BSA, and 10 000 U/mL penicillin-G, 10 000 µg/mL streptomycin 25 µg/mL amphotericin-B at 37°C and 5% CO2 in air.
Culture in 3-Dimensional Basement Membrane Matrix (Matrigel)
Growth factor reduced matrigel was used in the study, as described by us previously.10 First matrigel was allowed to liquefy at 4°C and then diluted with the culture medium in a 1:1 ratio (100 μL from each) and placed in 96-well format culture plates. Following transfer of isolated follicles into it, the plates were put in the incubator for 30 minutes to allow matrigel to solidify and then 100 μL of culture media was added on the top of matrigel. Half of the culture media was replaced every other day with the addition of JNK inhibitors given at 12.5-25-50-100 μmol/L concentrations.
Culture in Standard Culture Plate
Isolated preantral follicles were cultured in 100 μL droplets of culture media covered with mineral oil in 6 cm culture plates. Half of the culture media was replaced every other day with the addition of JNK inhibitors given at doses mentioned.
Follicle Measurements and Evaluation of Survival
Follicles were imaged every other day using Olympus IX 70 inverted microscope with digital imager under ×300 and ×150 magnifications. Follicle diameter was calculated using the mean of 2 perpendicular measurements. The percentage follicular growth was calculated according to the following formula: ([Follicle diameter on day 7 − diameter day 0] × 100/follicle diameter on day 0). Follicles with disrupted basal lamina or somatic cell layers, extruded, damaged or misshapen oocytes, and those, which did not attach to culture plates in the first 24 hours of culture period were not included in the analysis.
Reagents
Alpha-minimum essential media and DMEM-F12 were purchased from Gibco (Invitrogen Co, Carlsbad California). Bovine serum albumin, collagenase, Dnase-I, and mouse monoclonal vinculin antibody were purchased from Sigma (Sigma Co, St Louis, Missouri). Recombinant FSH was obtained from Serono (Serono Inc, Rockland, Maryland). Growth factor reduced matrigel was purchased from BD Bioscience (BD Bioscience, San Jose, California). Mouse monoclonal phospho-c-Jun (Ser63) antibody was purchased from Santa Cruz (Santa Cruz Biotech Inc Santa Cruz, California). JNK inhibitors, SP600125 and AS601245, were purchased from Calbiochem (EMD Bioscience San Diego, California). JNK inhibitors were dissolved in dimethyl sulfoxide (DMSO) as instructed by their manufacturers. For every 10 µmol/L concentration of JNK Inhibitor, 0.1% DMSO was include in the culture medium as instructed.
Cell Culture and Synchronization
Spontaneously immortalized granulosa cells were provided by Dr Burghardt and grown in DMEM-F12 supplemented with 10% FBS and antibiotics. For synchronization at G1/S, cells were serum starved for 48 hours and then treated with complete media containing 2 μg/mL aphidicolin for 18 hours. Then cells were rinsed (time 0) and harvested at 2-hour intervals. At 6 hours after aphidicolin release, cells were at G2/M transition.
Flow Cytometry Analysis
Cells synchronized at G1/S by aphidicolin were treated with complete media (0 hour). At sixth hour, a set of plates was treated with JNK inhibitors SP600125 and AS601245 (Calbiochem Inc.) at 25 and 50 μmol/L concentrations in complete media. At indicated time points, the cells were trypsinized, washed, and fixed with 70% ethanol over night at 4°C. Washed cells were then stained with 25 μg/mL propidium iodide in 4 mmol/L sodium citrate/0.1%TritonX-100, and 25 μg/mL DNase-free RNase A. Flow cytometry analyses were performed using FACS Calibur machine and the FlowJo program.
BrdU Uptake
Cells synchronized at G1/S by aphidicolin were washed 3 times and treated with complete media (0 hour). A subset of plates was treated with 50 μmol/L of SP600125 and AS601245 at time 0. At second hour, cells were incubated with 10 μmol/L of BrdU (Roche Applied Science, Germany) for 30 minutes at 37°C 5% CO2 in air, with and without JNK inhibitors and then fixed using 70% ice-cold ethanol for 20 minutes and washed 3 times with PBS. Then cells were treated with anti-BrdU working solution including anti-BrdU mouse monoclonal antibody and nucleases for denaturation of DNA at 37°C for 30 minutes and followed by incubation with anti-mouse immunoglobulin (Ig) fluorescein antibody at 37°C for 30 minutes. Cells were then stained with 4’,6-diamidino-2-phenylindole (DAPI) for immunofluorescence analysis.
Western Blot
Spontaneously immortalized granulosa cells were first serum starved for 48 hours and then stimulated with complete culture media including 10% FBS with and without JNK inhibitors at mentioned doses. Cell lysates were prepared at indicated time points using radioimmunopreceipitation assay (RIPA) buffer (sodium dodecyl sulfate 0.1%, sodium deoxycholate 0.5%, NP-40 1%. NaCl 150 mmol/L, Tris-HCl 50 mmol/L, EDTA 2.5 mmol/L, NaF 25 mmol/L, sodium pyrophosphate 10 mmol/L, sodium orthovanadate 1 mmol/L with protease inhibitors phenylmethylsulfonyl fluoride (PMSF) 100 mmol/L, aprotinin 1 μg/mL, leupeptin 1 μg/mL, and pepstatin 1 μg/mL) and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. After electrophoresis, proteins were transferred to nitrocellulose membrane using semi-dry blot device (Transblot, BioRad, Hercules California). The membrane was blocked with 5% dry milk in tris-buffered saline (TBS) and blotted with antibodies against phospho c-Jun (mouse monoclonal Santa Cruz Biotech Inc), and vinculin (mouse monoclonal Sigma St Louis, Missouri) antibodies overnight at 4°C. Then the membrane was blotted with horseradish peroxidase (HRP)-conjugated secondary antibody and images were developed using chemiluminescence device (BioRad).
Statistical Analysis
Follicular growth was expressed as percentage of the mean growth ± standard error of mean (SEM) of at least two independent experimental replicates. For comparison of growth, analysis of variance (ANOVA) or Kruskal-Wallis was used where appropriate. Growth bars were created using GraphPad Prism (GraphPad Prism, GraphPad Software Inc) and Microsoft excel (Office 2000, Microsoft Inc). P < .05 was considered significant.
Results
Inhibition of JNK Pathway Halts In Vitro Growth of Isolated Murine Preantral Follicles
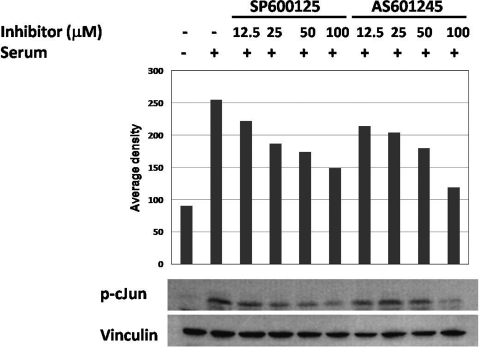
We first determined the inhibitory concentrations of JNK inhibitors in granulosa cells by Western blot. As shown in Figure 1 , both SP600125 and AS601245 inhibited phosphorylation of c-Jun in a dose-dependent manner with significant decrease of c-Jun phosphorylation at 25 and 50 μmol/L concentrations and an almost complete abolishment of the signal at 100 μmol/L dose after 1 hour.
Figure 1.
The inhibition of c-Jun phosphorylation in granulosa cells with JNK inhibitors SP600125 and AS601245 as quantified as an average density in Western blot. c-Jun phosphorylation was significantly decreased at 25 and 50 μmol/L, and almost abolished at 100 μmol/L concentrations of SP600125 and AS601245, respectively, 1 hour after treatment. JNK indicates c-Jun N-terminal kinase.
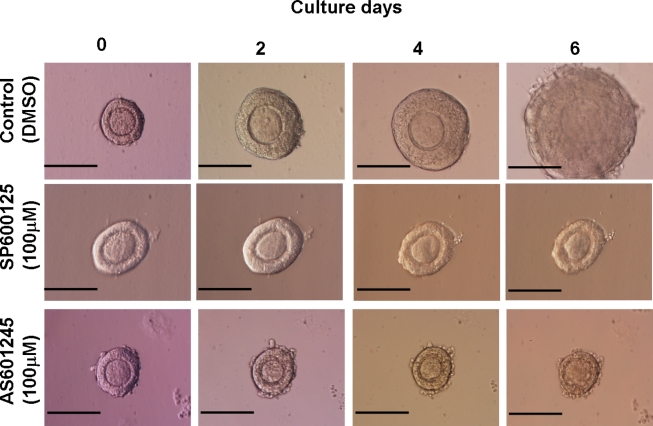
We then cultured isolated preantral follicles in matrigel for 6 days with either inhibitor at 25-50-100 μmol/L concentrations. Mean follicle diameters at days 0 and 6 and the mean percentages of growth after 6 days of culture were shown in Table 1 . The pictures of follicles cultured in matrigel are shown in Figure 2 . Control follicles grew 72.1% at the end of the 6-day culture period. However, follicles treated with SP600125 at 25 and 50 μmol/L grew 22.8% and 9.75%, respectively, compared to control follicles (P < .01). At 100 μmol/L concentrations, follicle growth is completely arrested (P < .0001; Figure 2). Similarly treatment of preantral follicles with AS601245 caused a dose-dependent inhibition of growth with no growth at 100 μmol/L (P < .0001; Table 1 and Figure 2).
Table 1.
The Number of Follicles, the Mean Diameter of Follicles on Days 0 and 6, and the Mean Percentage of Growth for Control and JNK Inhibitor Groups After 6 Days of Culture in Matrigela
| Groups | N | Follicle Diameter, Mean ± SEM (Day 0) | Follicle Diameter, Mean ± SEM (Day 6) | % Growth Mean ± SEM |
|---|---|---|---|---|
| Control (DMSO) | 13 | 120.6 ± 1.4 | 208.1 ± 7.4 | 72.1 ± 5.4b,c |
| SP600125 (25 μmol/L) | 10 | 130.9 ± 11 | 161.1 ± 15.1 | 22.8 ± 1.4 b |
| SP600125 (50 μ mol/L) | 11 | 125.8 ± 12.6 | 141.1 ± 20.7 | 9.75 ± 6.0 b |
| SP600125 (100 μ mol/L) | 12 | 113.6 ± 9.9 | 103.1 ± 5.3 | −8.02 ± 4.1 c |
| AS601245 (25 μ mol/L) | 9 | 145.5 ± 9.6 | 169.1 ± 13.2 | 16.2 ± 12.9 b |
| AS601245 (50 μ mol/L) | 10 | 107.2 ±19.4 | 117.2 ± 23.8 | 9.23 ± 6.4 b |
| AS601245 (100 μ mol/L) | 10 | 126.9 ± 10.7 | 113.2 ± 9.9 | −10.5 ± 1.8 c |
Abbreviations: JNK, c-Jun N-terminal kinase pathway; DMSO, dimethyl sulfoxide.
a Note the dose-dependent decrease in growth of preantral follicles treated with JNK inhibitors SP600125 and AS6012145 at 25, 50, and 100 μ mol/L concentrations.
b P < .01.
c P < .0001.
Figure 2.
Arrested growth and regression of diameter of follicles cultured in matrigel with JNK inhibitors at 100 μmol/L dose for 6 days compared to growing control follicle. Note the arrested growth and regression of follicles treated with JNK inhibitors in comparison to growing control follicle (scale bar 100 μ). JNK indicates c-Jun N-terminal kinase.
To rule out the possibility that the observed arrest in preantral follicle growth after JNK inhibitor treatment might have been specific to culture conditions, preantral follicles were cultured on standard culture plate as well as in matrigel with and without serum supplementation for 6 days. As shown in Supplementary Table and Figure, abolishment of JNK pathway inhibited preantral follicle growth in vitro, regardless of culture condition and the presence of serum, suggesting an indispensable role of JNK signaling pathway in in-vitro growth of preantral follicles.
Inhibition of JNK Pathway Impairs S Phase and Blocks Cell Cycle at G2/M Phase
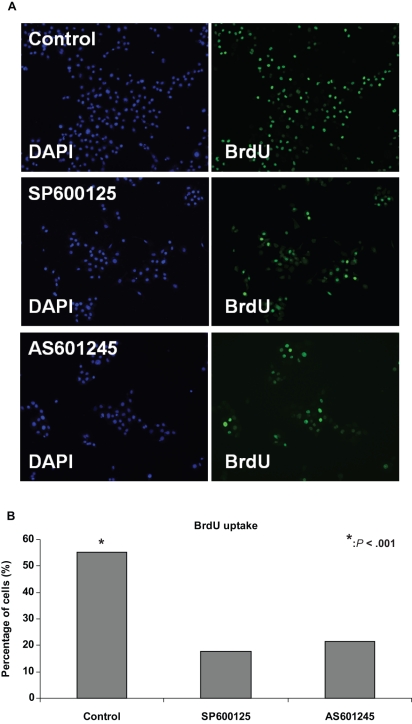
To further dissect the mechanism underlying the arrest in in-vitro growth of preantral follicles induced by JNK inhibitors, we analyzed the effect of inhibition of JNK pathway on cell cycle progression of granulosa cells. For this purpose, SIGCs were first synchronized at G1/S by aphidicolin, washed, and re-plated in serum-supplemented medium (time 0). The inhibitors were given at the beginning of S phase, and BrdU uptake analysis was performed using immune-fluorescence staining. As shown in Figure 3A and B, 17% and 21% of cells treated with 50 μmol/L SP600125 and AS601245, respectively, were BrdU positive compared to 55% of control cells (P < .001).
Figure 3.
BrdU uptake assay of granulosa cells by immune-fluorescence analysis. Granulosa cells synchronized at G1/S interphase were treated with 50 μmol/L SP600125 and AS601245 and BrdU uptake was compared between control and JNK inhibitor groups; 17% and 21 % of cells treated with 50 μmol/L SP600125 and AS601245, respectively, were BrdU positive compared to 55% of control cells (P < .001). JNK indicates c-Jun N-terminal kinase.
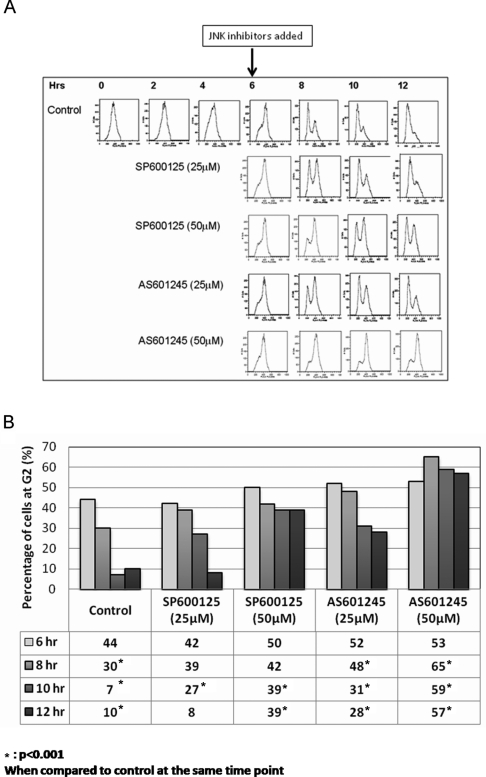
To investigate the effects of the inhibitors on G2/M transition, SIGCs were synchronized at G1/S by aphidicolin, washed, and re-plated in serum-supplemented medium (time 0). Six hours later, the cells progressed into G2 phase and were treated with the inhibitors at 25 and 50 μmol/L concentrations. Flow cytometry analysis revealed that the cells treated with SP600125 and AS601245 were arrested at G2/M phase in a dose-dependent fashion (Figure 4A ).
Figure 4.
Cell cycle analysis of granulosa cells by flow cytometry. A, Granulosa cells treated with 25 and 50 μmol/L SP600125 at sixth hour after aphidicolin release exhibit delay and arrest at G2/M transition, respectively. B, Percentage of cells at G2 during G2/M transition in control and JNK inhibitor groups. There is a dose-dependent increase (accumulation) of cells at G2 after treatment of JNK inhibitors, showing a block in cell cycle progression during G2/M transition. JNK indicates c-Jun N-terminal kinase.
As shown in Figure 4B, there is a dose-dependent accumulation of the cells at G2 phase when JNK pathway was interrupted during G2/M transition compared to control cells proceeding through G2/M phase regularly (Figure 4B). Although the percentage of cells at G2 were similar at sixth hour after aphidicolin release before the initiation of JNK inhibitor treatment, later during the following time points significantly less number of cells treated with JNK inhibitors completed mitosis compared to control cell.
Discussion
In this study, we showed that inhibition of JNK signaling pathway via its pharmacologic inhibitors SP600125 and AS601245 halts in vitro growth of mouse preantral follicles in a dose-dependent manner. Further analysis revealed that both the inhibitors impair S phase and G2/M transition of granulosa cell cycle. Regardless of the type of JNK inhibitor, culture condition or the presence of serum interruption of JNK signaling pathway had the same effect on follicle growth and granulosa cell cycle progression, suggesting a universal role of this pathway in granulosa cell kinetics and preantral follicle growth in vitro.
The JNK subgroup of MAPKs was originally implicated in stress response and apoptosis, but there is surmounting evidence that these kinases play a critical role in cell proliferation, control of the cell cycle, as well as in cancer.1 In addition to its well-known activators such as UV irradiation, osmotic shock or physical tension, proinflammatory cytokines,12 extracellular matrix components,13 and selected growth factors,14 JNK and its downstream c-Jun have been shown to play a role in progression through the G1 phase of the cell cycle in somatic cell lines12 and cell proliferation. Microinjection of Jun family member-specific antibodies blocks S-phase entry and DNA synthesis in fibroblasts.15,16 Homozygous jun−/− mouse embryos die at mid to late gestation with fibroblasts derived from heterozygous and homozygous mouse embryonic fibroblasts (MEFs), showing reduced growth rates in culture. The mutant embryos show altered liver erythropoiesis, impaired hepatogenesis, and generalized edema.2 Activation of JNK pathway has been observed in Jurkat cells at G2/M phase of cell cycle.17 We have recently shown that JNK activity and phosphorylation of c-Jun become elevated during the G2/M transition of the cell cycle in immortalized fibroblasts and ovarian granulosa cells. Pharmacological inhibition of JNK causes a profound cell cycle arrest at the G2/M transition in both cell types. Inactivation of JNK prior to mitosis prevents expression of aurora B and phosphorylation of histone-H3 at Ser 10 along with a delay in the activation of cdc-2 and prevents downregulation of Cyclin B1, suggesting that JNK signaling promotes entry into mitosis by promoting expression of aurora B and thereby phosphorylation of histone-H3.5 A recent study has shown that JNK inhibitors can act on the cell cycle in a manner that does not involve direct inhibition of JNK kinase activity. SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition.18 Therefore, it is possible that granulosa cells are or are not engaging in endoreplication and arrest in G2 rather than G2 arrest alone due to direct effects of the inhibitor on JNK.
Even though there is no data on the role of JNK pathway in human reproduction, we previously first showed that phospho-c-Jun was exclusively expressed in mitotic granulosa cells of follicles from transitional to antral stages3 and then presented our preliminary data on the effects of the inhibition of JNK pathway on in-vitro growth preantral follicle in a mouse model.8,9 In xenopus model JNK activity increases abruptly just prior to germinal vesicle breakdown during oocyte maturation, at the boundary between G2-phase and meiotic M-phase and remains high throughout early embryogenesis until early gastrulation.18 In porcine granulosa cells, JNK and ERK pathways are activated by phorbol 12-myristate 13-acetate, an activator of protein kinase C pathway critical for gonadotropin and cyclic adenosine monophosphate (cAMP)-mediated follicle growth.19
In summary, we showed in this study that pharmacologic inhibition of JNK caused a profound arrest in in vitro growth of preantral follicles and cell cycle progression of granulosa cells at both S phase and G2/M transition. This effect does not appear to be due to nonspecific inhibition of other MAPK pathways because 2 structurally different inhibitors of JNK phosphorylation showed the same effect. However, the possibility exists that JNK inhibition prevents the entry of cells into mitosis and leads to endoreplication of DNA from G2 phase. In this study, we did not determine whether the inhibition of follicle growth was reversible or due to apoptotic granulosa and or oocyte cell death. However in our earlier studies,5 we showed that such action was reversible in immortalized granulosa cells and due to follicle cell death. In addition, we did not see any morphological signs of follicle death in follicles after exposure to JNK inhibitors.
Taken together JNK signaling pathway with its critical role in in vitro growth of preantral follicles and mitotic control of granulosa cells might have pivotal roles in early stages of follicle growth for which controlling mechanisms are largely unknown.
Supplementary Material
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
This study was supported by NICHD grant KO8 43339 and NICHD/NCI grant R01 HD053112 to K.O.; and by NIH grant R01 HD043339 to K.O.
References
- 1. Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149 [DOI] [PubMed] [Google Scholar]
- 2. Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7(7B):1309–1317 [DOI] [PubMed] [Google Scholar]
- 3. Oktay K, Oktay M. Immunohistochemical analysis of tyrosine phosphorylation and AP-1 transcription factors c-Jun, Jun D, and Fos family during early ovarian follicle development in the mouse. Appl Immunohistochem Mol Morphol. 2004;12(4):364–369 [DOI] [PubMed] [Google Scholar]
- 4. Gaillard P, Jeanclaude-Etter I, Ardissone V, et al. Design and synthesis of the first generation of novel potent, selective, and in vivo active (benzothiazol-2-yl)acetonitrile inhibitors of the c-Jun N-terminal kinase. J Med Chem. 2005;48(14):4596–4607 [DOI] [PubMed] [Google Scholar]
- 5. Oktay K, Buyuk E, Oktem O, Oktay M, Giancotti FG. Jun amino terminal kinase regulates G2/M transition through a mechanism upstream of aurora-B. Cell Cycle. 2008;7(4):533–541 [DOI] [PubMed] [Google Scholar]
- 6. Stein LS, Stoica G, Tilley R, Burghardt RC. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res. 1991;51(2):696–706 [PubMed] [Google Scholar]
- 7. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386 [DOI] [PubMed] [Google Scholar]
- 8. Oktem O, Oktay K. The role of extracellular matrix and activin-A in in-vitro growth and survival of murine preantral follicles. Reprod Sci. 2007;14(4):358–366 [DOI] [PubMed] [Google Scholar]
- 9. Oktay K, Buyuk E, Oktem O. Deciphering Early Folliculogenesis: Jun Amino Terminal Kinase (JNK) plays a key role in preantral follicle growth. Fertil Steril. 2003;80(3):26–27 [Google Scholar]
- 10. Oktem O, Oktay K. Role of extracellular matrix, Activin-A and mitogen activated protein kinase (MAPK) signaling pathways in preantral follicle growth and survival in the mouse. Fertil Steril. 2005;84(S1):384–38516084879 [Google Scholar]
- 11. Oktay K, Karlikaya G, Ojakian GK, Oktay M. Interaction of extracellular matrix and activin-A in the initiation of follicle growth in the mouse ovary. Biol Reprod. 2000;63(2):457–461 [DOI] [PubMed] [Google Scholar]
- 12. Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145(7):1461–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072(2-3):129–157 [DOI] [PubMed] [Google Scholar]
- 14. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252 [DOI] [PubMed] [Google Scholar]
- 15. Kovary K, Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991;11(9):4466–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riabowol KT, Vosatka RJ, Ziff EB, Lamb NJ, Feramisco JR. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988;8(4):1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol. 1999;19(12):8469–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim JA, Lee J, Margolis RL, Fotedar R. SP600125 suppresses Cdk1 and induces endoreplication directly from G2 phase, independent of JNK inhibition. Oncogene. 2010;29(11):1702–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sriraman V, Modi SR, Bodenburg Y, Denner LA, Urban RJ. Identification of ERK and JNK as signaling mediators on protein kinase C activation in cultured granulosa cells. Mol Cell Endocrinol. 2008;294(1-2):52–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.