Abstract
The aim of this study was to compare the contractility of the anterior vaginal muscularis (AVM) from women with and without pelvic organ prolapse (POP). In vitro experiments were performed to measure the peak force generated in response to potassium chloride (KCl; 125 mmol/L) and phenylephrine by AVM tissue from women with and without POP. Cross-sectional areas and co-localization of α1A adrenergic receptor protein with smooth muscle α-actin in AVM strips were determined by histology and immunofluorescence, respectively. There were no differences in the mean amplitude of force generated in response to KCl normalized to either wet weight or muscle cross-sectional area (mN/mm2) between women with and without POP (P > .30). However, AVM from women with prolapse produced a significantly higher mean force to KCl normalized to total cross-sectional area compared to controls (P = .007). While the control samples demonstrated a consistent response to phenylephrine, there was no response to this stimulant generated by AVM tissue from women with POP. The proportion of co-localized α1A adrenergic receptors with smooth muscle α actin in AVM tissue was significantly less in women with POP compared to normal controls (P < .0001). Although there was significantly greater tissue stress generated by AVM from women with prolapse compared to controls, there were no differences in muscle stress. Absent response to phenylephrine by AVM from women with prolapse may be related to a lower expression of α1A adrenergic receptors in vaginal smooth muscle.
Keywords: vagina, smooth muscle, pelvic organ prolapse, contractility, adrenergic receptors
Introduction
Pelvic organ prolapse (POP) is a common pelvic floor disorder that is associated with an 11% lifetime risk of surgical treatment.1 In addition, 25% of those women treated surgically will require reoperation for recurrence.1 Several clinical risk factors for POP including parity, aging, menopause, neurologic damage, and connective tissue disorders have been described.2,3 However, little is known about the cellular mechanisms underlying this disorder.
Prior studies investigating the pathogenesis of POP described the changes in extracellular matrix and skeletal muscle seen in tissues of the pelvic floor including the vagina.4–9 Although data from collagen studies using human pelvic tissue have yielded conflicting results regarding total collagen,4,7 most research has confirmed that there is both an alteration in collagen subtypes and increased collagen turnover in women with POP.7,9
Smooth muscle, an integral part of the vaginal wall and endopelvic structures that support the pelvic viscera,10 has also been implicated in the pathophysiology of POP. Smooth muscle exhibits distinct contractile functionality depending on a variety of factors including the location within an organ. Furthermore, smooth muscle cells adapt and alter their contractile phenotype in response to mechanical stretch or trauma.11 Several studies have documented a decrease in the fractional area of smooth muscle in the anterior and posterior vaginal walls of women with prolapse compared to controls.12–14 In addition, there is also preliminary evidence that there are alterations in smooth muscle contractile and regulatory protein and gene expression in pelvic floor tissues.8,15,16 While these studies provide indirect evidence that smooth muscle contractility might be altered in women with POP, the direct relationship of these findings to vaginal smooth muscle contractility in POP has not been shown.
This study tested the hypothesis that the contractility of vaginal smooth muscle is altered in women with POP. The approach to our hypothesis is 2-fold. First, we aimed to compare the peak receptor-independent contractile response of the anterior vaginal muscularis (AVM) to potassium chloride (KCl) obtained from women with and without POP. Second, we compared the adrenergic receptor-dependent contractile properties of AVM in response to phenylephrine stimulation. We further confirmed the presence of α1A adrenergic receptor in smooth muscle of the anterior vaginal wall. This study provides additional evidence that changes in smooth muscle contractile properties in the anterior vaginal wall from patients with POP contribute to the pathophysiology of this disorder. This knowledge could be applied to develop new therapeutic approaches and protocols for more effective therapies targeting POP.
Materials and Methods
Human Tissue Acquisition
Following approval by the University of Pennsylvania Institutional Review Board, premenopausal women scheduled to undergo hysterectomy for benign indications were identified and prospectively recruited. Study participants were undergoing hysterectomy for apical and anterior vaginal wall prolapse that was at least stage III by preoperative pelvic organ quantification (POP-Q) examination between September 2005 and December 2007.17 Control participants were selected from patients who were undergoing hysterectomy during the same time period for nonprolapse benign conditions who did not have greater than stage I prolapse in any vaginal compartment by preoperative POP-Q examination. In an effort to mitigate the inherent variability anticipated in women with mild prolapse, women with stage II anterior wall prolapse were excluded in this study. Premenopausal status was defined as at least 3 menstrual periods in the preceding 12 months, and 1 period in the preceding 6 months.18 All preoperative POP-Q examinations were performed by the first author (G.M.N.) prior to surgery. Postmenopausal women and women with gynecological malignancies, connective tissue disorders, prior surgery for POP, history of hormone therapy within 12 months, or any neuromuscular disorder were also excluded.
Abdominal or vaginal hysterectomy was performed according to standard procedures as indicated.19 Immediately following excision of the uterus and cervix from the vagina, a full-thickness anterior vaginal wall biopsy was performed. Each full-thickness biopsy was approximately 1 to 2 cm (width) × 0.5 to 1 cm (depth) and taken from the central portion of the anterior vaginal cuff as previously described.12 Biopsies were immediately immersed and stored in Tyrodes physiologic buffer (125 mmol/L NaCl, 2.7 mmol/L KCl, 23.8 mmol/L NaHCO3, 0.5 mmol/L MgCl2.6H2O, 0.4 mmol/L NaH2PO4.H2O, 1.8 mmol/L CaCl2, and 5.5 mmol/L dextrose) at 4°C. Within 2 hours after biopsy collection, the specimen was divided into 2 portions. One portion was placed in Histochoice fixative for histology studies to confirm successful collection of a full thickness biopsy free of bladder tissue and for analysis of the fractional area of the vaginal muscularis within the vaginal wall. Biopsy specimens obtained from both groups were fixed in a similar fashion to ensure comparable results. The remaining portion of the biopsy was dissected free of the vaginal mucosa and used for same day in vitro studies of muscle strip contractility.
Physiology
Longitudinal vaginal muscularis strips (~2-3 mm × 7-10 mm) were dissected from the remainder of the anterior vaginal biopsy using a dissecting microscope (SMZ1000-Nikon, Japan) and then reimmersed in fresh Tyrodes buffer at 37°C and equilibrated with 95% O2 and 5% CO2. Anterior vaginal muscularis strips were immediately suspended in individual organ baths containing 10 mL of fresh Tyrodes buffer at 37°C. One end of each muscle strip was attached to a glass rod immersed in Tyrodes buffer; the other end was attached to a force transducer. Changes in muscle tension were measured on a Grass Model 7D Polygraph. To establish the viability of the dissected vaginal muscularis strips and to confirm a functional contractile apparatus in each sample, all samples were stimulated with KCl (125 mmol/L), a receptor-independent mechanism of contraction, at the beginning of each experiment. After 30 minutes, the length of optimal force was determined by increasing the length of each strip in 2 mm increments until maximal force in response to KCl (125 mmol/L) was obtained. The final maximal force in response to KCl was used for data analysis. The AVM tissue was then washed 3 times with 10 mL of fresh Tyrodes buffer and the strips were allowed to equilibrate in Tyrodes for 15 minutes prior to adding phenylephrine. Phenylephrine was added in a cumulative fashion to obtain final concentrations (1 μmol/L, 33 μmol/L, and 100 μmol/L). At the end of each experiment, muscularis strips were again stimulated with KCl to confirm an intact contractile apparatus. Peak force (in mg) of contraction per 100 mg of wet tissue weight was recorded after administration of each stimulant. At the conclusion of the organ bath experiments, muscle strips were weighed and placed in Histochoice fixative and later embedded in paraffin (AML laboratory, Rosedale, Maryland) for future histology and immunofluorescence experiments.
Histology/Morphometric Analysis:
Cross sections (5 μm) of full thickness biopsies underwent Masson trichrome staining (AML laboratory) to confirm full thickness biopsy. These biopsies were oriented in the axis parallel to the vaginal axis. Total and muscularis thickness of the entire anterior vaginal wall were measured (in mm) from slides of full thickness biopsies. Muscularis thickness was expressed as percenatge of total thickness for each sample. The AVM strips used in organ bath experiments were used to estimate the total and muscle cross-sectional areas in a plane perpendicular to that of the full thickness biopsies. Slides of cross-sections (5 μm) were obtained from the mid-section of the paraffin-embedded longitudinal AVM strips in order to calculate the total and muscle cross-sectional areas for normalization of force measurements. Images of Masson’s trichrome stained sections of the strips were viewed and captured using the Nikon Eclipse E800 (Melville, New York) fluorescence microscope and a RT Slider SPOT camera. Total and nonvascular smooth muscle cross-sectional areas of the strips were manually outlined with the assistance of Image Pro Plus Software as previously described (V4.5, Media Cybernetics, Silver Spring, Maryland).20 Cross-sectional areas were automatically computed within the regions of interest after appropriate calibrations were confirmed at ×10 and ×4 magnifications. The amplitude of contraction at peak force was then normalized to cross-sectional total area (tissue stress) and cross-sectional muscle area (muscle stress) and expressed as mN/mm2.
Immunofluorescence
Immunofluorescence microscopy was performed on 5 μm paraffin sections obtained from dissected AVM strips used for organ bath studies to detect and localize smooth muscle α actin and α1A-adrenergic receptor protein. Procedures for slide preparation have been described previously.20 Briefly, after epitope retrieval in preheated baths (95-100°F) of sodium citrate buffer (10 mmol/L sodium citrate, 0.05% Tween 20, pH 6.0) for 10 minutes, all AVM sections were incubated in a 1:300 dilution of monoclonal anti-smooth muscle α actin antibody preconjugated with fluorescein isothiocyanate ([FITC] F3777, Sigma, St Louis, Missouri) at room temperature for 2 hours. Following 3 washes in PBS, sections were incubated overnight at 4°C in a 1:200 dilution of a polyclonal anti α1A-adrenergic receptor antibody (sc-1477, Santa Cruz Biotechnology, Inc Santa Cruz, CA) followed by a 1:300 dilution of anti-goat IgG-Cy3 conjugate (C2821, Sigma-Aldrich, Inc St. Louis, MO) at room temperature for 1 hour. Slides were washed 3 times (10 minutes) with PBS. A drop of mounting medium with 4’,6-diamidino-2-phenylindole ([DAPI] Vectashield, Vector Laboratories, Inc, Berlingame, California) and a coverslip was then placed over each section. The edges of the coverslip were sealed and images were viewed under a Nikon Eclipse E800 fluorescence microscope. Images were captured using a RT Slider SPOT camera with fluorescence settings.
Image Analysis
In slides prepared as above, images were analyzed for co-localization of α1A adrenergic receptors and smooth muscle α actin. Digital fluorescent images capturing FITC and Cy3 signals were acquired separately using the Nikon Eclipse E800 (Melville, New York) fluorescence microscope and a RT Slider SPOT camera. Each AVM cross-section was divided into 4 quadrants, and fluorescent images (FITC, Cy3, and DAPI) were obtained within each quadrant at ×60 per sample. Exposure times and γ values were kept constant for all images. Regions of interest including nonvascular smooth muscle staining positive for smooth muscle α actin (FITC) were identified within each quadrant. Digital images were then merged and co-localization was detected within regions of interest using color segmentation software provided by Image Pro Plus. The pixel number and intensity of co-localized color (FITC plus Cy3) were recorded as well as the entire region of interest (FITC for smooth muscle α actin). Percentage of total co-localized area to total smooth muscle α actin area was then compared in AVM cross-sections from women with and without prolapse.
Statistical Analysis
Results are expressed as mean ± standard deviation for demographics and ± standard error of the mean for other continuous variables. Statistical comparisons of continuous variables between groups were conducted by an unpaired t test assuming unequal variances. Categorical data were compared using Fisher exact chi-square. We performed a sample size analysis using data from a prior study examining the maximal contractile response in the proximal rat vagina using vaginal strips of similar weight.20 Based on a mean contractile response of 11.13 ± 1.57 mN/mm2, sample size analysis showed that we would need a minimum of 6 samples in each group to have 80% power to detect a 25% difference in maximal force of contraction between groups.
Results
A total of 12 premenopausal women were included in this study (6 controls, 6 prolapse). No cases of invasive cancer were identified from pathology reports. All women in the prolapse group had at least stage III anterior vaginal wall prolapse, while 3 (50%) of /6 also had posterior wall prolapse. Demographic data are presented in Table 1 . There were no statistically significant differences in age, race, BMI, or parity between groups. In addition, there were no differences between groups regarding hysterectomy route.
Table 1.
Demographics
| Control (N = 6) | Prolapse (N = 6) | P Value | |
|---|---|---|---|
| Age (years) | 44.1 ± 6.2 | 48.3 ± 7.2 | .31 |
| Parity | 2.5 ± 3.0 | 2.6 ± 3.0 | .95 |
| BMI (kg/m2) | 29.4 ± 6.0 | 25.9 ± 5.4 | .30 |
| Race N (%) | .54 | ||
| Caucasian | 3 (50) | 4 (67) | |
| African American | 3 (50) | 1 (16) | |
| Asian | 0 (0) | 1 (16) | |
| Hysterectomy route N(%) | .99 | ||
| Vaginal | 2 (33) | 3 (50) | |
| Abdominal | 4 (67) | 3 (50) |
Abbreviation: BMI, body mass index.
Morphometric Analysis of Full Thickness Biopsy
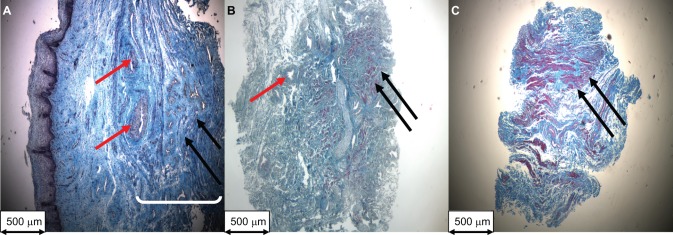
Histology was used to confirm that the biopsies were full thickness anterior vaginal wall (Figure 1A ) and to calculate cross-sectional areas (total and muscle) of the AVM strips used in organ bath experiments (Figure 1B-C). Total thickness of the anterior vaginal wall biopsies did not differ significantly between prolapse and control groups (1.86 ± 0.32 mm vs 1.65 ± 0.42 mm, respectively, P = .62). However, the mean proportional thickness of the vaginal muscularis layer was significantly lower in the prolapse group compared to the control group (31.04% ± 4.60% vs 58.43% ± 4.90%, P = .01).
Figure 1.
Representative Masson trichome staining of 5 μm sections (magnification ×40) of a (A) full thickness vaginal biopsy of the anterior vaginal wall from a control patient used to measure vaginal wall thickness, (B) a cross-section of an anterior vaginal muscularis (AVM) strip from a control patient, and (C) a cross-section of an AVM strip from a woman with prolapse. (B) and (C) AVM strips were used to measure total and smooth muscle cross-sectional areas (mm2). Red arrows: blood vessel; black arrows: vaginal smooth muscle; white bracket: vaginal muscularis layer.
Physiology
All vaginal muscularis tissue strips included in the analysis contracted to KCl (125 mmol/L) as shown in Table 2 . Mean peak force of KCl contractions normalized to wet tissue strip weight or cross-sectional muscle area in AVM prolapse samples were not significantly different compared to controls (P > .07). However, when force was normalized to total cross-sectional area (tissue stress in mN/mm2) prolapse strips generated significantly higher mean amplitude compared to controls (P < .01).
Table 2.
Peak Force of Contraction to KCl (125 mmol/L) for Vaginal Muscularis Tissue Strips From Women With and Without Prolapsea
| Prolapse, Mean ± SEM | Control, Mean ± SEM | P Value | |
|---|---|---|---|
| Force (grams/g tissue weight) | 17.0 ± 7.3 | 2.5 ± 0.8 | .07 |
| Force/cross-sectional total area (mN/mm2) | 5.33 ± 1.30 | 0.95 ± 0.05 | <.01 |
| Force/cross-sectional muscle area (mN/mm2) | 37.50 ± 11.01 | 25.04 ± 3.10 | .30 |
Abbreviations: KCl, potassium chloride; SEM, standard error of the mean.
a N = 6 in each group.
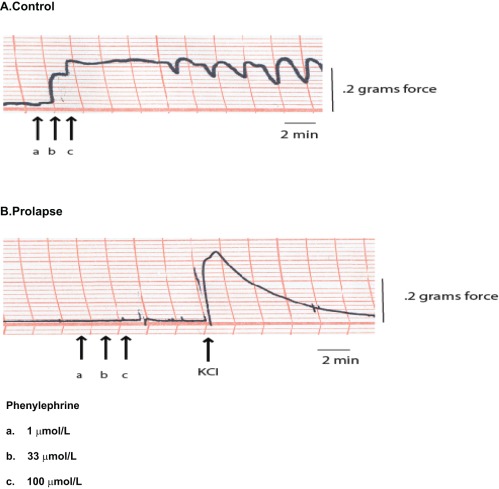
Control AVM strips consistently demonstrated a contractile response to phenylephrine (1μmol/L-100 μmol/L) as noted in Figure 2A . Contractile responses to phenylephrine by control samples demonstrated a variable pattern (phasic vs tonic) with phasic activity noted at high doses of phenylephrine (100 μmol/L). The control AVM strips demonstrated a maximum contractile response to phenylephrine (as percentage of maximum KCl) of 55% ± 14% and ranged from 10% to 117%. In contrast, we failed to detect a contractile response to phenylephrine (1 umol/L-100umol/L) in tissue strips obtained from prolapse biopsies (Figure 3B).
Figure 2.
Representative anterior vaginal muscularis (AVM) force tracing. (A) In an AVM strip from a control patient, there was a consistent response to phenylephrine, an α1 adrenergic agonist. (B) There was no response generated by phenylephrine by a representative AVM strip from a patient with prolapse. For each strip in the prolapse group, the response to KCl (125 mmol/L) stimulation after phenylephrine (arrow in B) indicated that the prolapse smooth muscle remained viable and able to generate force in response to a membrane-independent agonist.
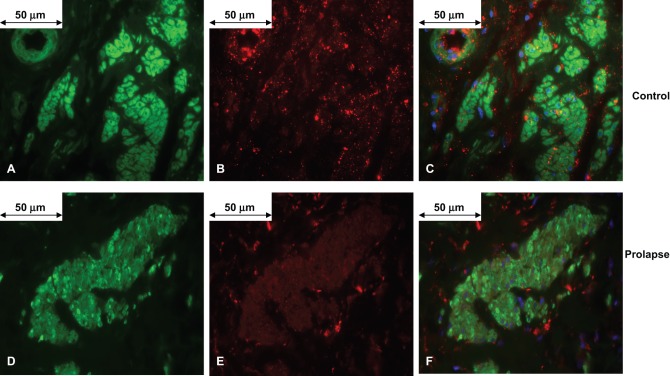
Figure 3.
Representative anterior vaginal muscularis (AVM) strip 5 μm sections confirming the presence of α1A adrenergic receptor in vaginal smooth muscle from women without (A-C) and with (D-F) pelvic organ prolapse (magnification ×600). In the control AVM, smooth muscle α actin-FITC is shown in green (A), α1A adrenergic receptor-CY3 is shown in red (B), and co-localization of the α1A adrenergic receptor to smooth muscle α actin is shown in orange, and nuclear staining with DAPI shown in blue (C). In an AVM from a patient with prolapse, smooth muscle α-actin (D) and α1A adrenergic receptor (E) were identified. Although, the intensity of staining of α1A adrenergic receptor was diminished in the prolapse AVM compared to controls, α1A adrenergic receptor was similarly co-localized with smooth muscle α actin in each strip (nuclear staining with DAPI in blue) (F). FITC indicates fluorescein isothiocyanate; DAPI, 4’,6-diamidino-2-phenylindole.
Immunofluorescence
Antibodies directed against smooth muscle α actin, used to confirm the presence of smooth muscle, was found in vascular and nonvascular smooth muscle in all dissected strips used for physiology (Figures 3A, D). Qualitatively, the immunofluorescent signal for α1A adrenergic receptor was significantly less in the prolapse samples (Figure 3E) compared to the control samples (Figure 3B). Image analysis was performed to estimate the percent co-localized α1A adrenergic receptor with nonvascular smooth muscle α actin in AVM cross-sections (Figure 3C, F). The percentage co-localized α1A adrenergic receptor to α actin was significantly less in AVM from women with POP compared to AVM from controls (1.92 ± 0.20% vs. 4.82 ± 0.23%, P < .0001).
Discussion
The results of this study provide direct evidence of in vitro contractile responses of human vaginal anterior muscularis to KCl (125 mmol/L) and the adrenergic agonist, phenylephrine. Although force of contraction (mN/mm2 cross-sectional muscle area) in response to KCl stimulation was not significantly different between control and prolapse AVM strips, we demonstrate that contractile responses to phenylephrine detected in control AVM strips are absent in strips obtained from prolapse biopsies. Studies examining the in vitro contractility of human vaginal muscularis are limited.21,22 The results of this study are an important contribution to further understand the physiological significance of the human vaginal muscularis and possible pathophysiological changes that occur with prolapse.
Previous studies have shown alterations in the expression of contractile proteins within the vaginal muscularis from women with POP compared to controls.15,16 Boreham et al (2001) demonstrated upregulation of the thin filament associated protein, caldesmon, in the vaginal muscularis of prolapsed patients.15 As caldesmon has been shown to decrease myosin ATPase activity,23 these authors postulate that caldesmon upregulation may result in a net inhibition of force of vaginal contraction in prolapse. Alternatively, Takacs et al (2009) demonstrated decreased caldesmon messenger RNA (mRNA) corresponding to a decrease in fractional smooth muscle area in vaginal smooth muscle from women with prolapse compared to controls.16 These authors speculate that this change in caldesmon expression is a compensatory mechanism to maintain force in the human vagina. Our finding that AVM strips from prolapse biopsies generated the same muscle stress as that of similarly aged controls lends indirect functional support for Takac’s hypothesis. However, a limitation of all human studies is that biopsies were obtained from only a late stage of prolapse. Animal studies of the time course of molecular and functional changes of the vaginal muscularis in response to prolapse would help to define the temporal regulation of caldesmon as a compensatory change to maintain force of contraction. Conversely, animal studies involving the overexpression of caldesmon within the vaginal muscularis may help to identify whether upregulation of caldesmon predisposes the vagina to prolapse.
Another important result of our study was that a contractile response to phenylephrine, which was detected in strips from control participants, was absent in prolapse samples. A corollary to our in vitro findings was that women with prolapse had a lower percentage of co-localized α1A adrenergic receptor with smooth muscle α actin compared to women without prolapse. These results suggest that the inability of muscle strips from prolapse samples to mount a response to receptor stimulation may be at least partially a result of a downregulation of vaginal α1A adrenergic receptor expression. Further studies are needed to elucidate the effect of prolapse on adrenergic innervation, receptor expression, and function. Such studies will help to determine whether compromised sympathetic regulation is one of the mechanisms by which the endopelvic fascial support mechanism fails in women with POP.
Our results of morphometric analysis of full thickness biopsies corroborate previous reports of a decreased percentage of fractional area of the vaginal muscularis of the vaginal wall in prolapse patients compared to controls.12,13,16 Several studies have indicated alterations in collagen subtypes7,24,25 and increased expression of matrix metalloproteinases4,9 in women with prolapse compared to controls, suggesting active collagen turnover is an important pathophysiologic mechanism in this disorder. It is possible that alterations in the extracellular matrix of vaginal tissue in women with prolapse explains the increased tissue stress (force normalized to total cross-sectional area) exhibited by AVM strips from women with POP in our study; however, additional basic research is required to establish this link.
Limitations of our study include the relatively small sample size and our inability to generalize our findings to postmenopausal women. However, in an effort to reduce potential confounders, we elected to narrowly define our study and investigate contractility in younger premenopausal women. Given that the incidence of POP increases with age, it will be important to test our hypothesis in older postmenopausal women as well. In addition, in an attempt to standardize our measurements, we tested AVM strips that were dissected longitudinally from biopsy specimens. Therefore, we have not accounted for the contractile function of the circumferentially oriented smooth muscle bundles.
Despite these limitations, our study provides useful data on the differences in the in vitro function of anterior vaginal wall smooth muscle tissue between women with and without anterior vaginal wall prolapse. To ensure the validity of our findings, we performed in vitro experiments within 2 hours of biopsy excision. Further, we normalized force of contraction to total and muscle cross-sectional areas to account for differences in strip weight. Larger studies examining vaginal α adrenergic receptor function as well as the smooth muscle myosin contractile and regulatory gene expressions in the vaginal wall are required to elucidate the precise mechanism of altered contractility in women with POP.
Conclusions
Our finding that AVM strips from prolapse biopsies generate the same muscle stress in response to KCl as control biopsies provides indirect support that molecular changes of the contractile apparatus of prolapsed vaginal muscularis may be a compensatory mechanism to maintain force. In addition, AVM from women with prolapse had an absent response to α1 adrenergic receptor agonist corresponding to a decrease in the expression of α1A adrenergic receptor compared to women without prolapse. These preliminary results may be useful for further research into ideal implant materials or therapeutic targets modulating smooth muscle contraction. Animal model studies that address the time course of morphometric, molecular, and functional changes of the vaginal wall are necessary to help elucidate the contribution of these changes to the pathogenesis of POP.
Acknowledgments
The authors would like to thank Drs Deborah Driscoll, Anna Malykhina, and Megan Schimpf for their assistance with manuscript preparation. The authors also thank Joseph Hypolite for his assistance with optimizing the in vitro experimental protocol.
Footnotes
Portions of this research was presented at the 26th Annual Scientific Meeting of the American Urogynecologic Society, September 2005, Atlanta, GA, and the Society of Urodynamics and Female Urology Annual Scientific Meeting, 2006, Freeport, Bahamas.
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: A.J.W. and L.A.A. have served as consultants for Pfizer, Inc. and Astellas, Inc. A.J.W. has additional financial relationships with Indevus, Novartis, BioXell, Allergan, Dynogen and Sanofi. This study was supported by the National Institutes of Health Grants P50-DK-052620 (S.C), T32-DK-007708 (A. J.W.(PI), G.M.N., and M.B.), and the Reproductive Scientist Development Award-UCSF K12-HD-00849-21 (G.M.N).
References
- 1. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark Al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–506 [DOI] [PubMed] [Google Scholar]
- 2. Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104(5):579–585 [DOI] [PubMed] [Google Scholar]
- 3. Smith AR, Hosker GL, Warrell DW. The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. Br J Obstet Gynaecol. 1989;96(1):24–28 [DOI] [PubMed] [Google Scholar]
- 4. Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347(9016):1658–1661 [DOI] [PubMed] [Google Scholar]
- 5. Ewies AA, Al-Azzawi F, Thompson J. Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod. 2003;18(10):2189–2195 [DOI] [PubMed] [Google Scholar]
- 6. Lin SY, Tee YT, Ng SC, Chang H, Lin P, Chen GD. Changes in the extracellular matrix in the anterior vagina of women with or without prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(1):43–48 [DOI] [PubMed] [Google Scholar]
- 7. Moalli PA, Shand SH, Zyczynski HM, Gordy SC, Meyn LA. Remodeling of vaginal connective tissue in patients with prolapse. Obstet Gynecol. 2005;106(5 pt 1):953–963 [DOI] [PubMed] [Google Scholar]
- 8. Visco AG, Yuan L. Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol. 2003;189(1):102–112 [DOI] [PubMed] [Google Scholar]
- 9. Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(2):80–87 [DOI] [PubMed] [Google Scholar]
- 10. Delancey JO, Starr RA. Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med. 1990;35(8):765–771 [PubMed] [Google Scholar]
- 11. Gunst SJ, Fredberg JJ. The first three minutes: smooth muscle contraction, cytoskeletal events, and soft glasses. J Appl Physiol. 2003;95(1):413–425 [DOI] [PubMed] [Google Scholar]
- 12. Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(6):1501–1508 [DOI] [PubMed] [Google Scholar]
- 13. Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63 [DOI] [PubMed] [Google Scholar]
- 14. Takacs P, Gualtieri M, Nassiri M, Candiotti K, Medina CA. Vaginal smooth muscle cell apoptosis is increased in women with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(11):1559–1564 [DOI] [PubMed] [Google Scholar]
- 15. Boreham MK, Miller RT, Schaffer JI, Word RA. Smooth muscle myosin heavy chain and caldesmon expression in the anterior vaginal wall of women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2001;185(4):944–952 [DOI] [PubMed] [Google Scholar]
- 16. Takacs P, Gualtieri M, Nassiri M, Candiotti K, Fornoni A, Medina CA. Caldesmon expression is decreased in women with anterior vaginal wall prolapse: a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(8):985–990 [DOI] [PubMed] [Google Scholar]
- 17. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17 [DOI] [PubMed] [Google Scholar]
- 18. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4(4):267–272 [PubMed] [Google Scholar]
- 19. Thompson JD, Warshaw JS. Hysterectomy Rock JA, Thompson JD. (Eds.), Te Linde's Operative Gynecology. 1997;8 ed. Philadelphia, PA: Lippincott-Raven, 771–854 [Google Scholar]
- 20. Basha M, Chang S, Smolock EM, Moreland RS, Wein AJ, Chacko S. Regional differences in myosin heavy chain isoform expression and maximal shortening velocity of the rat vaginal wall smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1076–R1084 [DOI] [PubMed] [Google Scholar]
- 21. Basha M, Labelle EF, Northington G, Wang T, Wein AJ, Chacko S. Functional significance of muscarinic receptor expression within the proximal and distal rat vagina. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1486–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uckert S, Ehlers V, Nuser V. F, et al. In vitro functional responses of isolated human vaginal tissue to selective phosphodiesterase inhibitors. World J Urol. 2005;23(6):398–404 [DOI] [PubMed] [Google Scholar]
- 23. Horiuchi KY, Chacko S. Effect of unphosphorylated smooth muscle myosin on caldesmon-mediated regulation of actin filament velocity. J Muscle Res Cell Motil. 1995;16(1):11–19 [DOI] [PubMed] [Google Scholar]
- 24. Soderberg MW, Falconer C, Bystrom B, Malmstrom A, Ekman G. Young women with genital prolapse have a low collagen concentration. Acta Obstet Gynecol Scand. 2004;83(12):1193–1198 [DOI] [PubMed] [Google Scholar]
- 25. Liapis A, Bakas P, Pafiti A, Frangos-Plemenos M, Arnoyannaki N, Creatsas G. Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol. 2001;97(1):76–79 [DOI] [PubMed] [Google Scholar]