Abstract
MicroRNAs (miRNAs) have emerged as key regulators of gene expression stability implicated in cell proliferation, apoptosis, and development, whereas their altered expression has been associated with various pathological disorders. The objective of this study was to assess the expression profile of miRNAs and their predicted target genes in placentas from patients with preeclampsia (PC) and preterm (PT) labor as compared to normal term (NT) pregnancies. Using microarray profiling of 820 miRNAs and 18,630 mRNA transcripts, the analysis indicated that 283 of these miRNAs and 9119 mRNAs were expressed in all placentas, of which the relative expression of 20 miRNAs (P < .05 and ≥1.5-fold) and 120 mRNAs (P < .05, and 2-fold cutoff) was differentially expressed in PT and PC as compared to NT. The expression of miR-15b, miR-181a, miR-200C, miR-210, miR-296–3p, miR-377, miR-483–5p, and miR-493 and a few of their predicted target genes: matrix metalloproteinases (MMP-1, MMP-9), a disintegrin and metalloproteinase domains (ADAM-17, ADAM-30), tissue inhibitor of metalloproteinase 3 (TIMP-3); suppressor of cytokine signaling 1 (SOCS1); Stanniocalcin (STC2); corticotropin-releasing hormone (CRH), CRH-binding protein (CRHBP); and endothelin-2 (EDN2) were validated in these cohorts using real-time polymerase chain reaction (PCR), some displaying an inverse correlation with the expression of their predicted target genes. Functional analysis indicated that the products of these genes regulate cellular activities considered critical in normal placental functions and those affected by PC and PT labor. In conclusion, the results provide further evidence that placentas affected by PC and PT labor display an altered expression of a number of miRNAs with potential regulatory functions on the expression of specific target genes whose altered expression and function have been associated with these pregnancy complications.
Keywords: placentas, preterm, preeclampsia, miRNA, mRNA, expression
Introduction
Preeclampsia (PC) and preterm labor (PT) are common pregnancy complications and leading causes of maternal and infant morbidity and mortality worldwide. Although the cause and effect relationship is unclear, familial and epidemiological studies have implicated a complex interplay between genetics and environmental factors and the outcome of PT labor complications.1–7 Infection and inflammation are among the most rigorously demonstrated pathologic processes that cause PT4,7–10 Conventional and large-scale gene expression profiling have identified altered expression of a number of genes, including several proinflammatory, angiogenic, and tissue turnover-related genes in placentas from pregnancies complicated by PT as compared to normal term (NT) deliveries.4,7–11 Other studies have also associated genetic polymorphisms in proinflammatory, angiogenic, and proteases genes in placentas from pregnancies complicated by PT birth.8
Similar studies have also supported an underlying genetic basis for PC.1,6,8,12 Preeclampsia is a pregnancy-specific disorder characterized by the failure of physiologic transformation of the spiral arteries in the placental bed and affects up to10% of all pregnancies1,3,12 As in PT labor, altered placental expression of a number of proinflammatory, angiogenic, and tissue turnover-related genes has been associated with pregnancies affected by PC6,10,11,13–25 In addition, maternally derived mediators have been postulated to alter the expression of these genes in trophoblasts and other maternal−fetal cell types.1,6,12,18–20,26–28
Accumulated evidence has also implicated a key regulatory function for microRNAs (miRNAs) in gene expression regulation.29–33 As a member of nonprotein coding short RNAs family miRNAs through binding to the 3′ untranslated region of specific target genes regulate their expression through translational regression.29–33 A large number of miRNAs have been identified and/or predicted, and in humans it is estimated that 30% of genes are potential target of miRNAs regulatory functions.29–33 Although, the biological significance of many of these miRNAs remains to be elucidated, functional studies have implicated their regulatory actions on various developmental processes, whereas their aberrant expression has been associated with developmental abnormalities, cell-cycle progression and apoptosis, inflammation and immune regulation, as well as cellular transformation and cancer.33–38 Recent studies have also reported the expression of a number of miRNAs in placentas and fetal membranes with altered expression in these tissues affected by PC.39–42
To further our understanding of the correlation between the expression of miRNAs and their target genes in placentas affected by pregnancy complications as compared to NT pregnancies, we profiled the expression of miRNAs and mRNAs in the same placentas from women with spontaneous PT and PC and from NT pregnancy undergoing elective cesarean section without labor. We further confirmed the expression of a few miRNAs and their predicted target genes in these tissues using real-time polymerase chain reaction (PCR) and through bioinformatic analysis assessed their potential biological functions.
Materials and Methods
Study Design
Placental tissues were obtained from patients (N = 21) in the following 3 groups: (1) PC (N = 7); 2) spontaneous PT delivery at ≤ 35 weeks gestation (N = 7); and (3) control group (NT elective cesarean section without labor; N = 7). The study was approved by the Institutional Review Board at the University of Florida, and all patients who participated provided written informed consent. The tissues were collected at Shands Hospital at the University of Florida.
Preeclampsia was defined as hypertension—systolic blood pressure, ≥140 mm Hg, or diastolic blood pressure, ≥ 90 mm Hg, on at least 2 occasions, at least 4 hours apart, and proteinuria of ≥300 mg in a 24-hour urine collection or urine dipstick measurement of ≥2+. Severe PC was defined as hypertension with systolic blood pressure ≥160 mm Hg, or diastolic blood pressure ≥110 mm Hg, plus mild proteinuria or mild hypertension plus severe proteinuria—a 24-hour urine collection with at least 5 g of protein or urine dipstick measurement of greater than or equal to 3+. Spontaneous PT delivery was defined as the presence of PT—regular uterine contractions of at least 3 contractions in 10 minutes that were associated with cervical changes—that resulted in delivery at ≤35 completed weeks of gestation. Control patients delivered normal infants at term without labor via elective cesarean section. Patients with diabetes mellitus, PT premature ruptured membranes without labor, multiple gestations, and fetal demise in utero or fetal anomalies were excluded (Table 1 ).
Table 1.
Demographic Characteristic of the Study Population
| Demographic | Preeclampsia (N = 7) | Preterm Labor (N = 7) | Control (N = 7) |
|---|---|---|---|
| Age, years a | 23.8 (20-26) | 28.3 (22-35) | 30 (21-38) |
| Nulliparity, n | 2 (29%) | 1 (14%) | 1 (14%) |
| Smoking, n | 0 (0%) | 2 (29%) | 4 (57%) |
| Weight, kg a | 98.4 (81.2-133.8) | 76.6 (58.1-102.1) | 96.7 (68.5-147.9) |
| Body mass index, a kg/m2 | 38.2 (32.3-54) | 30.8 (23-43.9) | 37.5 (27.6-59.6) |
| White race, n | 7 (100%) | 2 (29%) | 6 (86%) |
| Black race, n | 0 (0%) | 2 (29%) | 0 (0%) |
| Gestational age at delivery, a wk | 35 (31-39) | 28 (24-33) | 38.1 (37-39) |
| Birth weight, a g | 2687.5 (1193-4073) | 1203.8 (600-1901) | 3450.3 (2952-3934) |
a Values expressed as median (range).
Tissue Collection and miRNA and mRNA Expression Profiling
Immediately after delivery, the overlaying fetal membranes were removed and several small pieces of placental villous tissues were randomly collected from different areas, blotted on clean tissue paper to remove excess blood and placed in cryotubes containing RNAlater (Qiagen Inc, Valencia, California), at 4 to 1 volume of tissue. After several hours at room temperature, the tubes were stored in liquid nitrogen for further analysis. Total RNA was extracted from 2 to 3 small portions of each placenta using Trizol (Invitrogen, Carlsbad, California) and their quality and quantity were assessed using ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, Delaware) and Agilent 2100 Bio-analyzer (Agilent Technologies, Foster City, California).
Total RNA isolated from 16 placentas (NT, N = 6; PC, N = 7; and PT, N = 3) was used for miRNA expression profiling. The low molecular weight RNAs were isolated and 3′-end labeled with Alexa-647 fluorescent dye using Rapid Labeling Kit (Invitrogen) and profiled using custom-developed microarray by Ocean Ridge Biosciences (Jupiter, Florida). The array contained 1213 human probes (35- to 44-mer oligonucleotides), including 820 mature miRNAs (Sanger Institute mirBASE v11, Ncod3) and 393 Invitrogen “Novel” probes (Invitrogen), as well as negative controls (38 mismatch and shuffled controls and 87 nonconserved Caenorhabditis elegans probes) all spotted in triplicates. After hybridization, the arrays were scanned using a GenePix 4000A scanner (Axon Instruments, Union City, California) to obtain the spots intensities. The mean triplicate spot intensity values for each probe was determined, subtracted from the median background values, normalized relative to control miRNA (Ambion) added to each sample, and threshold and 95th percentile of negative controls were calculated based on negative controls. The above analysis resulted in the identification of a total of 570 miRNAs whose expression values were subjected to further statistical analysis. Prior to analysis the expression value of all the Invitrogen “Novel” probes were removed from the data set due to their exceedingly high level of expression and lack of any information about their biological significance. In addition, the mean expression values of the remaining 325 human mature miRNAs were subject to supervised assessment resulting in omitting the expression values of 42 miRNAs from the data set due to relatively low expression values. The expression value of remaining 283 miRNAs was subject to “R” analysis and analysis of variance (ANOVA) with Tukey test and 1.5-fold change cutoff.
Total RNA isolated from the above placentas (N = 12) was also subjected to gene expression profiling at the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida. Following amplification and complementary DNA (cDNA) synthesis, 5 μg of cDNA was reverse, the product was purified, and 20µg of complementary RNA ([cRNA]; 0.5 µg/µl) was fragmented, mixed with 300 µL of hybridization mixture and 200 µl of the mixture was hybridized to HumanRef-12 v3 Expression BeadChip (Illumina, Inc, San Diego, California) consisting of 24,526 oligonucleotide probe sets representing 18,630 transcripts. The beads were processed after meeting recommended criteria according to manufacturer’s protocol. The expression values were background subtracted and globally normalized using BeadStudio version 1.5.1.3 (Illumina) and probes with differential score of ≤13 were independently removed from each cohort. Prior to further analysis the expression values of 2 placentas, 1 from PC and 1 from PT group, were omitted from the data set due to the unusually low expression values. The mean gene expression values of the remaining tissues were then subject to supervised assessment which resulted in the selection of 9119 gene expression values that were subjected to “R” analysis as previously prescribed.43–45 Expression values having a statistical significance of P ≤ .05 (ANOVA, Tukey test) were selected and subjected to 2-fold change cutoff. These values were subjected to cluster and tree-view analysis.
MicroRNA Predicted Target Genes, Functional and Bioinformatic Analysis
Computational algorithms TargetScan (http://www.targetscan.org/), PicTar (http://pictar.bio.nyu.edu/), and miRanda (http://microrna.sanger.ac.uk/targets) were used for the selection of genes predicted as the targets miRNAs differentially expressed among these cohorts. The search results were downloaded to a local database and sorted based on their presence in all the algorithms. In addition, genes experimentally validated as target of these miRNAs were also identified through Pub-Med search and included in the list. The gene list (Supplement Table 2B) was used for data mining and bioinformatic analysis (Table 2 ). In each case, the predicted target genes for each miRNA was identified from the list of gene expression values obtained by microarray for the above cohorts using Microsoft Access.
Table 2.
Functional Network Categories of Differentially Expressed Genes
| Associated network functions | ||
| 1. Inflammatory response, cardiovascular disease | ||
| 2. Cell cycle, connective tissue development and function, cell-to-cell signaling and interaction | ||
| 3. Cell-to-cell signaling/interaction, cellular assembly/organization, cellular function/maintenance | ||
| 4. Embryonic development, tissue morphology | ||
| 5. Lipid metabolism, molecular transport, and small molecule biochemistry | ||
| Subgroup name | P Value (range)* | # Molecules |
| Diseases and disorders | ||
| Cardiovascular disease | 2.70E-05-3.04E-02 | 13 |
| Genetic disorder | 2.70E-05-3.00E-02 | 21 |
| Neurological disease | 2.70E-05-2.44E-02 | 17 |
| Skeletal and muscular disorders | 2.70E-05-2.44E-02 | 9 |
| Molecular and cellular functions | ||
| Lipid metabolism | 2.23E-04-3.04E-02 | 10 |
| Small molecule biochemistry | 2.23E-04-3.04E-02 | 13 |
| Cellular compromise | 3.71E-04-3.04E-02 | 6 |
| Cellular development | 4.13E-04-3.04E-02 | 24 |
| Vitamin and mineral metabolism | 1.64E-03-2.44E-02 | 4 |
| Physiological system development and function | ||
| Organismal functions | 3.71E-04-2.44E-02 | 6 |
| Skeletal and muscular system development/function | 4.13E-04-3.04E-02 | 9 |
| Nervous system development and function | 1.51E-03-3.04E-02 | 9 |
* P values indicate enrichment of functional subgroups within indicated categories with their associated number of molecules.
The differentially expressed genes and genes predicted as target of specific miRNAs were subjected to cluster and tree-view analysis as well as functional annotation and visualization using Database for Annotation, Visualization, and Integrated Discovery (http://www.david.niaid.nih.gov) software and Ingenuity Pathway Analysis (Ingenuity System, Redwood City, California).
Real-Time Reverse Transcription−Polymerase Chain Reaction
The expression of a number of miRNAs and mRNAs was confirmed using real-time PCR in 16 to 18 placentas (5 to 6 samples from each group), including all the tissues used for microarray. Complementary DNA was generated from 2 μg of total RNA using Taqman reverse transcription reagent and cDNA equivalent to 100 ng RNA in 50 μL reaction containing 1× Taqman Universal Master Mix, optimized concentrations of FAM-labeled probes, and specific forward and reverse primers (Assay on Demand, Applied Biosystems) was used for PCR. Real-time PCR was carried out using Applied Biosystems 7300 Fast Real-time PCR System at 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute for 40 cycles. The miRNAs and mRNAs expression values were analyzed using the comparative method following transformation and normalization to RU6B and18S rRNA expression, respectively, according to the manufacturer’s guidelines, with threshold cycle (Ct) set within the exponential phase of the PCR and converted into fold-change based on a doubling of PCR product in each PCR cycle. The final results were reported as relative expression by setting the expression values of miRNAs and mRNAs in one of the normal placenta at 1 and the expression value of other samples was calculated relative to this control.43,44
Statistical Analysis
The real-time PCR results are expressed as mean ± standard error of mean (SEM) and statistically analyzed using Student t test for comparison of 2 groups and ANOVA for multiple comparisons, with P < .05 considered significant.
Results
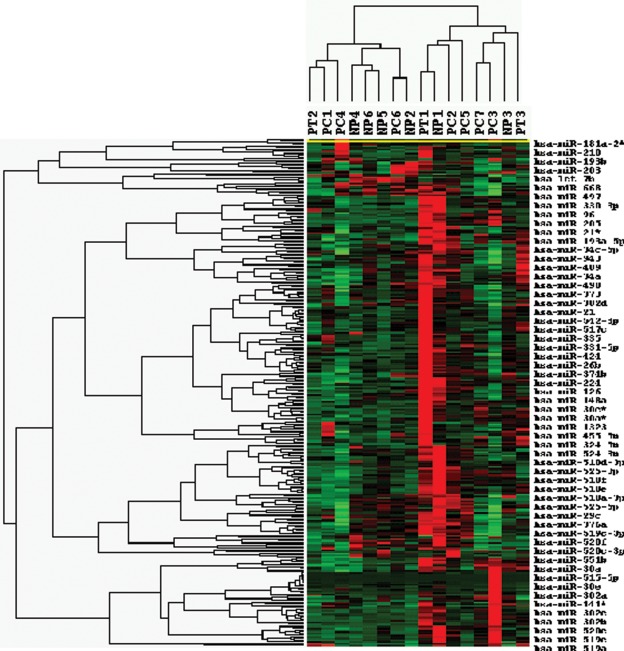
Total RNA isolated from placentas from NT, PC, and PT labor pregnancies was subjected to expression profiling of 820 miRNAs and 24,526 mRNAs. Of the 820 mature miRNAs, the expression of 325 was identified in all placentas from NT, PC, and PT (Supplement Table 1A). Following removal of the expression value of 42 miRNAs due to relatively low expression, statistical analysis among the 283 miRNAs, the expression of 20 miRNAs was identified as differentially expressed among NT, PC, and PT placentas (P < .05, Supplement Table 1B). However, selection based on 1.5-fold change cutoff identified 141 (113 upregulated and 23 downregulated) and 78 (30 upregulated and 48 downregulated) miRNAs with altered expression in PT and PC as compared to NT, respectively (Supplement Table 1C). In addition, 154 miRNAs were upregulated and 27 downregulated in PT as compared to PC (Supplement Table 1C). Cluster and tree-view analysis separated the expression of the 283 miRNAs (Supplement Table 1B) in these tissues into their respective subgroup some with overlapping expression (Figure 1 ). Supplement Table 1D illustrates the mean expression values, fold change differences, and the list of selective number of genes predicted as targets of these 20 miRNAs, including those verified by real-time PCR.
Figure 1.
Cluster and tree-view analysis of 283 differentially expressed miRNAs in placentas from PT, PC, and NT pregnancies (see Supplement Table 1B for the list). miRNAs represented by rows were clustered according to their similarities in pattern of expression in each tissue and dendrogram at the top of the image correspond to similarity among their expression in these cohorts (see Supplement Table 1 for the list of miRNAs). Each column represents data from a single cohort. miRNA indicates micro-RNA; PT, preterm; PC, preeclampsia; NT, normal term.
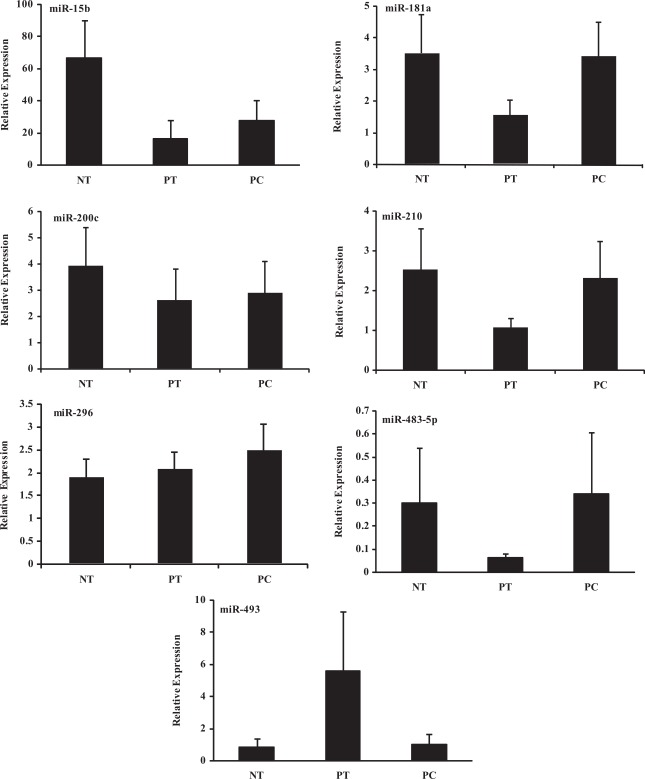
Based on P < .05, fold change cutoff, and predicted target genes, we selected and confirmed the expression of miR-15b, miR-181a, miR-200C, miR-210, miR-296–3p, miR-377, miR-483–5p, and miR-493 using real-time PCR (Figures 2 ). As indicated, there was considerable variation in the level of expression of these miRNAs within and between these cohorts with low expression of miR-377 (Figure not shown), and in some cases their relative expression reflected the microarray data (see Supplement Table 1D).
Figure 2.
Relative expression (mean ± SEM) of miR-15b, miR-181a, miR-200C, miR-210, miR-296, miR-483–5p, and miR-493 in placentas from spontaneous preterm delivery (PT, N = 5), preeclampsia (PC, N = 6), and at term (NT, N = 5) determined by real-time PCR. SEM indicates standard error of the mean; PCR, polymerase chain reaction.
Gene Expression Profiling
Total RNA isolated from 12 of the same placentas used for miRNA profiling was used for gene expression profiling. Of the 18 630 transcripts, the expression values of 9119 mRNAs were selected based on the initial analysis (see Materials and Methods) and subjected to statistical analysis (Supplement Table 2A) which identified 120 transcripts as either up- or downregulated among PC, PT, and NT placentas (P < .05; Supplement Table 2B). However, the analysis based on 2-fold change cutoff identified 2381 (571 upregulated and 1810 down-regulated) and 199 (165 upregulated and 34 downregulated) transcripts as differentially expressed in PC and PT as compared to NT, respectively (Supplement Tables 2C and 2D) and 4017 transcript (364 upregulated and 3653 downregulated) in PT as compared to PC (Supplement Table 2E). Cluster analysis separated these genes into their respective subgroups (see Figures in Supplement Tables 2A and 2B). Comparative analysis also identified 24 transcripts as differentially expressed in placentas from PC as compared to NT (Supplement Table 2F) which is in agreement with the report by Winn et al.11 Functional pathway analysis of the 120 up- and down-regulated genes by searching the functional annotations in Ingenuity database identified 5 associated networks with their specific enriched functional categories of the gene ontology (Table 2).
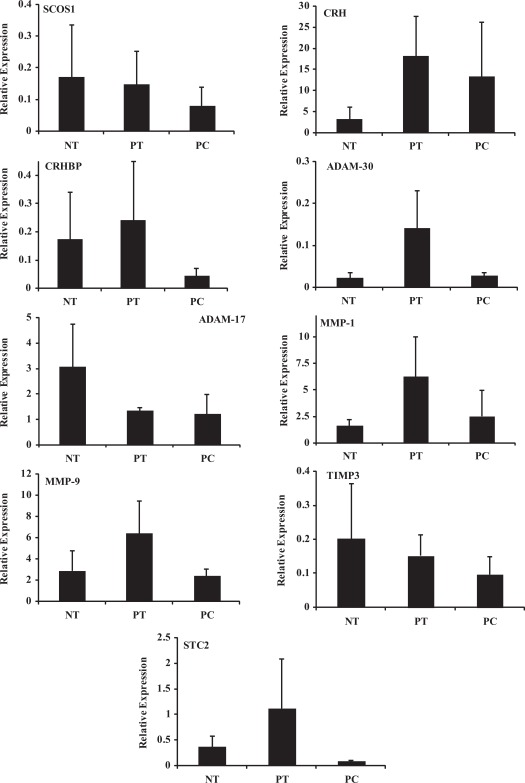
MicroRNA Predicted Target Genes
Based on computational algorithms, a few hundred genes have been predicted as targets of miR-15b, miR-210, miR-296–3p, miR-377, and miR-483, miR-493, and miR-200C. Supplement Table 1D illustrates the list of some of the genes, which include pregnancy associated protein A (PAPPA, PAPPA2); Stanniocalcin (STC1 and STC2); several MMPs, (TIMPs), and a disintegrin and metalloproteinase domain (ADAM), ADAM-17, -19, and -30; thrombospondin 1 (THBS1); activin receptors; CRH, CRHBP; SOCS1; EDN2, and EDN receptors; and so on We selected and confirmed the expression of MMP-1, MMP-9, TIMP-3, ADAM-17, ADAM-30, STC2, CRH, CRHBP, SOCS1, and END2 in placentas from NT, PC, and PT (Figures 3). Similar to the expression of miRNAs, there was a considerable variation in the level of expression of these genes in placentas from NT, PT, and PC. Such variations in the expression of miRNAs and mRNAs have been commonly observed among other tissues and is not unique to our study and in placenta is considered to reflect the heterogeneity of the fetal and maternal responses to PC and PT11 (Figures 3). There was no significant difference in the level of expression of these genes among these cohorts (Figure 3) with END2 expressed at very low levels (Figure not shown).
Figure 3.
Relative expression (mean ± SEM) of, SOCS1, CRH, CRHBP, ADAM-17, ADAM-30, MMP-1, MMP-9, TIMP-3, and STC2 in placentas from spontaneous preterm delivery (PT, N = 5), preeclampsia (PC, N = 6), and at term (NT, N = 5) determined by real-time PCR. SOCS1, suppressor of cytokine signaling 1; CRH, corticotropin-releasing hormone; CRHBP, CRH-binding protein; ADAM, a disintegrin and metalloproteinase domain; MMP, matrix metalloproteinase; TIMP-3, tissue inhibitor of metalloproteinase; STC2, stanniocalcin.
Using the gene microarray data set and through data mining, we identified the expression of number of genes predicted as targets of miR-15b, miR-200C, miR-210, 296–3p, miR-377, miR-483–5p, and miR-493 in placentas from the above cohorts (Supplement Table 3). A selective number of the genes targeted by these miRNAs are expressed in placentas from NT, PC, and PT and tree-view and cluster analysis separated these genes based on their expression values in these cohorts (see Figures embedded in Supplement Table 3). Pathway analysis further identified and mapped several interactive functional networks, ranging from 6 to 30 networks, among the regulatory actions of genes targeted by these miRNAs (Figures not shown).
Discussion
Through microarray profiling we identified the expression of large number of miRNAs and gene transcripts in placentas from NT, PC, and PT of which 20 miRNAs and 120 mRNAs were differentially expressed in the same placentas from these cohorts as compared to NT. We selected and confirmed the expression of a number of these miRNAs and mRNAs using real-time PCR. Despite considerable variations in their expression, placentas from PT and PC could be separated from NT by lower expression of miR-15b, miR-181, -210, -483–5p, and a trend toward higher miR-496 expression. Our results are in agreement with the previous studies examining the expression of few hundred miRNAs in human placentas were identified as differentially expressed in PC as compared to NT pregnancies.42,46 However, Zhu et al, reported several miRNAs, including miR-210 and miR-296, among upregulated, and miR-377 among downregulated miRNAs in placentas of patients with severe PC as compared with normal placentas.46 Our results further extend these observations and suggest potential involvement of these miRNAs in pregnancies affected by PT labor as compared to NT.
Gene expression profiling also identified several hundred differentially expressed transcripts in placentas from NT, PC, and PT labors. These results are in general agreement with previous reports2,4,8,10,11,13,14,16–19,21,47–52 more specifically, with a recent microarray in placentas from PC and NT pregnancies11 as presented in Supplement Table 2F. Functionally, many of these genes serve as mediators of proinflammatory, angiogenic, and tissue turnover, events considered to represent a hallmark of normal placental development and pregnancy complications (Supplement Table 2F). Several of these differentially expressed miRNAs are predicted to target the expression of many genes (Supplement Table 1D and Table 3), including those validated in our study as well as several number of MMP, insulin-like growth factor (IGF), activin receptor, platelet-derived growth factors (PDGF) receptor and collagens family, angiotensin II receptor (AGTR2), prostaglandin synthesizing enzymes, and pregnancy-associated plasma protein A (PAPPA) and PAPPA2. Co-expression and a degree of correlation between the expression of these miRNAs and mRNAs imply their potential regulatory function in various placental cellular activities. However, we observed a wide variation in the level of expression of miRNAs and mRNAs among placentas from PC, PT, and NT pregnancies. In addition to individual difference, factors such as the patients' race, body mass index (BMI), smoking status, and mode of labor, and so on, which have been shown to influence placental gene expression, could have influenced the miRNAs expression. Since the number of specimens in each category was inadequate to allow further insight, further studies are warranted to establish the relationship and potential influence of several factors.
Direct evidence for biological relevance of miRNAs expression and their alterations in placental cellular activities during normal and pathological disorders is rather difficult to achieve, although validating their true target genes in placental cells may help establishing their regulatory functions. Additionally, oxidative stress, a condition believed to cause poor placentation in preeclamptic pregnancies has been shown to alter the expression of several miRNAs, including miR-210 and miR377. Superoxide dismutases, SOD1 and SOD2, which catalyze the reduction of reactive oxygen species to less toxic moieties and their altered expression has been associated with several pregnancy complications, including PC, are validated as direct target of miR-377 regulatory function.53 miR-210 which targets the expression of HIF-1 is considered to serve as a potential marker of hypoxia54–57 Oxidative stress also alters the expression of STC1 and STC2 which functionally protect cells from apoptosis and their expression is highly correlated with embryo implantation and decidualization.58,59 As such, altered expression of miR-377 and miR-210, or even miR-24, miR-181, miR-30, and miR-483–5p and through functional regulation of their target genes, including HIF-1, STCs, and MMPs, may play a key role in placental cellular activities during normal development and placental complications. Unlike STC2 and ADAM-30, the expression of MMP-1, MMP-9, and ADAM-17 and several other member of secreted MMPs family, which are predicted as targets of a number of miRNAs, including miR-296–3p, miR-181, miR-431, and miR-512–3p, has been reported in normal placenta with their altered expression in PC and PT pregnancy complications.14,48,60–64
Existing evidence supports a key regulatory function of physiological inhibitors of MMPs, TIMPs, in various placental cellular activities during normal and pregnancy complication such as PC and PT labor.14,48,60–64 Interestingly, the expression of TIMP-3 was inversely correlated with miR-210 expression (predicted to target TIMP-3) in PC. TIMPs are also predicted target of other miRNAs, including miR-21, miR-181, and miR-483–5p (Supplement Table 1D) suggesting an array of regulatory genes as potential targets of these differentially expressed miRNAs. The rennin/angiotensin (Ang) system are also key players in the development of PC due to alteration in Ang I, Ang II, Ang-(1-7), and increased AGTR expression in chorionic villi.65 miR-15b, miR-181, miR-431, miR-493, miR-512–3p, and miR-654–3p are among several miRNAs predicted to target the expression of AGTR2, suggesting potential regulation of placental renin−angiotensin associated genes by these miRNAs. miR-15b expression has also been correlated with several immunological disorders and cell cycle arrest by targeting cyclin E1 in cervical and glioma carcinoma cell lines.66–68 Among the predicted genes targeted by miR-181a also include CRH which is expressed in the uterus, placenta, and the ovary and is considered to participate in decidualization, embryo implantation, and normal physiology of pregnancy, as well as in pregnancy complications and the onset of parturition.69 We confirmed the expression of CRH and CRHBP, a CRH binding protein, miR-181a and miR-200C which target CRHBP, and found varying levels of expression in placentas from NT, PT and PC group.
PAPPAs are abundantly expressed in the human placenta and though their proteolytic activities cleave insulin-like growth factor binding proteins (IGFBPs), with PAPPA acting on IGFBP4 and IGFBP5 cleavage, whereas PAPPA2 cleaves IGFBP5 and possibly IGFBP3, thus regulating local IGF release to promote feto-placental growth.70 Upregulated expression of PAPPA2 has been reported in placentas with pregnancy complications and considered possible marker of PC. Although we did not validate the expression of PAPPAs, the level of their expression detected by microarray was lower in PT and PC as compared to NT. In addition, miR-496, miR-512–3p, and miR-654–3p, which are predicted to target PAPPAs are expressed in PT PC NT placentas. Because of the biological importance of CRH and PAPPAs in placenta’s biological activities, further investigation is required to elucidate the regulatory function of the miRNAs that target their expression.
In conclusion, through expression profiling and confirmation by real-time PCR, we provided further evidence indicating that the expression of a number of miRNAs and their predicted target genes are altered in placentas from women with pregnancy complications as compared to NT pregnancies. Given the importance of miRNAs in gene regulation, detailed investigation is required to elucidate the biological significance of these differentially expressed miRNAs by validating their specific target genes relevant to placental developmental and functional activities.
Supplementary Material
This study was presented in part at the 34th annual meeting of the Society for Material and Fetal Medicine (SMFM), February 2009, San Diego, California.
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Departmental fund and in part by grant HD-58664 from the National Institute of Health.
Reference
- 1. Baumwell S, Karumanchi SA. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin Pract. 2007;106(2):C72–C81 [DOI] [PubMed] [Google Scholar]
- 2. DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med. 2007;25(1):40–51 [DOI] [PubMed] [Google Scholar]
- 3. Dildy GA 3rd, Belfort MA, Smulian JC. Preeclampsia recurrence and prevention. Semin Perinatol. 2007;31(3):135–141 [DOI] [PubMed] [Google Scholar]
- 4. Faye-Petersen OM. The placenta in preterm birth. J Clin Pathol. 2008;61(12):1261–1275 [DOI] [PubMed] [Google Scholar]
- 5. Genc MR, Schantz-Dunn J. The role of gene-environment interaction in predicting adverse pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. 2007;21(3):491–504 [DOI] [PubMed] [Google Scholar]
- 6. Gilbert JS, Nijland MJ, Knoblich P. Placental ischemia and cardiovascular dysfunction in preeclampsia and beyond: making the connections. Expert Rev Cardiovasc Ther. 2008;6(10):1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holst D, Garnier Y. Preterm birth and inflammation-The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Biol. 2008;141(1):3–9 [DOI] [PubMed] [Google Scholar]
- 9. Pennell CE, Jacobsson B, Williams SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. Am J Obstet Gynecol. 2007;196(2):107–118 [DOI] [PubMed] [Google Scholar]
- 10. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winn VD, Gormley M, Paquet AC, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150(1):452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112(2 Pt 1):359–372 [DOI] [PubMed] [Google Scholar]
- 13. Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol. 2008;199(5):566–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gack S, Marme A, Marme F, et al. Preeclampsia: increased expression of soluble ADAM 12. J Mol Med. 2005;83(11):887–896 [DOI] [PubMed] [Google Scholar]
- 15. Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol. 2007;29(2):163–167 [DOI] [PubMed] [Google Scholar]
- 16. Han JY, Kim YS, Cho GJ, et al. Altered gene expression of caspase-10, death receptor-3 and IGFBP-3 in preeclamptic placentas. Mol Cells. 2006;22(2):168–174 [PubMed] [Google Scholar]
- 17. Hawfield A, Freedman BI. Pre-eclampsia: the pivotal role of the placenta in its pathophysiology and markers for early detection. Ther Adv Cardiovasc Dis. 2009;3(1):65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9(6):480–485 [DOI] [PubMed] [Google Scholar]
- 19. Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nejatizadeh A, Stobdan T, Malhotra N, Pasha MA. The genetic aspects of pre-eclampsia: achievements and limitations. Biochem Genet. 2008;46(7–8):451–479 [DOI] [PubMed] [Google Scholar]
- 21. Okazaki S, Sekizawa A, Purwosunu Y, Farina A, Wibowo N, Okai T. Placenta-derived, cellular messenger RNA expression in the maternal blood of preeclamptic women. Obstet Gynecol. 2007;110(5):1130–1136 [DOI] [PubMed] [Google Scholar]
- 22. Lockwood CJ, Krikun G, Caze R, Rahman M, Buchwalder LF, Schatz F. Decidual cell-expressed tissue factor in human pregnancy and its involvement in hemostasis and preeclampsia-related angiogenesis. Ann N Y Acad Sci. 2008;1127:67–72 [DOI] [PubMed] [Google Scholar]
- 23. Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29(2):151–162 [DOI] [PubMed] [Google Scholar]
- 24. Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41(4):278–286 [DOI] [PubMed] [Google Scholar]
- 25. Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol. 2007;179(6):3391–3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LaMarca BD, Alexander BT, Gilbert JS, et al. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5(Suppl A):S133–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600 [DOI] [PubMed] [Google Scholar]
- 28. Nesin M. Genetic basis of preterm birth. Front Biosci. 2007;12:115–124 [DOI] [PubMed] [Google Scholar]
- 29. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163–6169 [DOI] [PubMed] [Google Scholar]
- 31. Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008;9(11):831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27(1):91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagen JW, Lai EC. microRNA control of cell-cell signaling during development and disease. Cell Cycle. 2008;7(15):2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene. 2006;25(46):6202–6210 [DOI] [PubMed] [Google Scholar]
- 35. Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng S, Calin GA, Croce CM, Coukos G, Zhang L. Mechanisms of microRNA deregulation in human cancer. Cell Cycle. 2008;7(17):2643–2646 [DOI] [PubMed] [Google Scholar]
- 37. Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7(20):3112–3118 [DOI] [PubMed] [Google Scholar]
- 38. Lee YS, Dutta A. MicroRNAs in Cancer. Annu Rev Pathol. 2008;4:199–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54(3):482–490 [DOI] [PubMed] [Google Scholar]
- 40. Donker RB, Mouillet JF, Nelson DM, Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod. 2007;13(4):273–279 [DOI] [PubMed] [Google Scholar]
- 41. Montenegro D, Romero R, Kim SS, et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J Pathol. 2009;217(1):113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3):261–266 [DOI] [PubMed] [Google Scholar]
- 43. Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13(11):797–806 [DOI] [PubMed] [Google Scholar]
- 44. Pan Q, Luo X, Chegini N. Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol. 2007;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13(11):797–806 [DOI] [PubMed] [Google Scholar]
- 46. Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661E1–E7 [DOI] [PubMed] [Google Scholar]
- 47. Carty DM, Delles C, Dominiczak AF. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18(5):186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cockle JV, Gopichandran N, Walker JJ, Levene MI, Orsi NM. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod Sci. 2007;14(7):629–645 [DOI] [PubMed] [Google Scholar]
- 49. Eastabrook G, Hu Y, von Dadelszen P. The role of decidual natural killer cells in normal placentation and in the pathogenesis of preeclampsia. J Obstet Gynaecol Can. 2008;30(6):467–476 [DOI] [PubMed] [Google Scholar]
- 50. Muhle RA, Pavlidis P, Grundy WN, Hirsch E. A high-throughput study of gene expression in preterm labor with a subtractive microarray approach. Am J Obstet Gynecol. 2001;185(3):716–724 [DOI] [PubMed] [Google Scholar]
- 51. Sitras V, Paulssen RH, Gronaas H, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009;30(5):424–433 [DOI] [PubMed] [Google Scholar]
- 52. Tromp G, Kuivaniemi H, Romero R, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1331–1338 [DOI] [PubMed] [Google Scholar]
- 53. Wang Q, Wang Y, Minto AW, et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22(12):4126–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Corn PG. Hypoxic regulation of miR-210: shrinking targets expand HIF-1’s influence. Cancer Biol Ther. 2008;7(2):265–267 [DOI] [PubMed] [Google Scholar]
- 55. Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69(3):1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fasanaro P, D’Alessandra Y, Di SV, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283(23):15878–15883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giannakakis A, Sandaltzopoulos R, Greshock J, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7(2):255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Law AY, Lai KP, Lui WC, Wan HT, Wong CK. Histone deacetylase inhibitor-induced cellular apoptosis involves stanniocalcin-1 activation. Exp Cell Res. 2008;314(16):2975–2984 [DOI] [PubMed] [Google Scholar]
- 59. Xiao LJ, Yuan JX, Song XX, Li YC, Hu ZY, Liu YX. Expression and regulation of stanniocalcin 1 and 2 in rat uterus during embryo implantation and decidualization. Reproduction. 2006;131(6):1137–1149 [DOI] [PubMed] [Google Scholar]
- 60. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75(2):346–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soleymanlou N, Jurisica I, Nevo O, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90(7):4299–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Spencer K, Vereecken A, Cowans NJ. Maternal serum ADAM12s as a potential marker of trisomy 21 prior to 10 weeks of gestation. Prenat Diagn. 2008;28(3):209–211 [DOI] [PubMed] [Google Scholar]
- 63. Stone AL, Kroeger M, Sang QX. Structure-function analysis of the ADAM family of disintegrin-like and metalloproteinase-containing proteins (review). J Protein Chem. 1999;18(4):447–465 [DOI] [PubMed] [Google Scholar]
- 64. Zhu H, Leung PC, MacCalman CD. Expression of ADAMTS-5/implantin in human decidual stromal cells: regulatory effects of cytokines. Hum Reprod. 2007;22(1):63–74 [DOI] [PubMed] [Google Scholar]
- 65. Anton L, Merrill DC, Neves LAA, et al. The Uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150(9):4316–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Choong ML, Yang HH, McNiece I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Exp Hematol. 2007;35(4):551–564 [DOI] [PubMed] [Google Scholar]
- 67. Wang X, Tang S, Le SY, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE. 2008;3(7):e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xia H, Qi Y, Ng SS, et al. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380(2):205–210 [DOI] [PubMed] [Google Scholar]
- 69. Grammatopoulos DK. Placental corticotrophin-releasing hormone and its receptors in human pregnancy and labour: still a scientific enigma. J Neuroendocrinol. 2008;20(4):432–438 [DOI] [PubMed] [Google Scholar]
- 70. Wang J, Qiu Q, Haider M, Bell M, Gruslin A, Christians JK. Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse. J Endocrinol. 2009;202(3):337–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.