Abstract
Glucose transport from the maternal to fetal side of the placenta is critical for fetal growth and development due to the absence of fetal gluconeogenesis. Human GLUT9, existing as 2 isoforms, is a novel member of the transporter family. This study investigated the localization and relative expression levels of these isoforms in the human term placenta from both control and diabetic patients. Placenta samples were collected from normal pregnancies and those complicated by maternal diabetes (White classifications A1, A2, and B). Antibodies specific for the different isoforms were used to detect expression. Both forms of the protein are expressed in syncytiotrophoblast cells. Subcellular fractionation revealed an asymmetrical expression pattern with GLUT9a on basal membranes, whereas GLUT9b localizes to microvillus membranes. Expression of both isoforms is significantly increased in placental tissue from diabetic pregnancies. Altered expression of GLUT9 in the placenta may play a role in the fetal pathophysiology associated with diabetes-complicated pregnancies.
Keywords: glucose transport, syncytiotrophoblast, microvillus membrane, basal membrane, maternal diabetes
Introduction
From approximately 10 weeks after conception to delivery, the placenta is responsible for transporting fuel to the developing fetus. Appropriate fetal growth and development depend not only on adequate maternal glucose levels but also on transplacental glucose transport. This transport process is facilitated by the expression of the GLUT family of (facilitated-diffusion) glucose transporters. The 14 members of this superfamily all contain 12 membrane-spanning helices and several conserved sequence motifs. The family can be divided into 3 subclasses based on structure and transport function. Class I contains the extensively characterized glucose transporters GLUT1 to GLUT4, which can be distinguished on the basis of their distinct tissue distributions (GLUT1, erythrocytes, brain microvessels; GLUT2, liver, pancreatic islets; GLUT3, neuronal cells; and GLUT4, muscle, adipose tissue) and their hormonal regulation (eg, insulin sensitivity of GLUT4). Class II consists of the fructose-specific transporter GLUT5 and 3 related proteins, GLUT7, GLUT9, and GLUT11. Class III, characterized by the lack of a glycosylation site in the first extracellular linker domain and by the presence of such a site in loop 9, comprises GLUT6, GLUT8, GLUT10, and GLUT12.
Like most tissues in the body, the placenta expresses GLUT1 (a class I transporter) throughout gestation. Although there are conflicting reports, it is believed that GLUT1 is responsible for transporting glucose through the syncytiotrophoblasts as it is expressed in both the microvillus (MVM) and basal membranes (BM) of these cells.1–3 GLUT3 has been shown to localize to the fetal endothelial capillary cells, however, due to the nature of these continuous capillaries, it is unlikely that glucose transported across this cell type significantly contributes to transplacental transport.4 The insulin responsive isoform, GLUT4, was shown to be expressed in the intervillous stromal cells, but its function remains somewhat elusive since insulin does not induce GLUT4 translocation or syncytial glucose transport in term placenta.5 In addition, 2 of the 3 GLUT11 splice variants have been shown to be expressed in the placenta at the message level, and GLUT12 has been localized to the stromal cells and fetal membranes.6–8 The role of both GLUT11 and 12 in placental glucose transport has not been established.
GLUT9, a relatively recently cloned member of the GLUT family, has been shown to exist as 2 splice variants, each with differential membrane targeting.9 The placenta is one of the few tissues that express both variants at the messenger RNA (mRNA) level, suggesting a possible role for both GLUT9a and GLUT9b in placental hexose transport.9 GLUT9 transports both glucose and fructose but with close to 3-fold higher affinity for glucose10; nevertheless it is possible that GLUT9 functions as a fructose transporter in the placenta.
It is estimated that 3% to 5% of pregnancies are complicated with gestational diabetes, yet many more exhibit some form of glucose intolerance.11 The GLUT glucose transporters are believed to play a role in the pathophysiology of diabetes as dysregulation of GLUT expression may be responsible for the fetal pathophysiologies observed in diabetes-complicated pregnancies. Offspring of mothers with gestational diabetes are more likely to have increased adiposity and body size as well as a higher risk of developing type II diabetes.12,13 In offspring from pregestational and gestational diabetics, both body mass index (BMI) and rate of impaired glucose tolerance were found to be higher than offspring from control women.14 Some of these effects may be due to dysregulated glucose levels in utero, as a result of altered placental GLUT expression.15
In this study, we show for the first time that both hGLUT9a and hGLUT9b are expressed in the human term placenta at the protein level. Furthermore, we demonstrate that hGLUT9a predominates on the BM of the syncytiotrophoblast, whereas hGLUT9b is expressed predominately on the microvillus membrane. In additions, the A form (hGLUT9a) increases under pregestational and gestational diabetes. The B form (hGLUT9b), however, only seems to increase in response to insulin treatment. These characteristics suggest a role for GLUT9 in the fetal pathophysiologies seen in such pregnancies.
Materials and Methods
Reagents and Antibodies
All chemicals were obtained from Sigma Chemical (St Louis, Missouri) unless otherwise noted. Three polyclonal human GLUT9 antibodies were used in this study. The first was raised in rabbits against a C-terminal peptide common to both hGLUT9a and hGLUT9b protein, and this antibody has been used and characterized previously.9,16 The other 2 GLUT9 polyclonal antibodies were raised against N-terminal peptides in rabbits. The GLUT9a antibody was raised against DTSHARPPGPGRALLEC, and the GLUT9b was raised against KSRGEDEESDSAKKC. These have previously been characterized.17–19 Both antibodies were peptide purified before use. Preimmune sera for both antibodies were used as a negative control as well.
Placental Tissue Preparation
Placental tissue was collected from normal term deliveries occurring at Barnes-Jewish Hospital, St Louis, Missouri. Sample collection was approved by the Washington University Medical Center Human Studies Committee (#03-0778). On receipt, various portions from throughout the placenta, excluding the fetal membranes, were excised. Samples were flash frozen, paraffin embedded, or embedded in tissue freezing media (Electron Microscopy Science, Washington, Pennsylvania) and flash frozen in liquid nitrogen for generation of frozen sections. A Leica Microsystems (Wetzlar, Germany) microtome was used to produce 11 µm sections. Paraffin embedding and sectioning was done by the OB/GYN Histology Core at Washington University School of Medicine.
Immunohistochemical Staining
Paraffin-embedded sections were stained using an ABC-alkaline phosphatase kit from Vector Laboratories (Burlingame, California) and following manufacturer’s protocol. Slides were deparaffinized using an alcohol series. Heat-induced epitope retrieval was achieved by microwaving the slides in 0.01 mol/L sodium citrate pH 6.0 for 10 minutes. The primary antibodies were prepared in blocking buffer (2.5% normal goat serum, 0.1% BSA in PBS) for each of the samples. Samples were probed overnight for hGLUT9 (C-terminal antibody),9 hGLUT9a (see above; 20 µg/mL), hGLUT9b (see above; 15 µg/mL), hGLUT1 (1:100, positive control, Chemicon International, Temecula, California), hGLUT3 (1:500, negative control, gift from Dr J Moley, Washington University School of Medicine). Red AP Substrate in 100 mmol/L Tris HCl pH 8.4 (Vector Laboratories) was used for visualization. Counterstaining was performed using hematoxylin (Zymed Laboratories, South San Francisco, California). Slides were dehydrated using an alcohol series and mounted using Histamount Mounting Solution (Zymed Laboratories). Results were visualized using a Nikon microscope with camera head attachment.
Immunofluorescent Staining
Frozen sections were permeabilized with 0.2% saponin/phosphate buffered saline (PBS), washed in PBS, then nonspecific binding was blocked by incubation with 2% bovine serum albumin (BSA) in 0.01% saponin/PBS for 30 minutes. Cells were incubated for 1 hour with antiserum for the respective transporter. Peptide purified antibodies raised to a portion of the N-termini of hGLUT9a and hGLUT9b were used in a concentration of 15 and 10 µg/mL in 2% BSA/0.01% saponin/PBS, respectively. The slides were then probed with goat anti-rabbit Alexa 488 antibody (1:200 in 2% BSA in 0.01% Saponin/PBS). Nuclei were stained with 1 mmol/L ToPro-3 iodide (1:500 in PBS; Molecular Probes, Eugene, Oregon). Human GLUT1 and GLUT3 antibodies were used for positive and negative controls, respectively, at a concentration of 10 μg/mL. Similarly, preimmune sera for each of the GLUT9 antibodies were also used as a negative control at the same concentrations as their corresponding peptide purified antibody. Mounting was done using Vectashield (Vector Laboratories) and examined by confocal microscopy using a Nikon C1 confocal microscope (Nikon, Inc., Melville, NY).
Placental Syncytial Membrane Preparation
Placental tissue from control and diabetic pregnancies was obtained under procedures approved by the New Jersey Medical School Institutional Review Board, as described previously.15 Criteria for inclusion were (1) diagnosis of White class A or B diabetes (see below), (2) maternal age between 18 and 38 years, (3) absence of medical or obstetric complications other than diabetes, (4) term delivery (37 weeks gestation), and (5) singleton pregnancy. Exclusion criteria included the existence of nephropathy, retinopathy, hypertension (essential or pregnancy-induced), and conditions that might indicate altered uteroplacental blood flow or substrate delivery. Normal, age-matched control placental tissue was obtained and processed in a manner identical to the diabetic tissue. Demographics on the patients were described previously.15
Placental tissue obtained at delivery was placed on ice before preparation. Microvillous membrane and BM vesicles were prepared according to methods described previously.2,20 The only modification was the inclusion of protease inhibitory components (0.5 mmol/L ethylenediamine tetraacetate, 0.5 mg/mL aprotinin, and 0.5 mg/mL leupeptin) in the standard preparative buffer (250 mmol/L sucrose and 10 mmol/L HEPES/Tris, pH 7.4). Microvillous membrane and BM fractions were stored at −80°C until use.
Western Immunoblot Analysis
Isolated membrane fractions were solubilized in Laemmli buffer and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes, blocked with 5% dry milk in Tris-buffered saline/Tween 20 (TBS-T), and probed with an antibody raised against the human GLUT9a N-terminus (1.5 µg/mL in 1% dry milk/TBS-T), or the human GLUT9b N-terminus (1.0 µg/mL in 1% dry milk/TBS-T). Blots were then probed with a horseradish peroxidase-coupled goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Inc, Santa Cruz, California) and detection was performed using the SuperSignal Dura Western kit (Pierce Biotechnology, Inc, Rockford, Illinois). Blot quantitation after scanning was carried out using NIH Image. For the data generated from each set of Western, pairs of samples (BM and MVM fractions) from 3 different patients for the 4 different groups were run on separate immunoblots. The experiments were all run 3 times. Statistical analysis was done using analysis of variance (ANOVA) with Fisher post hoc testing on the sets of data for different patients and for the different antibodies. Values are expressed as averages + standard error of the mean (SEM).
Results
hGLUT9a and hGLUT9b Antibody Specificity
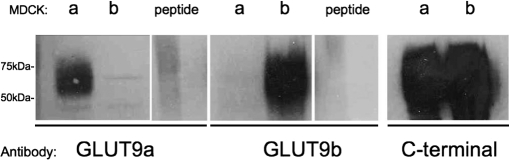
Total cell lysates from Madin-Darby canine kidney (MDCK) cells overexpressing either hGLUT9a or hGLUT9b, as described previously,9 were subjected to SDS-PAGE and immunoblotted with the hGLUT9a-specific antibody, the hGLUT9b-specific antibody, or the hGLUT9 antibody to the C-terminus, which detects both forms (Figure 1 ). Each of the N-termini-specific antibodies detected the correct protein overexpressed in MDCK cells, however, the alternative protein was not detected with the same antibody, demonstrating specificity. The C-terminal antibody detected both proteins as would be expected. In the presence of preimmune sera (not shown) or antibody with excess antigenic peptide (Figure 1), the corresponding proteins were not detected.
Figure 1.
Specificity of hGLUT9a and hGLUT9b antibodies. Expression of hGLUT9a was detected as a broad band at approximately 70 kDa by a polyclonal antibody raised against the N-terminus of hGLUT9a in MDCK cells overexpressing hGLUT9a but not detected in MDCK cells overexpressing hGLUT9b. Peptide competition blocked this specific detection when added in excess. Expression of hGLUT9b was detected by a polyclonal antibody raised against the N-terminus of hGLUT9b in MDCK cells overexpressing hGLUT9b but not detected in MDCK cells overexpressing hGLUT9a. Peptide competition blocked this specific detection when added in excess. Finally, both overexpressing hGLUT9a and hGLUT9b were detected in MDCK cells using a polyclonal antibody to the C-terminus of hGLUT9, common to both splice variants. MDCK indicates Madin-Darby canine kidney.
Both GLUT9a and GLUT9b are Expressed in the Human Term Placenta
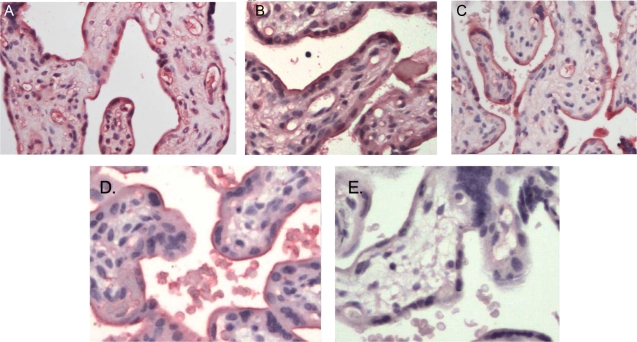
Using antibodies raised to the specific N-termini of the 2 splice variants of hGLUT9, both GLUTs were detected in term placenta (Figure 2 ). Both hGLUT9a (Figure 2A) and hGLUT9b (Figure 2B) were detected. Using the C-terminal GLUT9 antibody, the proteins were detected in a similar distribution (Figure 2C). Polyclonal hGLUT1 antibody was used as a positive control and appeared in the syncytiotrophoblast cells (Figure 2D). Conversely, hGLUT3 antibody was used as a negative control. No GLUT3 protein was detected in the syncytiotrophoblasts as previously reported15 (Figure 2E). Preimmune sera also failed to detect a signal.
Figure 2.
GLUT9a and GLUT9b protein are expressed in human full-term placenta. Immunohistochemistry using primary antibodies to nonsimilar motifs in the N-termini of both proteins shows that GLUT9a (A) and GLUT9b (B) are both expressed in term placentas. Using a primary antibody to a similar motif in the C-termini of both proteins, hGLUT9 demonstrated a similar distribution (C). Polyclonal antibody to hGLUT1 was used as a positive control (D), whereas hGLUT3 polyclonal antibody was used as a negative control (E).
GLUT9a is Predominantly Expressed in the Basal Membrane of the Syncytiotrophoblasts
Three sets of Western blots were performed comparing paired MVM and BM obtained from the same 3 placentas as reported previously15 and 2 control placentas. Details of these patients are given elsewhere.15 The immunoblots of MVM and BM from normal, term placentas detect hGLUT9a as a band at approximately 70 kDa as reported previously.9 GLUT9a is enriched in BM compared to MVM (Figure 3A). Immunofluorescent staining confirms no MVM staining (Figure 4A); however, localization of GLUT9a appears to be predominantly cytoplasmic with some BM labeling. This splice variant is also expressed in the endothelial cells from the fetal capillaries. Immunofluorescent labeling with hGLUT1 and hGLUT3 confirmed correct staining patterns, with hGLUT1 on both microvillus and basal membrane and hGLUT3 not in the syncytiotrophoblast cells.
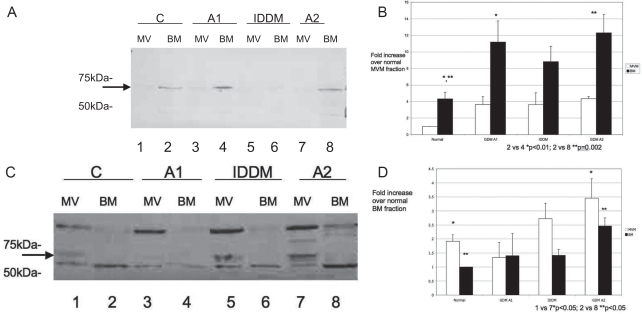
Figure 3.
A and B, GLUT9a is expressed predominantly in the basal membrane (BM) fraction. Following subcellular fractionation, GLUT9a was observed as a ~70 kDa band, predominantly in BM from both control (lanes 1 and 2) and diabetic (lanes 3 and 8) placentas. A lower degree of expression was seen in the microvillous membrane (MVM) fraction, however the trend of higher expression in the diabetic placentas also was seen. This expression was quantitated (B) and demonstrated that GLUT9a protein was significantly higher in the BM of gestational diabetics (GDM A1, lane 4; GDM A2, lane 8) compared to control (lane 2; *P < .01, **P = .002 by analysis of variance [ANOVA]). Although GLUT9a protein trended toward significance in the BM of placenta from the insulin-dependent diabetes mellitus (IDDM) patients (lane 6; P = .053), this difference compared to control BM (lane 2) was not significant. For each experiment, 1 patient placenta per group was examined. The experiments were repeated a total of 3 times.C and D, GLUT9b is expressed predominantly in the MVM fraction. GLUT9b was seen as a ~70 kDa doublet band observed predominantly in MVM from term placentas of control (lanes 1 and 2) and diabetic patients (lanes 3-8). GLUT9b protein was significantly higher in the placenta from the insulin-controlled gestational diabetic patients (GDM A2) in both MVM (lane 7) and BM (lane 8) compared to control (lanes 1 and 2; P < .05, respectively, ANOVA). The trend between IDDM patient placental MVM and BM as compared to control patient MVM and BM was also toward significance.
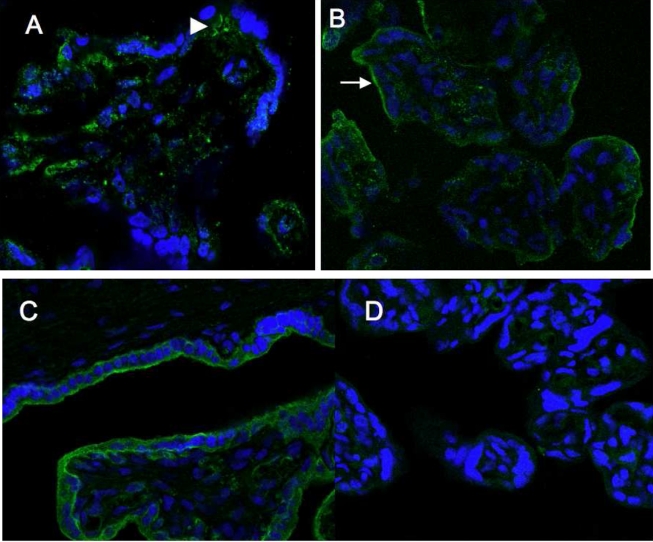
Figure 4.
GLUT9a and GLUT9b are expressed in different locations in term placenta. A, By immunofluorescent microscopy, GLUT9a appears to be expressed predominantly on the basal membrane (BM) and cytoplasm of the syncytiotrophoblast cells and also in fetal capillaries. B, GLUT9b appears to be localized in intracellular compartments and on the microvillous membrane (MVM) or apical surfaces of the syncytiotrophoblast cells. Small arrows indicate MVM surface; large arrows indicate BM surface. GLUT1 is expressed on both MVM and BM surfaces as a positive control (C), whereas GLUT3 protein is not expressed in syncytiotrophoblasts (D).
GLUT9b Expression Predominates in the MVM of Syncytiotrophoblasts
In contrast to GLUT9a, there is an enrichment of GLUT9b in MVM when compared to BM preparations from the same control placenta (Figure 3C). Therefore, GLUT9b predominates on the MVM in term control syncytium. Immunofluorescent staining of normal placenta confirms this observation (Figure 4B). In addition, cytoplasmic labeling is detected in a vesicular compartment.
Upregulation of GLUT9a and GLUT9b in Syncytium from Diabetes-Complicated Pregnancies
To determine relative expression levels of each splice variant between placenta from different diabetic patients, MVM and BM were isolated from placental tissue and the membrane fractions were run on SDS-polyacrylamide gels and then blotted on nitrocellulose membranes. These blots show the presence of a ~70 kDa band corresponding to GLUT9 in all samples (Figure 3A and C). Quantitation based on total protein levels in paired samples revealed an increase in BM GLUT9a expression in both gestational and pregestational diabetic patients compared to control BM (Figure 3B, lanes 4 and 8 vs lane 2, P < .01; lane 6 trended toward significance P = .053). GLUT9a expression in MVM also appeared to be greater in diabetic versus normal placental fractions; however, this was not a significant increase. All samples in all experiments were normalized to control MVM for GLUT9a quantification.
For GLUT9b, the transporter band appears as a doublet, demonstrating the highly glycosylated nature of some GLUTs (Figure 3C). Both upper band, when present, and lower band were used in the quantification. An increase in GLUT9b expression was observed in the MVM fraction from insulin-treated gestational (GDMA2) compared to control MVM (Figure 3D, lane 1 vs 7; P < .05); there was also a trend toward significance for increased expression in the pregestational diabetics (insulin-dependent diabetes mellitus (IDDM); lane 1 vs 5; P = .09). GLUT9b protein was significantly higher in the BM placental fractions from the insulin-controlled gestational diabetic patients (GDMA2; lane 8) compared to control (lane 2; P < .05). There was also a trend toward increased GLUT9b expression in the BM placental fractions from the IDDM group compared to the control group (lane 2 vs 6; P = .053). All samples in all experiments were normalized to control BM for GLUT9b quantification.
Discussion
In this study, we show for the first time the presence of both splice variants of hGLUT9 at the protein level in human term placenta. Previous studies have demonstrated the presence of GLUTs 1, 3, 4, and 12, and the message for 2 GLUT11 splice variants in term placenta.1,2,4–7 However, GLUT9 is the first of the class II GLUTs to be shown on the protein level. We also demonstrate asymmetric localization of the 2 splice variants with the “a” form predominating on the basal membrane and the “b” form primarily on the microvillus membrane. Furthermore, analysis of placentas from diabetic pregnancies (White Class A1 and A2) demonstrates a significantly increased expression of both GLUT9a and GLUT9b in the presence of maternal diabetic conditions.
Augustin and colleagues have previously shown the 2 splice variants of GLUT9 to localize to different regions of polarized epithelial cells.9 The “a” form is targeted to the basolateral membrane whereas the “b” form is observed primarily in the apical membrane. In this study, we have shown similar localization within the placental syncytium, via both immunofluorescent microscopy and Western blot of membrane fractions. This article, therefore, confirms the observed location of GLUT9 in an overexpressing model to be representative of that in vivo.
The observed asymmetric targeting is of interest because of the homeostatic implications of the polarized transporter localization. The placental syncytium functions as the primary barrier between maternal and fetal circulations and therefore transport into and out of this cell layer is likely to be highly regulated. The basal membrane is believed to be the rate-limiting membrane for the transport of glucose from maternal to fetal blood. This conclusion stems first from the observation that there is substantially less GLUT1 observed on the basal membrane when compared to the GLUT1-enriched microvillus membrane. The second piece of evidence is drawn from measurements of transport across BeWo choriocarcinoma cell monolayers (a trophoblast model), in which variations in the basal membrane content of active glucose transporters produced corresponding changes in transcellular transport, whereas alterations in microvillous transporters did not.21 In support of this hypothesis, in vivo investigations have shown an upregulation in basal membrane GLUT1 expression over gestation, corresponding to the increase in fetal glucose demand, in contrast to the stable expression of microvillous GLUT1.2 Similarly, in diabetic patients, syncytial basal membrane GLUT1 expression and activity have been shown to increase, whereas MVM expression and activity do not change.15,22 Our study is particularly relevant to this proposed mechanism in that GLUT9a was found to predominate on the basal membrane, suggesting an alternative transport system for glucose through this membrane. Additionally, GLUT9a expression increased when the pregnancy was complicated with diabetes. Hyperglycemia is one of the conditions which links all 3 diabetic populations studied in this report. In mice, hyperglycemia has been shown to increase the expression of GLUT9 in the kidney and liver.23 Therefore, GLUT9 may function as an overflow transporter, providing additional basal membrane glucose transport and demonstrating upregulation when maternal circulating glucose levels are high.
Class II GLUTs, including GLUT9, are known to transport fructose in addition to glucose. Animal and human studies have demonstrated the production and transport of fructose by the placenta.24,25 GLUT5, the primary enteric fructose transporter has a high affinity for fructose (Km = 5 mmol/L),26 however, GLUT5 has not been shown in the human placenta. GLUT9, the transporter shown to be expressed in the placenta in this study, has an even higher affinity for fructose (Km = 0.42 mmol/L).10 Therefore, it is possible that GLUT9 may function as a fructose transporter in the human placenta.
Diabetics have been shown to have higher circulating levels of serum fructose.27 With the presence of a fructose transporter in the placenta, this may lead to higher fetal fructose levels. In bovine and porcine models, fetal fructose levels were reported to be 3- to 4-fold higher than maternal fructose levels under normal fed conditions.28,29 In 1 human study, a 40% fructose solution was administered to mothers just prior to delivery and cord blood fructose levels were analyzed. In this model, which may reflect the diabetic conditions, cord fructose levels increased 3-fold in response to elevated maternal fructose levels.30 These findings support our hypothesis that in diabetic women, fetal fructose levels may rise due to the presence of increased GLUT9a and b on the BM and MVM surfaces of the term placenta. Fructose has been shown to induce insulin-resistance and thus play a role in the downstream effects of metabolic syndrome.31,32 Brownlee has suggested a unifying mechanism to explain diabetic complications, which focuses on increasing reactive oxygen species (ROS).33 Elevated fructose has also been shown to increase the production of ROS by inhibiting glyceraldehyde 3-phosphate dehydrogenase (GAPDH).34,35 Although speculative, it is possible that increased transport of fructose via GLUT9 in the placenta leads to elevated fructose levels in utero and increased ROS production within the placental or fetal compartment. In this way, an increase in GLUT9 in the placenta may contribute to diabetic complications in pregnancy.
In conclusion, we have shown hGLUT9a and hGLUT9b to be expressed in the human placenta at term. The “a” form appears predominantly at the basal membrane of the placental syncytium and the “b” form is expressed on the MVM. Additionally, we have shown an increase in the expression of the both forms when the pregnancy is complicated by maternal diabetes. This increase suggests a role for GLUT9 in placental hexose transport and the fetal pathophysiologies associated with diabetes-complicated pregnancies.
Footnotes
The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Grant from NIH R01 HD04390 (KHM) and a research grant from the American Diabetes Association (KHM) .
References
- 1. Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA. Quantitation and immunolocalization of glucose transporters in the human placenta. Placenta. 1995;16(7):623–633 [DOI] [PubMed] [Google Scholar]
- 2. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562 [DOI] [PubMed] [Google Scholar]
- 3. Takata K, Kasahara T, Kashahara M, Ezaki O, Hirano H. Localization of erythrocyte/HepG2-type glucose transporter (GLUT1) in human placental villi. Cell Tissue Res. 1992;267(3):407–412 [DOI] [PubMed] [Google Scholar]
- 4. Hauguel-de Mouzon S, Challier JC, Kacemi A, Cauzac M, Malek A, Girard J. The GLUT3 glucose transporter isoform is differentially expressed within human placental cell types. J Clin Endocrinol Metab. 1997;82(8):2689–2694 [DOI] [PubMed] [Google Scholar]
- 5. Xing AY, Challier JC, Lepercq J, et al. Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta. J Clin Endocrinol Metab. 1998;83(11):4097–4101 [DOI] [PubMed] [Google Scholar]
- 6. Gude NM, Stevenson JL, Rogers S, et al. GLUT12 expression in human placenta in first trimester and term. Placenta. 2003;24(5):566–570 [DOI] [PubMed] [Google Scholar]
- 7. Scheepers A, Schmidt S, Manolescu A, et al. Characterization of the human SLC2A11 (GLUT11) gene: alternative promoter usage, function, expression, and subcellular distribution of three isoforms, and lack of mouse orthologue. Mol Membr Biol. 2005;22(4):339–351 [DOI] [PubMed] [Google Scholar]
- 8. Gude NM, Stevenson JL, Murthi P, et al. Expression of GLUT12 in the fetal membranes of the human placenta. Placenta. 2005;26(1):67–72 [DOI] [PubMed] [Google Scholar]
- 9. Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking. J Biol Chem. 2004;279(16):16229–16236 [DOI] [PubMed] [Google Scholar]
- 10. Manolescu A, Augustin R, Moley KH, Cheeseman CI. A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity. Mol Membr Biol. 2007;24(5-6):1–9 [DOI] [PubMed] [Google Scholar]
- 11. Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21(2):103–113 [DOI] [PubMed] [Google Scholar]
- 12. Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care. 1999;22(8):1284–1291 [DOI] [PubMed] [Google Scholar]
- 13. Lindsay RS, Dabelea D, Roumain J, Hanson RL, Bennett PH, Knowler WC. Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes. 2000;49(3):445–449 [DOI] [PubMed] [Google Scholar]
- 14. Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21(suppl 2):B142–B149 [PubMed] [Google Scholar]
- 15. Gaither K, Quraishi AN, Illsley NP. Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter. J Clin Endocrinol Metab. 1999;84(2):695–701 [DOI] [PubMed] [Google Scholar]
- 16. Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology. 2004;145(3):1435–1443 [DOI] [PubMed] [Google Scholar]
- 17. Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology. 2009;150(12):5302–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim ST, Moley KH. The expression of GLUT8, GLUT9a, and GLUT9b in the mouse testis and sperm. Reprod Sci. 2007;14(5):445–455 [DOI] [PubMed] [Google Scholar]
- 19. Kim ST, Moley KH. Regulation of facilitative glucose transporters and AKT/MAPK/PRKAA signaling via estradiol and progesterone in the mouse uterine epithelium. Biol Reprod. 2009;81(1):188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jansson T, Powell TL, Illsley NP. Non-electrolyte solute permeabilities of human placental microvillous and basal membranes. J Physiol. 1993;468:261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine. 2002;19(1):13–22 [DOI] [PubMed] [Google Scholar]
- 22. Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999;180(1 pt 1):163–168 [DOI] [PubMed] [Google Scholar]
- 23. Keembiyehetty C, Augustin R, Carayannopoulos MO, et al. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol. 2006;20(3):686–697 [DOI] [PubMed] [Google Scholar]
- 24. Hagerman DD, Villee CA. The transport of fructose by human placenta. J Clin Invest. 1952;31(10):911–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bertolini M, Wallace C, Anderson G. Expression profile and protein levels of placental products as indirect measures of placental function in in vitro-derived bovine pregnancies. Reproduction. 2006;131(1):163–173 [DOI] [PubMed] [Google Scholar]
- 26. Rand EB, Depaoli AM, Davidson NO, Bell GI, Burant CF. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am J Physiol. 1993;264(6 pt 1):G1169–G1176 [DOI] [PubMed] [Google Scholar]
- 27. Kawasaki T, Akanuma H, Yamanouchi T. Increased fructose concentrations in blood and urine in patients with diabetes. Diabetes Care. 2002;25(2):353–357 [DOI] [PubMed] [Google Scholar]
- 28. Meznarich HK, Hay WW, Jr, Sparks JW, Meschia G, Battaglia FC. Fructose Disposal and oxidation rates in the ovine fetus. Exp Physiol. 1987;72(4):617–625 [DOI] [PubMed] [Google Scholar]
- 29. Pere MC. Maternal and fetal blood levels of glucose, lactate, fructose and insulin in the conscious pig. J Anim Sci. 1995;73(10):2994–2999 [DOI] [PubMed] [Google Scholar]
- 30. Morris ED, Wood C, Archer GD. The effect on cord blood glucose levels of the intravenous administsration of fructose to the mother. J Obstet Gynecol Br Commonw. 1964;71:766–767 [DOI] [PubMed] [Google Scholar]
- 31. Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49(6):1155–1163 [DOI] [PubMed] [Google Scholar]
- 32. Lee MK, Miles PD, Khoursheed M, Gao KM, Moossa AR, Olefsky JM. Metabolic effects of troglitazone on fructose-induced insulin resistance in the rat. Diabetes. 1994;43(12):1435–1439 [DOI] [PubMed] [Google Scholar]
- 33. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625 [DOI] [PubMed] [Google Scholar]
- 34. Delbosc S, Paizanis E, Magous R, et al. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179(1):43–49 [DOI] [PubMed] [Google Scholar]
- 35. Zhao W, Devamanoharan PS, Varma SD. Fructose induced deactivation of antioxidant enzymes: preventive effect of pyruvate. Free Radic Res. 2000;33(1):23–30 [DOI] [PubMed] [Google Scholar]