Abstract
Inflammation is a defensive process by which the body responds to both localized and systemic tissue damage by the induction of innate and adaptive immunity. Literature from human and animal studies links inappropriate in utero inflammation to preterm parturition and fetal injury. The pathways by which such inflammation may cause labor, however, are not fully understood. Any proinflammatory agonist in the amniotic fluid will contact the fetal skin, in its entirety, but a potential role of the fetal skin in the pathways to labor have not previously been explored. We hypothesized that the fetal skin would respond robustly to the presence of intra-amniotic lipopolysaccharide (LPS) in our ovine model of in utero inflammation. In vitro and in utero exposure of fetal ovine keratinocytes or fetal skin to Escherichia coli LPS reliably induced significant increases in interleukin 1β (IL-1β), IL-6, tumor necrosis factor α (TNF-α), and IL-8 expression. We demonstrate that, in utero, this expression requires direct exposure with LPS suggesting that the inflammation is triggered directly in the skin itself, rather than as a secondary response to a systemic stimuli and that inflammation involves Toll-like receptor (TLR) regulation and neutrophil chemotaxis in concordance with an acute inflammatory reaction. We show that this response involves multiple inflammatory mediators, TLR regulation, and localized inflammatory cell influx characteristic of an acute inflammatory reaction. These novel data strongly suggests that the fetal skin acts as an important mediator of the fetal inflammatory response and as such may contribute to preterm birth.
Keywords: skin, inflammation, preterm birth, uterine infection
Introduction
Preterm birth (birth before 37 weeks of gestational age [GA]) remains possibly the greatest challenge facing clinical obstetrics in the developed world. Preterm births continue to account for approximately 13% of live births in North America and Australia.1,2 Very-low-birth-weight infants (birth before 28 weeks gestation) account for approximately 1% of all live births and a sizeable percentage (estimated at 50%-70%) of total neonatal deaths.3,4 Infants born preterm may require, depending on the degree of their prematurity, significant and expensive periods of hospitilization and are at an elevated risk of developing a host of complications including bronchopulmonary dysplasia and acute respiratory distress syndrome. In addition to the immediate risks associated with preterm delivery, there is a growing appreciation that exposure to adverse early life and in utero conditions can increase an individual’s predisposition to the development of a host of chronic diseases in adult life.5–8 Of the numerous etiologies, intrauterine infection and the subsequent production of inflammatory cytokines and chemokines has the most clearly established causal association with spontaneous preterm birth.9
Gomez and colleagues demonstrated that elevated fetal plasma interleukin 6 ([IL-6] >11 pg/mL) is an independent risk factor for the development of severe neonatal morbidity.10 This finding, now known as fetal inflammatory response syndrome (FIRS), correlates strongly with preterm delivery in the presence of intrauterine infection. Microbial invasion of the amniotic cavity by microorganisms from the genital tract is thought to be the most prevalent cause of fetal inflammation, chorioamnionitis, and funisitis.11,12
Animal studies further reinforce the infection—inflammation—preterm parturition hypothesis.13 Intrauterine infection with Escherichia coli or administration of the Toll-like receptor (TLR) 4 agonist lipopolysaccharide (LPS) caused intrauterine inflammation and preterm parturition in mice, hamsters, and rhesus macaques.14,15 We previously demonstrated that injection of E. coli LPS to the amniotic cavity of merino ewes reliably induced fetal inflammation and chorioamnionitis.16,17 More recently, we demonstrated that deliberate infection of the ovine fetus with the human genital tract mycoplasma Ureaplasma species led to fetal inflammation and chorioamnionitis.18
Amniotic fluid is in contact with the fetal lung, gastrointestinal (GI) tract, skin, and chorioamnion in utero. One of the major outstanding questions in relation to inflammation and preterm birth relates to the relative contributions of fetal and uterine inflammation to the induction of the parturition process, and which fetal tissues are most involved in the causal pathways. We previously demonstrated that intra-amniotic administration of LPS induced a rapid (within 2 hours) influx of inflammatory cells into the fetal lung leading to proinflammatory cytokine (IL-1β, IL-6) expression that peaks in the respiratory tissues at approximately 48 hours postinjection.19
The fetal skin is exposed, in toto, to the amniotic fluid and thus any invading microorganisms that it might contain. Despite its likely exposure to proinflammatory agonists, its significant size and the demonstrated ability of keratinocytes from adult skin to launch a vigorous response to infection, the fetal skin remains almost completely unstudied with respect to a potential role in fetal inflammation.20–22 An autopsy study involving 12 preterm infants demonstrated that dermatitis can be a component of the fetal inflammatory response induced by chorioamnionitis.23 However, it is not known whether the fetal skin response is caused by a fetal systemic inflammation or is due to localized contact with bacterial agonist. We have tested the hypothesis that the developing fetal skin is able to mount a robust proinflammatory response following in utero exposure of fetal sheep to E. coli LPS. Here we present the first data from a controlled study demonstrating that the fetal skin is able to respond to the presence of E. coli LPS in the amniotic fluid by the production of significant levels of proinflammatory cytokines and chemokines.
Materials and Methods
Animals
Date-mated merino ewes with singleton pregnancies were bred as previously described.16 All procedures involving animals were performed in Western Australia, following review and approval by the animal care and use committees of the Cincinnati Children’s Hospital (Cincinnati, Ohio) and The University of Western Australia. At the conclusion of each experimental protocol, animals were heavily sedated with intravenous injection of metadomidine (0.12 mg/kg) and ketamine (12 mg/kg). The fetus was then immediately surgically delivered, and both ewe and lamb were euthanized with pentobarbitone (100 mg/kg).
Intra-Amniotic Administration of LPS
Intra-amniotic administration of LPS from E. coli (055:B5, Sigma Aldrich, Australia) was performed under ultrasound guidance as previously described, with successful intra-amniotic targeting verified by electrolyte analysis of 2 mL of amniotic fluid.24 To investigate the effects of LPS on the fetal skin, animals were randomly assigned to receive 10 mg of LPS in 2 mL (n = 6) or saline (n = 4) 2 days (122 days GA) before surgical delivery at 124 days GA.
Surgical Delivery of Intratracheal LPS
A separate group of animals were used to investigate the systemic effects of LPS in the absence of direct skin exposure. We selectively delivered LPS into the fetal lung by ligating the fetal trachea prior to introducing an osmotic pump (Alzet 200D1, Bioscientific Pty, Australia) containing 1 mg of LPS. Briefly, the anesthetized ewe was subjected to a laparotomy to expose the uterus and a small incision was made in the uterine wall allowing externalization of the fetal head. The fetal trachea was exposed and ligated using a 2.0 silk suture. A fine catheter, attached to an Alzet pump containing 1 mg LPS in 200 μL saline (24 hour release) was placed in the trachea and sealed in place with surgical adhesive (n = 6). To effectively separate the fetal lung from the amniotic cavity and to allow fetal lung fluid to collect (preventing lung distention), a catheter with sealed bag (sited in the uterine space) was tightly sutured to the trachea. Controls had identical surgical procedure with the only difference being saline loaded in the Alzet pump (n = 4). The surgical site was closed and the animal allowed to recover for 2 days (GA at time of surgery = 122 days, n = 6) before surgical delivery.
Primary Keratinocyte Cell Culture
Primary ovine keratinocytes were collected from fetuses immediately following surgical delivery and post-mortem at 124 days gestation (term = 150 days). The fetal skin was quickly shaved and dissected free of the underlying fascia before being stored at 4°C in 10 mL of serum-free Dulbecco's modified Eagle's medium (DMEM) and prior to processing. Strips of fetal skin were rinsed and then incubated for 3 minutes in 70% ethanol. The decontaminated tissue was rinsed 3 times and incubated in sterile phosphate buffered saline (PBS) containing 5 mg/mL streptomycin and 5 mg/mL penicillin before being cut into 5 mm2. The tissue pieces were incubated in a 3% solution of povodine/iodine for 10 minutes, rinsed 3 times in sterile PBS, and incubated for 48 hours at 4°C in a 10 mL solution of dispase containing 5 mg/mL streptomycin and 5 mg/mL penicillin. Following incubation, the epidermal layer was removed from the underlying dermis using watchmaker’s forceps and transferred to a 10 mL solution of PBS containing 0.1% trypsin in 1 mmol/L EDTA. The epidermal sheets were incubated in a shaking water bath for 15 minutes at 37°C before the reaction was halted by the addition of keratinocyte growth media (KGM) containing 5% fetal bovine serum (Invitrogen, Australia). Cells were pelleted by centrifugation at 1000 rpm for 5 minutes. Cell pellets were resuspended in 5 mL of KGM and assessed for viability using trypan blue staining before being seeded into 25 cm2 flasks (total culture volume 10 mL) and cultured at 37°C in a 5% CO2 atmosphere in serum-free KGM containing 5 mg/mL streptomycin and 5 mg/mL penicillin. All fetal ovine keratinocytes used in this study were passage 3 and were taken from fetuses at 124 days of GA (term = 150 days).
In vitro LPS Inflammation assays
Primary ovine keratinocytes were seeded into 12 well culture plates at a density of 2 × 105 cells/well in 2 mL of serum-free KGM containing 5 mg/mL streptomycin and 5 mg/mL penicillin (Invitrogen, Australia). Cells were grown until >95% confluent. Cells were then exposed to either saline (20 μL/well) or LPS (1 μg/well in 20 μL saline). Each exposure was performed in triplicate at the following time points: 0 minutes, 30 minutes, 60 minutes, 90 minutes, 2 hours, 3 hours, and 6 hours.
RNA Isolation and Complementary DNA Generation
Total RNA was isolated from cells and frozen tissue using Trizol (Sigma Aldrich, Australia). Cells, or frozen tissue were finely milled in liquid nitrogen and were added directly to 1 mL of trizol and processed in accordance with the manufacturer’s instructions. Complementary DNA (cDNA) was generated using BIOSCRIPT (BIOLine, Australia), according to manufacturer’s instructions.
Quantitative Polymerase Chain Reaction
Primer pairs unique to this study were IL-1β (F: ACG AAC ATG TCT TCC GTG AT; R: ACC AGG GAT TTT TGC TCT CT) and IL-6 (F: ACC TGG ACT TCC TCC AGA AC; R: TTG AGG ACT GCA TCT TCT CC). Primer efficiencies for all reactions in this study were calculated from the raw data of 15 randomly selected runs as published previously and found to be >90%.25 Quantitative PCR analysis of cytokine and chemokine expression was performed on a Corbett Robotics Rotorgene 3000 using 2 × power SYBR master mix (ABI, Australia) in a final volume of 20 μL. Fluorescence was normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data were processed to generate fold change estimates using a modified 2−ΔΔCT method.26 The remaining primer sequences were derived from studies previously published by Chang and Sow.27,28
Tissue Immunocytochemistry
Fetal ovine skin was dissected, embedded in Optimal Cutting Temperature Compound (OCT), and immediately frozen in liquid nitrogen-cooled isopentane. OCT embedded skin was transversely oriented and sectioned on a Leica CM1900 cryostat to a thickness of 9 μm. Transverse skin sections were snap fixed in 500 μL of a 1:1 solution of ice-cold acetone:methanol, then blocked for 2 hours at room temperature in 1% newborn calf serum (FCS) in PBS containing 0.1% triton X-100. For immunofluorescence analysis, primary antibodies against CD18 (Abnova, Sapphire Bioscience Pty, Australia) or TLR4 (AbCam, UK) were diluted 1:150 in PBS containing 1% newborn calf serum, 0.1% triton X-100 and applied to fixed sections. After overnight (16 hours) incubation at 4°C, sections were rinsed repeatedly in PBS followed by incubation with secondary antibody: Alexa fluor 488/594 anti-mouse immunoglobulin G ([IgG] Biolegend, Australia) diluted 1:600 in PBS containing 1% newborn calf serum, 0.1% triton X-100 and incubated for 2 hours at room temperature. Sections were rinsed repeatedly in PBS before being mounted using biogel aqueous mounting medium (Proscitech, Australia) and sealed with hard-set permamount (Proscitech). Staining specificity was demonstrated by the absence of staining in sections prepared without primary antibody. Sections for hemotoxylin and eosin staining were processed using a standard protocol.
Results
Primary Fetal Ovine Keratinocyte Cultures
Cultures of primary fetal ovine keratinocytes were validated on the basis of morphology and the expression of the keratinocyte-specific differentiation marker involucrin. Figure 1A shows immunofluorescent analysis of a 9-μm cryosection of fetal epidermis, demonstrating keratinocyte-specific expression of involucrin. The characteristic cobblestone morphology of cultured primary fetal ovine keratinocytes exhibited in Figure 1B is consistent with the morphology expected for a primary culture of epidermal keratinocytes. The cultures demonstrated uniform expression of involucrin indicating pure cultures of keratinocytes. The morphological differences between cells in Figure 1B and C derive from the low-density culture of primary epidermal keratinocytes on glass slides (C).
Figure 1.
Validation of primary fetal ovine keratinocyte culture. A, Immunohistological stain of transverse skin section demonstrating epidermal staining for involucrin. B, Confluent fetal ovine keratinocytes displaying characteristic cobblestone morphology. C, Immunohistological stain of primary fetal ovine keratinocytes demonstrating cytoplasmic staining for involucrin.
In Vitro Exposure to LPS Induces Cytokine Expression in Primary Fetal Ovine Keratinocytes
Cultures of primary fetal ovine keratinocytes were assessed for their ability to express proinflammatory cytokines following exposure to E. coli LPS. Data contained within Figure 2 demonstrate that primary fetal ovine keratinocytes have the capacity to mount a multifactorial response to LPS immunostimulation (Figure 2). Interleukin 1β, IL-6, IL-8, and tumor necrosis factor α (TNF-α) messenger RNA (mRNA) expression is greatly upregulated 100 minutes postexposure relative to saline control. Cytokine mRNA expression peaked after 3 hours for all 4 transcripts analyzed and returned to baseline at 6 hours poststimulation.
Figure 2.
Quantitative PCR analysis of cytokine expression profile in primary fetal ovine keratinocytes exposed to 1 ug LPS over a 6-hour time course. All data points represent mean of 3 individual readings. Error bars represent 95% confidence interval about the mean. Red line represents ddCt = 1 (no difference between control and treatment groups). PCR indicates polymearase chain reaction; LPS, lipopolysaccharide; IL, interleukin; TNF, tumor necrosis factor.
Direct Intra-Amniotic Exposure to LPS is Both Sufficient and Necessary to Induce Cytokine Expression in the Fetal Skin
Intra-amniotic administration of E. coli LPS induced increased mRNA expression for IL-1β (Figure 3A) and IL-8 (Figure 3B) in the skin of 5/6 and 6/6 fetuses 48 hours following treatment, respectively. In contrast, LPS exposure limited to the fetal trachea and lungs failed to consistently induce either IL-1β or IL-8 expression in the fetal skin 48 hours following catheterization. Analysis of pulmonary epithelia demonstrated increased expression of IL-1β mRNA and neutrophil influx (data not shown, S. Kallapur, personal communication), suggesting a localized acute inflammatory response. The surgical controls did not have increased Il-1β or IL-8 mRNA expression relative to nonsurgical controls exposed to intra-amniotic saline (data not shown), demonstrating that the surgical procedure by itself did not induce inflammation in the skin.
Figure 3.
Quantitative PCR analysis of cytokine expression in fetal skin following exposure to 10 mg intra-amniotic LPS. A, IL-1β. B, IL-8. All data points represent mean of 3 individual readings. Error bars represent 95% confidence interval about the mean. *represents replicates with lower limit of 95% confidence interval > ddCt = 1. Red segmented line represents ddCt = 1 (no difference between control and treatment groups). PCR indicates polymearase chain reaction; LPS, lipopolysaccharide; IL, interleukin, 2D = two day, IA = intra-amniotic, IT = intra-tracheal.
Direct Intra-Amniotic Exposure to LPS is Sufficient to Induce Neutrophil Influx and TLR4 Regulation in the Fetal Ovine Skin
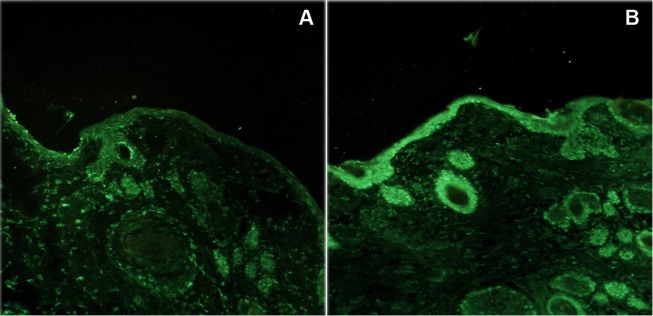
Hemotoxylin and eosin staining of skin from fetuses exposed to intra-amniotic or intra-tracheal saline failed to induce epidermal cellular infiltration 48 hours following treatment (Figure 4A and C). In contrast, the intra-amniotic administration of LPS yielded epidermal infiltration of regular basophilic (dark purple) staining cells 48 hours after treatment (Figure 4B). There was no recruitment of these cells to the dermis of fetuses when LPS exposure was surgically restricted to the respiratory tract (Figure 4D). To characterize the cellular infiltrate, skin sections were stained with an antibody specific for CD18 (β2-integrin), a marker of neutrophil activation.29 Little or no epidermal staining was detectable in the saline exposure groups and the intratracheal only LPS group (Figure 5A, C, and D). Robust staining for CD18 was detected in the dermis of fetal skin exposed to intra-amniotic LPS (Figure 5B). Finally, we assessed TLR4 expression in fetal skin following in utero exposure to LPS (Figure 6). Immunohistochemical analysis of skin 48 hours following LPS (Figure 6A) exposure demonstrated a strong increase in TLR4 staining in comparison with skin exposed only to saline (Figure 6B) exposure.
Figure 4.
Hemotoxylin and eosin analysis of transverse section of fetal skin 48 hours posttreatment. Main fields represent a 200× magnification, inset B* represents a 400× magnification. A, Intra-amniotic saline treatment. B, Intra-amniotic LPS treatment. C, Intratracheal saline treatment. D. Intratracheal LPS treatment. Note the striking epidermal infiltration of basophilic cells in B (arrows). A similar infiltration is absent in D. Images representative of n = 5 animals (LPS treatment) or n = 3 animals (saline treatment). LPS indicates lipopolysaccharide.
Figure 5.
Immunohistological analysis of transverse section of fetal skin 48 hours posttreatment stained for CD18. All fields represent a 200× magnification. A, Intra-amniotic saline treatment. B, Intra-amniotic LPS treatment; C) Intratracheal saline treatment; D) Intratracheal LPS treatment. Note the marked increase in epidermal CD18 staining (arrows) in B, correlating with basophilic infiltrate seen in Figure 4. A similar increase in staining is absent in D. Images representative of n = 5 animals (LPS treatment) or n = 3 animals (saline treatment). LPS indicates lipopolysaccharide.
Figure 6.
Immunohistological analysis of transverse section of fetal skin 48 hours posttreatment stained for TLR4. All fields represent a 200× magnification. A, Intra-amniotic saline treatment. B, Intra-amniotic LPS treatment. Note the marked increase in staining intensity and distribution in B. Images representative of n = 5 animals (LPS treatment) or n = 3 animals (saline treatment). LPS indicates lipopolysaccharide; TLR, Toll-like receptor.
Discussion
We report data demonstrating the importance of the skin in the development of fetal inflammation. Inflammation of the fetal and maternal tissues following microbiological invasion of the uterine cavity is one of the most important causes of preterm birth. Between 25% and 40% of all preterm births (delivery before 37 weeks gestation) are associated with intrauterine infection.9
By exposing the fetal and maternal tissues to E. coli LPS, a number of investigators have studied the evolution and effects of intrauterine inflammation in animal models including mice, rabbits, nonhuman primates, and sheep.14–17 We and others have clearly demonstrated that intra-amniotic administration of LPS induces chorioamnionitis, inflammation, and maturation of the fetal lung in conjunction with elevated levels of fetal and amniotic cytokine/chemokine expression. Despite our appreciation of the role played by the innate immune system and inflammation in the premature induction of parturition, there remains a number of unresolved questions in relation to the timing and origin of the inflammatory stimuli that drive these processes.
In this study, we demonstrate the importance of the skin in the development of fetal inflammation. The fetal skin is exposed in its entirety to the amniotic environment and thus any colonizing microorganisms. Previous histological investigation of the developing fetal skin in humans demonstrated that the outer cornified stratum corneum, which plays an important barrier function by virtue of its high lipid and keratin composition, is largely absent in the first 2 trimesters of gestation.20 We suggest that even a low-grade inflammatory response in the fetal skin is likely to have significant systemic impact on the fetus due to the skin possessing both a large net surface area and ample vascularization.
Keratinocytes constitute approximately 95% of the adult epidermis; accordingly it is not unreasonable that keratinocytes are significant constituent of the fetal skin. Our analysis of fetal epidermis at 124 days gestation using antibodies against involucrin (a keratinocyte-specific marker of differentiation) demonstrated robust epidermal staining (Figure 1). Adult human keratinocytes respond to E. coli LPS via TLR4-driven signaling and express a diverse complement of innate immunoreceptors.30 We established a reliable system for culturing fetal epidermal keratinocytes and demonstrated that these cells possess the capacity to respond to LPS in vitro (Figure 2). Interleukin 1β, IL-6, and TNF-α constitute a triumvirate of acute-phase proinflammatory cytokines that drive the development of an inflammatory response following tissue injury or exposure to a host of microbial pathogens.31 Analysis of mRNA transcript expression for these cytokines and IL-8 demonstrated significant increases as early as 100 minutes after exposure. Elevated expression of cytokine transcripts generally persisted for up to 4 hours. Initial studies of IL-8 demonstrated its importance as a neutrophil chemotactant, and it has subsequently been suggested that this cytokine plays a role in the chemotaxis of both basophils and activated eosinophils.32,33 Interleukin 8 is also involved in maturation of the inflammatory response of neutrophils; specifically the induction of morphological alterations, increased expression of adhesion molecules (including CD18) enhanced tissue migration (especially into epidermal and dermal tissues), increased superoxide and lipid production along with lysosomal enzyme release.34,35 This acute increase in expression followed by return to baseline is in concordance with our own studies and those of other investigators conducted in the fetal lung and other fetal endothelial tissues.19
Having established that the fetal keratinocyte is able to mount a proinflammatory response to a TLR4 agonist, we sought to study the ability of the fetal skin to respond to E. coli LPS in vivo. By either completely exposing the fetus to LPS via intra-amniotic injection or restricting LPS exposure to the respiratory tract following surgical ligation of the trachea, we were able to demonstrate that the fetal skin expressed elevated levels of IL-1β and IL-8, 48 hours after treatment but only when directly exposed to LPS. This observation has 2 important implications for the skin as a mediator of fetal inflammation. First, it demonstrates agreement between our in vitro and in vivo studies and suggests that the fetal skin has the ability to respond, in a proinflammatory manner, to the presence of TLR4 agonist in the amniotic fluid. Second, the absence of an inflammatory response (as detected by IL-1β and IL-8 expression) in animals where LPS exposure was restricted to the lungs provides the first evidence that the presence of bacterial agonist in the amniotic milieu is both sufficient and necessary for the fetal skin to generate a localized inflammatory response.
We previously demonstrated that localized increases in cytokine expression in lung tissue are rapidly accompanied by cellular infiltration as part of the developing inflammatory response.19 In parallel, we observed an inflammatory response to LPS in the fetal skin characterized by the extensive recruitment of CD18 positive cells following direct exposure to LPS. The results of this investigation clearly show a marked elevation in CD18 staining in the dermis of fetal skin directly exposed to LPS via intra-amniotic administration. The administration of saline (irrespective of route) or intratracheal LPS failed to induce an alteration in CD18 staining. From both our light and immunofluorescence analyses, we can conclude that direct exposure of the fetal skin to LPS induces an epidermal infiltrate and that activated neutrophils comprise a significant component of this infliltrate. Therefore, the fetal skin is capable of generating a localized de novo inflammatory response. Activated neutrophil infiltration is a well-characterized response (most notably in epidermal and dermal tissues) of proinflammatory cytokine expression, in particular in response to localized increases in the expression of IL-8.
A further well-characterized effect of LPS-driven immune stimulation is the induction of alterations in TLR-4 receptor expression. TLR-4 is the primary innate immune receptor for gram-negative LPS. TLR4 activation occurs via the formation of a complex of adaptor proteins including LPS-binding protein CD14 and MD2 with LPS.36 Intracellular adaptor proteins MyD88 and Mal regulate cytokine/chemokine expression via Nuclear factor κB (NFκB) signaling and interferon 1 expression via the Trif Related Adaptor Molecule (TRAM) and TIR domain-containing adaptor inducing IFN (TRIF) signaling pathways.37,38 Several investigators reported increases in TLR expression following exposure of cells to LPS or inflammation.39 Our immunohistological analyses identified increased spatial distribution and intensity of TLR4 staining of fetal skin in animals directly exposed to LPS relative to saline control alone. Interestingly, it appears that a number of epidermal appendages adjacent to tissues directly exposed to LPS also demonstrate enhanced TLR4 expression. These images suggest that either direct LPS exposure itself or the resultant, locally acting inflammation increases TLR4 expression, demonstrating that the fetal skin is able to mount a proinflammatory response following direct exposure to LPS (Figure 6).
Spontaneous preterm birth is likely the common “end point” of inflammation subsequent to intrauterine inflammation. Infection of the amniotic cavity results in the production of proinflammatory cytokines and chemokines that, through a variety of downstream mediators including vascular endothelial growth factor (VEGF), matrix metalloproteinases, and prostaglandins, induce degradation of the fetal membranes, cervical remodeling, and bring uterine quiescence to an end. Both “preterm birth” and “infection-derived inflammation” are enormously broad terms covering an extended range of interconnected inflammatory mediators and signaling systems activated in response to an as yet loosely defined (but most likely large) number of contributing microorganisms potentially acting from before conception up to 36 weeks ofGA.
One of the very important questions that remains unanswered is the relative importance of maternal versus fetal inflammation and, more specifically, which tissues (chorioamnion/placenta/fetal lung/fetal skin) are responsible for generating the numerous cytokines and chemokines that initiate and drive the pro-parturition inflammatory processes we know to be causative of preterm birth. To this end, we hypothesize that the developing fetal skin may play a role in the induction of fetal inflammation in response to intrauterine inflammation. We have demonstrated that the fetal skin is capable of generating an inflammatory response in utero to E. coli LPS. We show that that this response involves proinflammatory cytokine (IL-1β, IL-6, IL-8, and TNF-α) expression, the recruitment of activated neutrophils, and modulation of TLR4 expression and requires direct exposure to bacterial agonist.
Having demonstrated its ability to respond to bacterial agonist, we propose that the fetal skin is an important site of the inflammation that also contributes to the FIRS. The uncornified nature of the developing fetal skin at early GAs is likely to render it permissive to the transepidermal passage of compounds (including cytokines and chemokines) from the fetus into the amniotic environment.
There are a number of clinical implications of the current study. Using proteomic approaches, investigators have recently demonstrated that desquamated skin epidermal cells from neonates collected by an adhesive tape had increased expression of several chemokines/cytokines.40 In the context of chorioamnionitis-induced fetal inflammatory response, skin inflammation may contribute to both products of inflammation in the amniotic fluid and fetal blood. Additionally, skin inflammation may further compromise the already poor barrier function in the preterm skin. Thus, infants with exposure to chorioamnionitis may have increased predispostion to nosocomial infection due to potential alterations in skin barrier function. Further extrapolation of the data presented in this study is presently hindered by our limited understanding of the effect/effects of inflammation on the maturation of the fetal skin, putative induction of systemic inflammation, and the degree of concordance between the developmental trajectories of human and ovine skin in utero. In addition to the above, we suggest that the functional maturation of the fetal skin (with specific reference to the development of a mature stratum corneum following programmed terminal differentiation of epidermal keratinocytes) at differing GAs may affect the proinflammatory role played by the fetal skin in the setting of an in utero infection.
Despite these acknowledged limitations, we suggest that the data presented herein indicates for the first time that the fetal skin may be an important source of the both fetal inflammation and proinflammatory compounds that then act on the membranes, cervix, and uterus to ultimately induce preterm labor.
Acknowledgments
This work was supported by grants from the Ramaciotti Foundations (Australia), the Women and Infants Research Foundation (Western Australia), and the Financial Markets Foundation for Children to MWK and by NIH grant HD 57869 to SGK. The authors wish to thank Sara Ritchie for invaluable assistance with animal husbandry.
Footnotes
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Ramaciotti Foundations (Australia), the Women and Infants Research Foundation (Western Australia) and the Financial Markets Foundation for Children to MWK and by NIH grant HD 57869 to SK.
References
- 1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507 [DOI] [PubMed] [Google Scholar]
- 2. Tracy SK, Tracy MB, Dean J, Laws P, Sullivan E. Spontaneous preterm birth of liveborn infants in women at low risk in Australia over 10 years: a population-based study. BJOG. 2007;114(6):731–735 [DOI] [PubMed] [Google Scholar]
- 3. Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5(2):89–106 [DOI] [PubMed] [Google Scholar]
- 4. Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network January 1995 through December 1996 NICHD Neonatal Research Network. Pediatrics. 2001;107(1):E1. [DOI] [PubMed] [Google Scholar]
- 5. Roseboom TJ, Van der Meulen JHP, Ravelli ACJ, Osmond C, Barker DJP, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res. 2001;4(5):293–298 [DOI] [PubMed] [Google Scholar]
- 6. Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J. 1990; 301(6746):259–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorner G, Plagemann A. Perinatal hyperinsulinism as possible predisposing factor for diabetes mellitus obesity and enhanced cardiovascular risk in later life. Horm Metab Res. 1994;26(5):213–221 [DOI] [PubMed] [Google Scholar]
- 8. Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14(1):2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57 [DOI] [PubMed] [Google Scholar]
- 10. Gomez R, Romero R, Ghezzi F, Bo HY, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202 [DOI] [PubMed] [Google Scholar]
- 11. Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations other markers of inflammation and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808 [DOI] [PubMed] [Google Scholar]
- 12. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SGG. Preterm birth infection and inflammation advances from the study of animal models. Reprod Sci. 2010;17(7):619–628 [DOI] [PubMed] [Google Scholar]
- 14. Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol. 1996;174(2):754–759 [DOI] [PubMed] [Google Scholar]
- 15. Adams-Waldorf KM, Persing D, Novy MJ, Sadowsky DW, Gravett MG. Pretreatment with toll-like receptor 4 antagonist inhibits lipopolysaccharide-induced preterm uterine contractility cytokines and prostaglandins in rhesus monkeys. Reprod Sci. 2008;15(2):121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186(5):1062–1068 [DOI] [PubMed] [Google Scholar]
- 17. Moss TJM, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189(6):1771–1776 [DOI] [PubMed] [Google Scholar]
- 18. Moss TJM, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192(4):1179–1186 [DOI] [PubMed] [Google Scholar]
- 19. Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179(12):8491–8499 [DOI] [PubMed] [Google Scholar]
- 20. Holbrook KA, Odland GF. The fine structure of developing human epidermis: light scanning and transmission electron microscopy of the periderm. J Invest Dermatol. 1975;65(1):16–38 [DOI] [PubMed] [Google Scholar]
- 21. Sugita K, Kabashima K, Atarashi K, Shimauchi T, Kobayashi M, Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147(1):176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26 [DOI] [PubMed] [Google Scholar]
- 23. Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49(5):506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jobe AH, Newnham JP, Willet KE. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182(2):401–408 [DOI] [PubMed] [Google Scholar]
- 25. Kemp MW, Edwards B, Burgess M, et al. Syncoilin isoform organization and differential expression in murine striated muscle. J Struct Biol. 2009;165(3):196–203 [DOI] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 27. Chang J-S, Russell GC, Jann O, Glass EJ, Werling D, Haig DM. Molecular cloning and characterization of Toll-like receptors 1-10 in sheep. Vet Immunol Immunopathol. 2009;127(1-2):94–105 [DOI] [PubMed] [Google Scholar]
- 28. Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Dev Comp Immunol. 2009;33(6):761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnaout MA. Leukocyte adhesion molecules deficiency: its structural basis pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990;114:145–180 [DOI] [PubMed] [Google Scholar]
- 30. Song PI, Park Y-M, Abraham T, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119(2):424–432 [DOI] [PubMed] [Google Scholar]
- 31. Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity inflammation and cancer. Trends Mol Med. 2008;14(3):109–119 [DOI] [PubMed] [Google Scholar]
- 32. Yoshimura T, Matsushima K, Oenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987;139(3):788–793 [PubMed] [Google Scholar]
- 33. Djeu JY, Matsushima K, Oenheim JJ, Shiotsuki K, Blanchard DK. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990;144(6):2205–2210 [PubMed] [Google Scholar]
- 34. Schroder J-M, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139(10):3474–3483 [PubMed] [Google Scholar]
- 35. Schroder J-M. The monocyte-derived neutrophil activating peptide (NAP/interleukin 8) stimulates human neutrophil arachidonate-5-lipoxygenase but not the release of cellular arachidonate. J Exp Med. 1989;170(3):847–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu Y-C, Yeh W-C, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151 [DOI] [PubMed] [Google Scholar]
- 37. Honda K, Yanai H, Mizutani T. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signalling. Proc Natl Acad Sci U S A. 2004;101(43):15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol. 2008;9(4):361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raicevic G, Rouas R, Najar M, et al. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum Immunol. 2010;71(3):235–244 [DOI] [PubMed] [Google Scholar]
- 40. Narendran V, Visscher MO, Abril I, Hendrix SW, Hoath SB. Biomarkers of epidermal innate immunity in premature and full-term infants. Pediatr Res. 2010;67(4):382–386 [DOI] [PubMed] [Google Scholar]