Abstract
This study was conducted to determine whether gestational changes in maternal uterine artery reactivity are primarily driven by local vs. systemic factors. Rats underwent surgical ligation of one oviduct, thereby restricting implantation and pregnancy to one uterine horn while maintaining a gestational endocrine milieu. Uterine arcuate arteries were isolated and cannulated to evaluate reactivity. Vessels from the implanted horn were significantly more sensitive to phenylephrine and less sensitive to sodium nitroprusside than those from the non-implanted horn; endothelial basal calcium levels were only increased in the implanted horn. Conversely, there were no differences in sensitivity to acetylcholine, or its effects on endothelial cell calcium, although efficacy was greater in vessels from the implanted vs. non-implanted horn. These findings demonstrate that local factors are predominant in inducing changes in vascular smooth muscle function, while endothelial adaptations result from an interplay between local and systemic factors, with distinct effects attributable to each.
Keywords: pregnancy, uterine arteries, endothelium, vascular smooth muscle
Introduction
Although vascular changes in the mammalian uterus are prominent, required for species propagation, and well described during pregnancy, little is known about the signaling mechanisms through which these processes develop and are regulated.1 Gestational vascular adaptations that result in the significant (10- to100-fold) increases in uterine blood flow that are essential for normal pregnancy outcome include both structural (growth and remodeling) and functional (altered tone and reactivity) changes in arteries and veins.1
The underlying physiological mechanisms likely involve a combination of both physical and molecular signals. For example, changes in intravascular pressure and shear stress within a vessel can each produce distinct effects on arterial structure and reactivity.2–5 At the same time, circulating sex hormones and growth factors have also been shown to alter vascular structure and reactivity within the uterus of both pregnant and nonpregnant animals.6–8
Many investigators have interrogated the uterine vasculature to understand how its reactivity is altered during pregnancy, and the picture that emerges is complex. Experiments have demonstrated that the smooth muscle in uterine arteries is more sensitive to adrenergic stimulation and less sensitive to nitrate signaling (eg, sodium nitroprusside, SNP);9–11 conversely, endothelial cell-mediated vasodilatation is generally augmented in pregnant animals, most likely due to an upregulation of receptors, intracellular calcium signaling pathways, and vasodilator release.12
The purpose of this study was to elucidate the role of local versus systemic factors on changes in uterine vascular reactivity during pregnancy. The null hypothesis (no difference in vascular reactivity in vessels from the pregnant vs nonpregnant horn) was based on the sizeable and compelling body of experimental evidence that substantiates major effects of both sex steroids (estrogens and progestins) and altered growth factors on vascular reactivity.
By using a rat model in which placentation is surgically restricted to one uterine horn,8 we tested the hypothesis that local factors associated with placentation have more influence on reactivity than the systemic endocrine changes of pregnancy.
The objective of this study was to better understand the influences that guide the cellular (endothelial, vascular smooth muscle) adaptations that result in altered vessel reactivity, as these have an important effect on uteroplacental perfusion. Specifically, we were interested in determining whether they were primarily local or systemic in nature, as this knowledge would be helpful in guiding subsequent studies designed to identify their source, molecular nature, and alterations in the signal transduction pathway. Failure of uterine vascular adaptation during pregnancy is associated with gestational diseases such as preeclampsia and intrauterine growth restriction (IUGR). While the mechanisms underlying this process of arterial growth and altered reactivity are not well characterized, an understanding of the underlying mechanisms would have therapeutic potential.
Materials and Methods
Animals
Animals used for this experiment are a subset from a larger cohort of experimental animals used in a study that examined local versus systemic influences on uterine arterial remodeling.8 Briefly, nonresorbable silk suture was used to tie off one oviduct in thirteen 8-week-old Sprague Dawley rats using routine surgical preparation and anesthesia procedure. Surgery was performed by Charles River Laboratories (CRL, Kingston, New York, and Wilmington, Massachusetts). The advantage of this approach is that it allows ligation without any disruption of the uterine blood supply as the rodent oviduct is discrete and poorly vascularized. The horns are anatomically separated at the cervical end by a septum, hence, eggs released from the nonligated horn cannot be transferred to the ligated side and pregnancy is thereby restricted to 1 horn.
Animals were received at the University of Vermont at 9 weeks of age, with staples approximating a cranial-caudal incision in the right flank. Staples were removed 7 days postoperatively, and the rats were acclimated for at least 1 week before breeding. At 10 to 15 weeks (12 ± 1.7 wks) of age, rats were bred overnight in isolated pairs using metabolic cages at the Small Animal Care Facility, which is affiliated with CRL and fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AALAC). If a seminal plug was observed the following morning, that day was designated day 1 of pregnancy. Pregnancy was confirmed by observation and/or palpation on days 11 to 16 by CRL veterinary technicians. Post-mortem evaluation confirmed an empty right uterine horn and a silk ligature in the upper portion of the oviduct. Rats were singly housed and feed and water were provided ad libitum. All experiments and procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Chemicals
All chemicals were purchased from Fisher Scientific (Fair Lawn, New Jersey) unless otherwise specified. The composition of the isotonic buffer was HEPES (10 mmol/L), NaCl (141.8 mmol/L), KCl (4.7 mmol/L), CaCl2 (2.8 mmol/L), MgSO4 (1.7 mmol/L), KH2PO4 (1.2 mmol/L), Na2-EDTA (0.50 mmol/L), and glucose (5.0 mmol/L). The pH was adjusted to 7.40 at 37.0°C by adding 10 mol/L NaOH (Mallinckrodt Baker, Paris, Kentucky).
To measure endothelial cell [Ca2+]i, we used a physiological salt solution (PSS) containing 119 mmol/L NaCl, 4.7 mmol/L KCl, 24.0 mmol/L NaHCO3, 1.2 mmol/L KH2PO4, 1.6 mmol/L CaCl2, 1.2 mmol/L MgSO4, 0.023 mmol/L EDTA, and 11.0 mmol/L glucose. For the Fura-2AM (Fura-2AM-acetoxymethyl ester, Invitrogen, Carlsbad, California) calibration procedure, we used a solution of the following composition: 140 mmol/L KCl, 20 mmol/L NaCl, 5.0 mmol/L HEPES, 5.0 mmol/L EGTA, 1.0 mmol/L MgCl2, 5.0 µmol/L nigericin, and 10 µmol/L ionomycin, pH = 7.1.
Phenylephrine (Phe), sodium nitroprusside (SNP), and acetylcholine (ACh) were purchased from Sigma (St Louis, Missouri) and stock solutions prepared in distilled, de-ionized water before each experiment. Stock solutions were maintained under darkened conditions at 4°C or on ice at all times.
Papaverine and diltiazem ((+)-cis-diltiazem·HCl; 100 and 10 µmol/L, respectively) were obtained from Sigma and used to prepare a relaxing solution in HEPES buffer. The use of a combination of PDE inhibitor and a calcium channel blocker assures complete vessel relaxation. Pluronic acid was purchased from Invitrogen. Fura-2AM was dissolved in dehydrated DMSO as a 1.00 mmol/L stock solution, frozen in small aliquots, and used within 1 week of preparation. Ionomycin and nigericin were prepared as 10.0 mmol/L stock solutions in methanol and kept at −20°C until use.
Tissue Harvesting and Litter Characteristics
Rats were utilized on day 20 of pregnancy (rat gestation = 22 days) at 15.0 ± 1.7 weeks of age. Prior to necropsy or tissue harvesting, animals were weighed and euthanized with Nembutal (pentobarbital sodium, 60 mg/mL, Ovation Pharmaceuticals, Deerfield, Illinois) by intraperitoneal injection, followed by decapitation in a small animal guillotine. Uteri were harvested en bloc, pinned in a Petri dish containing HEPES buffer at 4 C, and utilized immediately for experiments.
After vessel dissection, fetuses were counted in each horn. In some animals (n = 6), fetuses and placentas were carefully dissected away from the uterus and weighed without membranes and umbilical cords. Representative subgroup data are presented herein, as not all measurements were performed on all animals (n values as indicated).
Vessel Dissection and Equilibration
Uterine arcuate arteries from the implanted and nonimplanted uterine horns from the same uterus were dissected free of connective tissue in chilled HEPES buffer, and each end was cannulated with buffer-filled, hollow, pulled-glass pipettes at room temperature in a custom-built arteriograph.
Intralumenal pressure was measured and maintained using a pressure servo system (Living Systems Instrumentation, Burlington, Vermont) with an in-line transducer that was calibrated before each experiment. Each vessel was then rinsed free of any residual blood (≤10 mm Hg) and maintained at 10 mm Hg for 30 minutes, followed by a 30-minute equilibration at 50 mm Hg. This pressure was chosen as it is a reasonable approximation of physiologic pressures experienced in vivo, and to avoid the complications of myogenic tone, which normally appears in these vessels at pressures between 55 and 60 mm Hg.11
All concentration-response experiments were run at a transmural pressure of 50 mm Hg. A thermostat maintained the superfusate temperature flowing through the vessel chamber of the arteriograph at 37 ± 0.2°C. Once experiments were completed, vessels were rinsed free of vasoactive compounds and re-equilibrated at 50 mm Hg in relaxing solution containing 10 µmol/L diltiazem and 100 µmol/L papaverine for 15 to 30 minutes. Transmural pressure was then reduced to determine each vessel’s opening pressure (usually 2-4 mm Hg), to allow accurate measurement of the inner diameter and wall thickness in a minimally stressed state.
Vascular Reactivity Experiments
Vasoconstriction experiments were completed by sequentially exposing equilibrated vessels to increasing concentrations of Phe (10−9 to 10−4 mol/L) using 6 vessel-pairs; here, and elsewhere, n values reflect number of pairs. Vasodilatation experiments were similarly performed using vessels that were preconstricted with Phe to ~80% of their fully constricted state, after which time vessels were exposed to increasing concentrations of ACh (10−8 to 10−5 mol/L, n = 5) or SNP (10−9 to 10−4 mol/L, n = 5) until the maximal response was obtained and recorded. EC50 values (concentration required to produce a half-maximal effect) were determined from each vessel’s concentration-response curve. Vasodilatation efficacy for ACh and SNP was defined as the ratio of maximal vessel dilatation obtained during the reactivity experiments compared to the fully relaxed diameter obtained in relaxing solution at the end of each experiment.
A composite vessel concentration-response curve for each compound was created by averaging the relative vessel constriction or dilation response for each concentration of drug. Separate EC50 values were generated for each individual vessel using standard curve analysis (Sigma Plot), and within-animal pair-wise comparisons of these values between the implanted and nonimplanted horns were made for each compound studied.
Data Acquisition
Vessel diameter and wall thickness measurements were obtained using an inverted microscope (Nikon TMS, MVI, Avon, Massachusetts) with a calibrated CCD camera (Sony XC-73) attached to a video dimension analyzer (Living Systems Instrumentation), and by post-acquisition processing of digitally recorded film clips (AVI format) or extracted CCD regions-of-interest. Data acquisition and experimental control utilized National Instruments' (Austin, Texas) LabVIEW software and hardware (PCI-6221 16–bit DAC/ADC card and PCI-1405 analog frame grabber) with a Windows-based platform (Microsoft Corp., Redmond, Washington).
Endothelial Cell Loading With Fura-2AM and Measurement of Endothelial Cell [Ca2+]i in Pressurized Arteries
In a separate set of experiments, after a 20-minute equilibration period in PSS at 10 mm Hg and 37°C, endothelial cells were loaded with the Ca2+-sensitive dye Fura-2AM (5 µmol/L) by intralumenal perfusion of Fura-2AM-containing solution at room temperature for 5 minutes followed by 10 minutes of washout with regular PSS. Background fluorescence and arterial autofluorescence were measured before the loading procedure. Ratiometric measurements of Fura-2AM fluorescence from endothelial cells were performed using a photomultiplier system (IonOptix Inc, Milton, Massachusetts).
Heat-polished glass cannulas were used in all experiments to prevent accidental damage of the endothelial cell (EC) layer during cannulation, and to avoid diffusion of Fura-2 into the smooth muscle cell (SMC) layer. A similar protocol was used in a previous study where preferential loading of ECs with fura-2 was confirmed by nearly complete disappearance of fluorescent signal after arterial denudation.12 Background-corrected ratios of 510 nm emission were obtained at a 5-Hz sampling rate from arteries alternately excited at 340 and 380 nm. The arterial lumen diameter was simultaneously monitored using the SoftEdge Acquisition Subsystem (IonOptix Inc.). All experimental protocols were started following an additional 20-minute equilibration period at 10 mm Hg to allow intracellular de-esterification of Fura-2AM. Intralumenal pressure was then elevated to, and maintained at, 50 mm Hg for the remainder of the experiment. After stabilization of arterial diameter (typically, 5-7 minutes), ACh was applied in 4 increasing concentrations (0.03; 0.1; 0.3; and 10 µmol/L) for a period of 5 minutes each.
Calculations and Statistical Analysis
Endothelial cell [Ca2+]i was calculated using the following equation:13 [Ca2+]i = Kdβ(R − R min)/(R max − R), where R is the experimentally measured ratio (340 nm/380 nm) of fluorescence intensities, R min is a ratio in the absence of [Ca2+]i, R max is a ratio at Ca2+-saturated Fura-2A conditions, and β is a ratio of the fluorescence intensities at 380 nm excitation wavelength at R min and R max. R min, R max, and β were determined by an in situ calibration procedure from the arteries treated with the ionophores nigericin (5 µmol/L) and ionomycin (10 µmol/L). Calibration was performed using vessels loaded intralumenally with Fura-2AM (n = 9). These values were then pooled and used to convert the ratio values into a [Ca2+]i. Kd (the dissociation constant for Fura-2A) was 282 nmol/L, as determined by using in situ titration of Ca2+ in Fura-2A-loaded small arteries.14
Statistical Analysis
Statistical analysis utilized SigmaPlot with integrated SigmaStat (Systat Software, Inc., San Jose, California) software, with preliminary analysis and verification from Microsoft Office Excel 2000 and LabVIEW. Data were represented as mean ± standard error and analyzed using paired t-tests or signed-rank tests to determine significant (P < .05) differences between the implanted and nonimplanted horns in individual animals.
Results
Maternal-Fetal Animal Data and Vessel Dimensions
Maternal body weights averaged 374 ± 7 g (n = 13), and litter size was 12.9 ± 1.7 pups (n = 13 litters). Pup and placental weights were 2.58 ± 0.14 g and 463 ± 11 mg, respectively (n = 6 litters, 76 pups).
Unstressed lumen diameter and wall thickness of vessels harvested from the implanted horn (measured at opening pressure, 2-4 mm Hg) were significantly larger than those from contralateral nonimplanted horns (135 ± 6 vs 93 ± 6 µm, P < .001 and 58 ± 3 vs 46 ± 3 µm, P < .01; n = 13), as previously noted.8
Reactivity Data
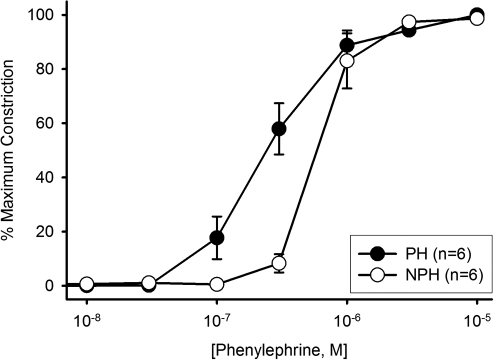
As shown in Figure 1 , vasoconstrictor sensitivity to Phe was significantly increased in arteries from the implanted horn, as evidenced by a 2-fold reduction in the EC50 (PH vs NPH: 0.28 ± 0.05 vs 0.62 ± 0.01 µmol/L; P = .03), consistent with previously reported observations under similar conditions in pregnant versus nonpregnant animals.10 There were no differences in efficacy between the 2 horns, with maximal constrictions of ≈85% in all vessels.
Figure 1.
Phenylephrine (Phe) concentration-response curves of uterine arcuate arteries from implanted (PH) versus nonimplanted (NPH) uterine horns (n = 6 vessel pairs). Based on the EC50 values (shown in text), vessels from the PH were significantly (P = .03) more sensitive to Phe constriction than their NPH counterparts. All means are ± SEM.
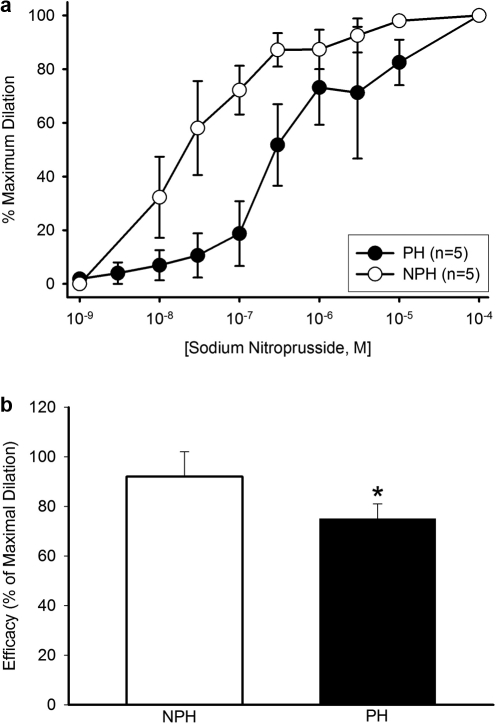
Vessels from implanted versus nonimplanted horns showed significantly decreased vasodilator sensitivity to SNP (Figure 2A), as demonstrated by the 11-fold increase in the EC50 values (PH vs NPH: 1.68 ± 0.83 vs 0.15 ± 0.08 µmol/L; P = .03). Moreover, the efficacy of SNP was significantly less in the implanted vs nonimplanted horn (75 ± 6 vs 92 ± 10%, respectively; P = .02, Figure 2B). These changes are consistent with previously-reported reductions in uterine artery sensitivity to SNP in pregnant versus nonpregnant animals.15
Figure 2.
A, Sodium nitroprusside (SNP) concentration-response curves of uterine arcuate arteries from implanted (PH) versus nonimplanted (NPH) uterine horns (n = 5 vessel pairs). Based on the EC50 values (shown in text), vessels from the PH were significantly (P = .03) more sensitive than their NPH counterparts. (B) Efficacy (SNP dilation relative to maximally-relaxed diameter) was significantly reduced in vessels from the PH versus NPH (P = .02).
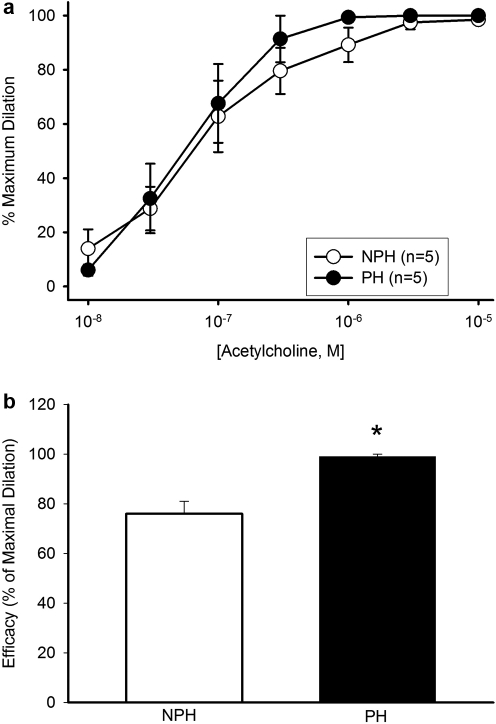
Vasodilator sensitivity to ACh was not altered in the pregnant horn (PH vs NPH: 0.10 ± 0.03 vs 0.06 ± 0.02 µmol/L; P = .60, Figure 3A). However, the extent of maximal vasodilatation (efficacy) was significantly increased in the implanted vs nonimplanted horn (99 ± 1 vs 76 ± 5%, P = .02; Figure 3B).
Figure 3.
A, Acetylcholine (ACh) concentration-response curves of uterine arcuate arteries from implanted (PH) versus nonimplanted (NPH) uterine horns (n = 5 vessel pairs). Based on the EC50 values (shown in text), differences were not significant (P = .55). B, Efficacy (ACh dilation relative to maximally-relaxed diameter) was significantly increased in vessels from the PH versus NPH (P = .02).
Changes in Basal Versus ACh-Stimulated Endothelial Cell [Ca2+]i.
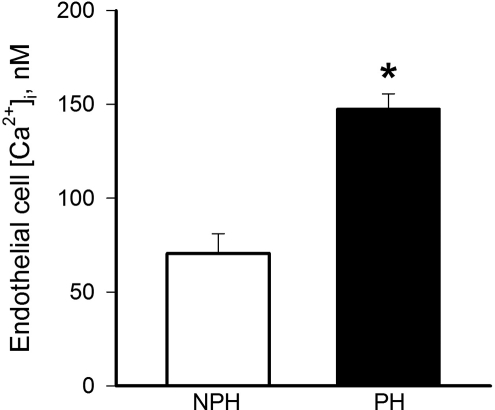
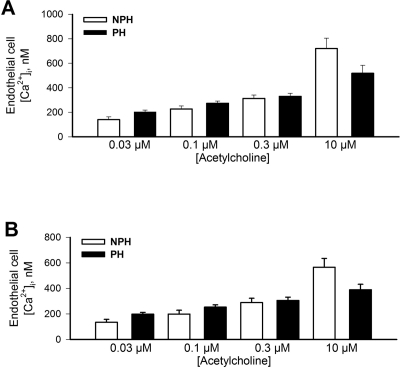
There was a significant (2-fold) increase in basal (unstimulated) level of endothelial cell [Ca2+]i in vessels from the implanted (147 ± 8 nmol/L, n = 7) versus the nonimplanted horn (71 ± 10 nmol/L, n = 7; Figure 4 ) that was similar to earlier findings in late pregnant versus nonpregnant animals.9 The application of ACh increased endothelial cell [Ca2+]i in a concentration-dependent manner, although there were no significant differences detected between horns in either the initial transient (Figure 5A) or sustained (Figure 5B) responses.
Figure 4.
Endothelial cell calcium measured under basal (unstimulated) conditions was significantly (P = .01) elevated in uterine arcuate arteries from implanted (PH) versus nonimplanted (NPH) uterine horns (n = 7).
Figure 5.
A, Transient increases in endothelial cell calcium following stimulation with acetylcholine (Ach; concentrations shown on x-axis) in uterine arcuate arteries from the implanted (PH) versus nonimplanted (NPH) horns (n = 7). No significant differences (P < .05) were detectable. B, Similar data showing sustained increases in endothelial cell calcium following ACh stimulation (n = 7); no significant differences (P > .05) were detectable.
Discussion
The results presented here suggest that local factors associated with fetal/placental implantation elicit differential regulation of vascular reactivity based on arterial wall cell type. Specifically, the alteration of vascular smooth muscle sensitivity to both contractile (Phe) and vasodilatory (SNP) stimuli in the implanted horn suggests that vascular smooth muscle adaptation is predominantly influenced by local rather than systemic factors. The situation regarding the endothelium is less clear. As the results indicate, sensitivity to acetylcholine was similar in vessels from both horns, with values that were comparable to those previously reported in pregnant animals.9
Because the arterial diameter response integrates both endothelial and vascular smooth muscle influences (ie, reflects both the amount of a vasodilator released by the endothelium and the sensitivity of vascular smooth muscle to its effect), and in view of the aforementioned differences in nitrodilator sensitivity, it is difficult to attribute the effect to either cell type alone. The pattern of increased basal endothelial calcium in vessels from the pregnant horn is consistent with that measured earlier in uterine vessels from pregnant versus nonpregnant animals,9 indicating that local rather than systemic factors play the predominant role in determining this parameter. On the other hand, both transient and sustained changes in endothelial calcium in response to ACh were similar in vessels from either horn. Here, the underlying mechanism involves muscarinic receptor (most likely M3 subtype)16 activation, and the results suggest that upregulation of EC signaling may be stimulated by systemic, rather than local factors. In this regard, the fact that the endothelium is in direct contact with the blood and is therefore exposed to humoral signals and hemodynamic forces such as shear stress supports this type of action.
In terms of systemic influences, sex steroids are known to modulate specific physiologic processes such as receptor expression or the “gain” of subsequent transduction mechanisms in the cell. There are several existing reports linking steroids to muscarinic receptor expression in nonreproductive tissues,17 although the situation in the uterine circulation is not known. Moreover, estrogen-sensitive muscarinic receptor expression and its signal transduction in the uterine arterial endothelium have been linked to multiple vasodilatory pathways, for example, nitric oxide, prostanoids, and endothelium-derived hyperpolarization factor (EDHF).18 Muscarinic receptors may also be present on vascular smooth muscle and, in some vessels (e.g. pig intramyocardial coronary arteries), ACh stimulation may elicit constriction.19 In the arteries used in this study, however, application of ACh to denuded vessels is without effect,12 minimizing this potential complication.
The observation that some pregnancy-related endothelial cell adaptations are sensitive to localized implantation whereas others are not underscores the complexity of both cell- and pathway-specific gestational vascular adaptation in mammals.
From a practical standpoint, the advantage of this particular experimental approach is that it allows the use of paired vessels (one from an implanted uterine horn and one from a nonimplanted horn) from the same animal, eliminating significant interanimal biologic variation and increasing the power of experiments. While there are some complexities in their interpretation, the results themselves are unambiguous and parallel previously-documented changes in uterine vascular reactivity in pregnant versus nonpregnant animals. The differences in basal and stimulated endothelial cell calcium levels are also quite consistent with values observed in vessels from nonpregnant versus late pregnant animals under similar experimental conditions,9 confirming the utility of the experimental model.
Our findings also underscore the interplay between the cells in the vascular wall in determining reactivity. The elevation in basal endothelium calcium in the pregnant horn supports a fundamental enhancement of vasodilator activity in view of its association with both NO and EDHF signaling. The reduced sensitivity to SNP in the pregnant horn links well to the selective increase in basal calcium, as reduced VSM sensitivity to nitrites in response to increased NO signaling is a well-established phenomenon, and one that we have previously reported in uterine vessels during pregnancy.15 The different response in vessels from the two horns also speaks to local influences being predominant in this particular adaptation. At the same time, reactivity to ACh was similar in vessels from both horns, as was the extent of provoked calcium release, suggesting that muscarinic signaling may be upregulated by systemic rather than local factors.
Last, it is worth noting that vessel remodeling is also attributable to local factors, and that vessels feeding the pregnant horn are both wider and longer than their counterparts on the nonpregnant side.8 It would be remiss to not note that changes in structure have implications for reactivity vis-a-vis altered forces (tension, stress) within the vascular wall. Within the uterine circulation, the structural adaptations characteristic of gestation predict an increase in arterial wall tension and stress, hence, a reduction in constrictor reactivity along with increased vasodilator responses in the pregnant horn. As our results indicate, this is clearly not the case for phenylephrine or acetylcholine, showing that nonbiophysical factors are equally important, for example, changes in receptor expression and downstream signal transduction mechanisms, and future studies will attempt to define the mechanistic basis for the observed differences in vessel behavior.
Acknowledgments
The authors gratefully acknowledge the research support of the National Heart, Lung, and Blood Institute (NIH/NHLBI RO1 HL79253, 73895 and RO1 HL088245) and the institutional support from the University of Vermont Department of Obstetrics and Gynecology.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: NIH/NHLBI RO1 HL79253 73895 (G.O.) and RO1 HL088245 (N.G.).
References
- 1. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda). 2009;24(1):58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben Driss A, Benessiano J, Poitevin P, Levy BI, Michel JB. Arterial expansive remodeling induced by high flow rates. Am J Physiol. 1997;272(2):H851–H858 [DOI] [PubMed] [Google Scholar]
- 3. Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996;74(7):834–841 [PubMed] [Google Scholar]
- 4. Tuttle JL, Nachreiner RD, Bhuller AS, et al. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281(3):H1380–1389 [DOI] [PubMed] [Google Scholar]
- 5. Alexander BT, Kassab SE, Miller MT, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37(4):1191–1195 [DOI] [PubMed] [Google Scholar]
- 6. Anderson SG, Hackshaw BT, Still JG, Greiss FC,, Jr Uterine blood flow and its distribution after chronic estrogen and progesterone administration. Am J Obstet Gynecol. 1977;127(2):138–142 [DOI] [PubMed] [Google Scholar]
- 7. Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+. Am J Physiol Endocrinol Metab. 2009;296(3):E503–E512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuller R, Barron C, Mandala M, Gokina N, Osol G. Predominance of local over systemic factors in uterine arterial remodeling during pregnancy. Reprod Sci. 2009;16(5):489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol. 2003;189(5):1489–1493 [DOI] [PubMed] [Google Scholar]
- 10. D'Angelo G, Osol G. Regional variation in resistance artery diameter responses to alpha-adrenergic stimulation during pregnancy. Am J Physiol. 1993;264(1):H78–H85 [DOI] [PubMed] [Google Scholar]
- 11. Osol G, Cipolla M. Interaction of myogenic and adrenergic mechanisms in isolated, pressurized uterine radial arteries from late-pregnant and nonpregnant rats. Am J Obstet Gynecol. 1993;168(2):697–705 [DOI] [PubMed] [Google Scholar]
- 12. Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol. 2006;290(5):H2124–H2135 [DOI] [PubMed] [Google Scholar]
- 13. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260(6):3440–3450 [PubMed] [Google Scholar]
- 14. Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(pt 1):199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu Y, Zhang H, Gokina N, et al. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat L-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol. 2008;294(2):C564–C571 [DOI] [PubMed] [Google Scholar]
- 16. Beny JL, Nguyen MN, Marino M, Matsui M. Muscarinic receptor knockout mice confirm involvement of M3 receptor in endothelium-dependent vasodilatation in mouse arteries. J Cardiovasc Pharmacol. 2008;51(5):505–512 [DOI] [PubMed] [Google Scholar]
- 17. Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FM. Effects of 17beta-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor alpha in rat hippocampus. Eur J Pharmacol. 2010;634(1-3):192–200 [DOI] [PubMed] [Google Scholar]
- 18. Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459(6):841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakayama K, Osol G, Halpern W. Differential contractile responses of pressurized porcine coronary resistance-sized and conductance coronary arteries to acetylcholine, histamine and prostaglandin F2 alpha. Blood Vessels. 1989;26(4):235–245 [DOI] [PubMed] [Google Scholar]