Abstract
Objective: Neutrophil gelatinase-associated lipocalin (NGAL) is a ubiquitous lipocalin that serves as a critical component of innate immunity and a transport shuttle for numerous substances (retinoids, arachidonic acid, prostaglandins, fatty acids, steroids, iron, and MMPs). Despite the well-documented association between intra-amniotic infection/inflammation (IAI) and preterm birth, NGAL expression in the uterus has not previously been examined. This study investigates NGAL expression at the maternal-fetal interface in vivo and in vitro. Methods: Neutrophil gelatinase-associated lipocalin expression in term placenta with/without IAI was examined by immunohistochemistry. Trophoblast and decidual stromal cells were retrieved from elective cesarean, purified, and depleted of leukocytes. On days 1 (cytotrophoblast cells) and 4 (syncytiotrophoblast), cells were stimulated with/without interleukin 1β (IL-1β; 1 ng/mL), tumor necrosis factor α (TNF-α; 1 ng/mL), or lipopolysaccharide (LPS; 1 μg/mL). Neutrophil gelatinase-associated lipocalin messenger RNA (mRNA) and protein expression were measured by immunocytochemistry/Western blot and RT-qPCR, respectively. Results: Under basal conditions, NGAL is expressed in trophoblast, but not decidua. Trophoblast NGAL is significantly upregulated in tissues with evidence of IAI vs controls. NGAL expression was increased after stimulation with all 3 pro-inflammatory mediators in day 1 (cytotrophoblast) but not day 4 cells (syncytiotrophoblast). IL-1β and TNF-α (not LPS) upregulated NGAL gene expression in cytotrophoblast (not syncytiotrophoblast) cells. Conclusions: Intra-amniotic infection/inflammation is associated with increased expression of NGAL in trophoblast tissues in vivo. IL-1β, TNF-α, and LPS stimulated NGAL in cytotrophoblast cells (not syncytiotrophoblast and decidua) in vitro. These data suggest that, in keeping with its role as a mediator of innate immunity, NGAL may have a central role to play in IAI-induced preterm birth.
Keywords: NGAL, intra-amniotic infection, preterm birth, human, pregnancy
Introduction
Intra-amniotic infection/inflammation (IAI)—also referred to as chorioamnionitis—is a major cause of perinatal morbidity and mortality. Although strongly associated with such adverse pregnancy events as preterm birth, stillbirth, and cerebral palsy,1–4 the molecular mechanisms by which IAI leads to such pregnancy complications are not well understood. Vaginal organisms appear to ascend first into the choriodecidual space, then into the amnion, then the umbilical cord and amniotic fluid, and ultimately to the fetus.2,3 However, the poor correlation between clinical and histologic chorioamnionitis5 and the fact that IAI may occur early in pregnancy or even before conception and remain indolent for many weeks and months6 suggests that pro-inflammatory intermediates are involved.
Lipocalins are a family of small extracellular proteins that are structurally and functionally diverse, and which are characterized by their ability to bind both small hydrophobic molecules—such as retinol, arachidonic acid, prostaglandins, fatty acids, steroids, iron, and matrix metalloproteinases (MMPs)—as well as specific cell surface receptors.7–9 Such properties suggest that lipocalins serve primarily as transport proteins transferring biologically hazardous molecules in a safe and controlled manner between cells. Members of the lipocalin family include, among others, apolipoprotein D, quiescience-specific protein, purpurin, alpha-1-microglobulin, and neutrophil gelatinase-associated lipocalin (NGAL).
Neutrophil gelatinase-associated lipocalin is a 25-kDa lipocalin that was originally purified from activated human neutrophils but has since been shown to be made by other immune cells, hepatocytes, adipocytes, and renal tubular cells. It exists in monomeric, homodimeric, and heterodimeric forms bound to human neutrophil gelatinase. In addition to serving as a transport shuttle for small compounds, NGAL also appears to comprise a critical component of innate immunity to bacterial infection.10,11 To this end, NGAL interacts with specific receptors as a complex with iron-siderophores (Holo-NGAL) or alone (Apo-NGAL). After internalization, Holo-NGAL releases its iron into the cytoplasm of the cell leading to downstream regulation of critical iron-dependent gene pathways that are responsible for protecting the cell from injury or death. NGAL is then destroyed within the cell or recycled as Apo-NGAL. This shuttle may also work in reverse, with Apo-NGAL capturing intracellular iron-siderophores and transporting them to the extracellular space thereby depleting the cell of its iron reserves and leading to apoptosis. This latter mechanism likely explains the strong antibacterial properties of NGAL.10
In addition to its role as a transport shuttle for small compounds7–9 and a regulator of innate immunity,11,12 NGAL is also an emerging biomarker for acute kidney injury13–16 and cancer.17 Despite these observations, the expression of NGAL within the uterus has not previously been examined. This study investigates the expression and regulation of NGAL at the maternal-fetal interface both in vivo and in vitro.
Materials and Methods
Specimens
Tissues for immunohistochemistry were collected from women with or without IAI and prepared as previously described.18,19 In all cases, IAI was confirmed by the presence of polymorphonuclear leukocyte infiltration of the fetal membranes, decidua, and umbilical cord at the time of histologic examination by a single placental pathologist (PT) who was blinded to the clinical status of the women. In control tissues, IAI was excluded on the basis of clinical criteria (absence of fever, maternal and/or fetal tachycardia, uterine tenderness, and foul lochia) as well as routine laboratory investigations (white cell count) and the absence of histologic chorioamnionitis. All tissues were collected in accordance with the requirements of the Human Investigations Committee of Yale University School of Medicine in New Haven, CT, and the Institutional Review Board of the University of Siena in Siena, Italy.
Immunohistochemical Studies
Serial sections (4 μm) of paraffin-embedded endometrial tissues were cut, deparaffinized, rehydrated, and washed in Tris-buffered saline (20 mmol/L Tris-HCl, 150 mmol/L NaCl, pH 7.6 [TBS]). Antigen retrieval was performed in citrate buffer (10 mmol/L, pH 6.0) under slow boil for 8 min. Endogenous peroxidase activity was quenched by incubating the slides in 3% in H2O2 for 15 minutes. The slides were then incubated in 10% normal donkey serum (Jackson ImmunoResearch, West Grove, Pennsylvania) diluted in PBS/0.1% Tween 20 with an avidin blocking kit (Vector Laboratories, Burlingame, California) for 1 hour at room temperature in a humidifying chamber. After blotting off excess serum, slides were incubated overnight at +4°C with primary antibodies prepared in PBS/0.1% Tween 20 and biotin blocking. Primary antibodies included rat anti-human NGAL (1:200 dilution [R&D Systems, Minneapolis, Minnesota]), mouse anti-human vimentin (Clone V9; 1:200 [Dako, Glostrup, Denmark]), and mouse anti-human cytokeratin-7 (Clone OV-TL 12/30; 1:200 [Dako]). Thereafter, slides were washed and incubated with secondary antibody (biotinylated donkey anti-rat or biotinylated donkey anti-mouse [Jackson ImmunoResearch]) for 30 minutes at room temperature. After further washing, the antigen-antibody complex was detected using an avidin-biotin-peroxidase kit with 3,3-diaminobenzidine tetrahydrochloride dehydrate (Vector Laboratories [DAB]) as the chromogen. Slides were counterstained with hematoxylin (Sigma, St Louis, Missouri) and mounted. Negative controls for each section were prepared by substituting preimmune serum primary for the corresponding preimmune antibody. Neutrophil gelatinase-associated lipocalin immunostaining intensity was evaluated semiquantitatively using AxioVision and the corresponding digital image processing software (Carl Zeiss Microimaging, Inc, Thornwood, New York) according to the following scale: 0+ (no staining), 1+ (weak but detectable staining), 2+ (moderate or staining), or 3+ (intense staining). For each slide, a computer-generated Histologic Score (H-SCORE) value (in relative light units) was determined as previously described18,19 by calculating the sum of the number of cells that stain at each intensity scale and multiplying that value by the weighted intensity scale using the following formula: H-SCORE = Σ π (i + 1), where ‘i’ is the intensity scale and π is the percentage of cells staining at the scale. For each slide, 5 fields and at least 100 cells per area were evaluated under a light microscope at ×400 magnification. Results are reported as mean ± SEM from a minimum of 5 separate readings from 3 separate tissue sections.
Isolation and Purification of Term Trophoblast Cells
Isolation of extravillous trophoblast cells from the chorion of term placenta was performed as previously described.20–22 In brief, trophoblast cells were removed from the placenta by scraping, washed in PBS (GIBCO, San Francisco, California), and subjected to 3 digestions in 0.125% Trypsin-EDTA buffer (GIBCO) in a shaking water bath (200 rpm) at 37°C for 15, 30, and 30 minutes, respectively. An equal volume of Dulbecco Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS; Gemini Bio-Products, Woodland, California) was added to inactivate the trypsin. The mixture was strained through a 32-μm gauze sieve to remove undigested tissue, and centrifuged at 1500 rpm for 10 minutes. The supernatant was collected and centrifuged at 1500 rpm for 10 minutes. The pellet was resuspended in DMEM with 10% FBS. Contaminating red blood cells were removed by resuspending the cell pellet in Hank’s balanced salt solution (HBSS [GIBCO]), layering this suspension over a discontinuous (25%:30%:40%:45%) Percoll gradient (Sigma), and centrifuging at 2000 rpm for 25 minutes. The 40%:45% interface was removed, and the trophoblast cells were purified by immunomagnetic microsphere separation. To this end, the enriched cell fraction was incubated with anti-CD45 (which binds to immune cells [R&D Systems]) and anti-CD9 monoclonal antibodies (which bind to fibroblasts [R&D Systems]) conjugated to immunomagnetic beads (Dynabeads 450 [Dynal, Oslo, Norway]) at 4°C with rotation for 15 minutes twice. Thereafter, contaminating immune and fibroblast cells were magnetically separated from the negative cell fraction, and the unbound cells were collected, washed, and cultured to confluence in a 95% air:5% CO2 incubator at 37°C in DMEM supplemented with 10% FBS.
Flow cytometry
The purified term trophoblast cell population was characterized by flow cytometry as previously described.20 In brief, 2 × 106 cells for each antibody reaction were fixed with 4% paraformaldehyde for 15 minutes at room temperature. Cells were washed and incubated with primary antibody resuspended in washing buffer. Primary antibodies (all mouse monoclonals) included anti-vimentin (Clone V9, diluted in 1:50 [Dako]), anti-cytokeratin 7 (OV-TL 12/30, diluted 1:50 [Dako], Denmark), anti-CD45 (diluted in 1:50 [R&D Systems]), or isotype-matched control antibody (R&D Systems). After 2 washings, secondary antibody (FITC-conjugated goat anti-mouse IgG [BD Biosciences, Bedford, Massachusetts] diluted 1:100) was added for 15 minutes in the dark. After the addition of 400 μL of sterile PBS per tube, samples were analyzed for 1 minute in a flow cytometer (FACS Calibur, Beckton Dickinson, San Jose, California) using CellQuest software (Becton Dickinson). Both forward scatter (FSC) and sideward scatter (SSC) were set on logarithmic gain. Cytotrophoblast cells were identified on the basis of their size and density. The immunomagnetic microsphere purification resulted in term trophoblast cell preparations that contained <1% contaminating fibroblasts (vimentin-positive cells) and <0.4% contaminating CD-45-positive immune cells (data not shown).
Isolation and Culture of Term Decidual Stromal Cells
Term decidual stromal cells (DSCs) were prepared as previously described.19,23–25 In brief, decidual tissues collected by the scraping of fetal membranes from uncomplicated term cesarean deliveries were minced and digested in Ham’s F-10 medium containing 10% charcoal-stripped calf serum (SCS; Flow Laboratories, Rockville, Maryland) and 25 mg/mL collagenase-deoxiribonuclease (200 U/mg; Worthington Biochemical Corp., Freehold, New Jersey) for 75 minutes. The resultant digestate was passed through a 23-gauge needle to dissociate remaining cell clusters, purified on a Percoll gradient, grown to confluence in a 95% air: 5% CO2 incubator at 37°C, and passaged until flow cytometry revealed that DSCs were more than 99% free of contaminating CD45-positive immune cells (data not shown).
Experimental Incubations for Term Trophoblast and DSCs
Term trophoblast cells were retrieved from elective cesarean and purified as described. These cells differentiate spontaneously into syncytiotrophoblast over a period of several days in culture. A total of 5 × 105 term cytotrophoblast cells were plated in T25 cells (Falcon) and cultured at 37°C. On days 1 (cytotrophoblast cells) and 4 (syncytiotrophoblast), cells were stimulated with or without interleukin 1β (1 ng/mL [IL-1β]; R&D Systems), tumor necrosis factor α (1 ng/mL [TNF-α]; R&D Systems), or lipopolysaccharide (1 μg/mL [LPS]; Sigma) for 24 h. The experimental paradigm was chosen based on prior dose- and time-course experiments.19,23–25
For term DSCs, experimental incubations were performed after the cells were treated with 10–8 mol/L estradiol (E2) and 10–7 mol/L medroxyprogesterone acetate (MPA) for 7 days as previously described.19,23–25 Thereafter, the cultures were washed twice with HBSS to remove residual serum and switched to a serum-free defined medium containing insulin/transferrin/selenium (BD Biosciences), 5 μmol/L FeSO4, 0.5 μmol/L ZnSO4, 1 nmol/L CuSO4, 50 μg/L ascorbic acid (Sigma), and 50 ng/mL epidermal growth factor (BD Biosciences) with or without IL-1β (1 ng/mL), TNF-α (1 ng/mL), and LPS (1 μg/mL). After 24 hours, cells were scrapped into ice-cold extraction buffer with protease inhibitor cocktail (Roche Applied Science, Indianapolis, Indiana) and frozen at –80°C until further analysis.
In select experiments, term DSCs and term trophoblast from days 1 (cytotrophoblast cells) and 4 (syncytiotrophoblast) were stimulated with or without IL-1β (1 ng/mL), TNF-α (1 ng/mL), or LPS (1 μg/mL) for 6 hours and RNA extracted for RT-qPCR analysis.
Confocal Immunoflourescence Microscopy
Purified trophoblast cells were grown on 6-chamber slides (BD Falcon). On days 1 (cytotrophoblast cells) and 4 (syncytiotrophoblast), cells were stimulated with or without IL-1β (1 ng/mL), TNF-α (1 ng/mL), or LPS (1 μg/mL) for 24 hours. Thereafter, slides were fixed with 4% paraformaldehyde for 15 minutes, permeabilized in 0.5% Triton X-100, and blocked with donkey serum. Neutrophil gelatinase-associated lipocalin expression was detected by incubating with rat anti-human NGAL (1:50 dilution [R&D Systems]) overnight at 4°C. Additional primary antibodies included mouse anti-human vimentin (1:50 dilution [Dako]) and mouse anti-human cytokeratin-7 (1:50 dilution [Dako]) to identify decidual and trophoblast cells, respectively. Slides were then incubated with secondary antibody conjugated with Texas Red or Cy5 (1:500 dilution [Jackson Immuno Research Laboratories, West Grove]). DAPI staining was used to identify the nuclei. Negative controls were prepared by substituting the primary antibody with the corresponding preimmune serum or incubation with secondary antibody only. Images were taken using a Laser Scanning Microscope (LSM 510 [Carl Zeiss, Jena, Germany]) with a ×40 1.4 oil immersion lens and the corresponding digital image processing software (Carl Zeiss Microimaging). Similar experiments were performed using term DSCs.
Western Blot
Term trophoblast and DSCs cells were isolated and stimulated as described above. Thereafter, cells were lysed in denaturing lysis buffer (Fermentas, Beverly, Massachusetts) containing 0.2 μg/mL PMSF and a protease inhibitor cocktail (Roche Applied Science). Total protein content was measured using the Bradford protein assay (Bio-Rad Laboratories, Hercules, California). Western blot analyses were performed using standard protocols as previously described.26 Equal amounts of protein lysate (35 μg) were separated on 4% to 15% Tris-HCl linear gradient gel (BioRad) and transferred to nitrocellulose membranes (Life Sciences, Boston, Massachusetts). After blocking, membranes were incubated overnight at +4°C with monoclonal rat anti-human NGAL (1:250 dilution [R&D Systems]) or monoclonal mouse anti-human heat-shock protein-90 (HSP-90; 1:1000 dilution [R&D Systems] to confirm equal loading of protein) followed by a 1 hour treatment at room temperature with the appropriate secondary antibody (horseradish peroxidase-conjugated anti-mouse IgG or horseradish peroxidase-conjugated anti-rat IgG at 1:10 000 dilution [Jackson ImmunoResearch Laboratories]). The blots were developed and the intensity of the bands analyzed using the enhanced chemiluminescence system according to the instructions of the manufacturer (PerkinElmer Life Sciences). Results were reported as NGAL/HSP-90 ratio. As a negative control, membranes were incubated with secondary antibody alone to validate the specificity of the signal.
RNA Isolation and RT-qPCR
Total RNA was extracted from term trophoblast and DSCs using RNeasy kit (Qiagen, Valencia, California). For each sample, 3 μg RNA was reverse transcribed using the SuperScript II First-Strand Synthesis System kit (Invitrogen, Carlsbad, California) and PCR Mastercycler (Eppendorf, Westbury, New York). Approximately 30 ng of complementary DNA (cDNA) were used for each reaction and all reactions were performed in duplicates. Prevalidated human primers that spanned exons were identified from TaqMan Assay-On-Demand (Applied Biosystems, Foster City, California). The primer sets used were NGAL (catalogue no. Hs00194353_m1 [LCN2]) and 18S (Hs99999901_s1; Applied Biosystems). RT-qPCR was performed using TaqMan One-Step RT-PCR Master Mix Reagents on an ABI Prism 7500 (Applied Biosystems) according to the instructions of the manufacturer. All reactions were multiplexed with the housekeeping 18S gene, which provided an optimized flourochrome-labeled control, thereby enabling data to be expressed in relation to an internal reference to control for differences in sampling. Data were obtained as cycle threshold (Ct) values (the cycle number at which logarithmic PCR plots cross a calculated threshold line) and used to determine ΔCt value (Ct of target gene—Ct of housekeeping gene). Visual representations of data were carried out by converting ΔCt values to fold-change data relative to ΔCt values for control (water). The 2–ΔΔC T was used to determine fold-change differences between samples. The PCR products were separated by gel electrophoresis in 1.5% agarose and visualized by ethidium bromide staining on an UV transilluminator. Negative controls were included for both RT (vehicle in place of RNA template and/or reverse transcriptase) and PCR reactions (vehicle in place of primers and/or cDNA).
Statistical Analysis
All data sets were subjected to normality testing using the Kolmogorov-Smirnov method. The H-score data sets were analyzed using the Holm-Sidak test for both pairwise comparisons and comparisons versus a control group. The data are reported as mean ± SEM (for normally distributed data) or as median and range (for nonnormally distributed data). Comparisons between 2 groups were performed using Student t-tests or Mann-Whitney rank sum tests. Statistical analyses were carried out using raw ΔCt values. Statistical calculations were performed using SigmaStat for Windows, version 3.0 (Jandel Scientific Corp., San Rafael, California). P < .05 indicated a statistically significant difference.
Results
In Vivo Localization and Regulation of NGAL Expression at the Maternal-Fetal Interface
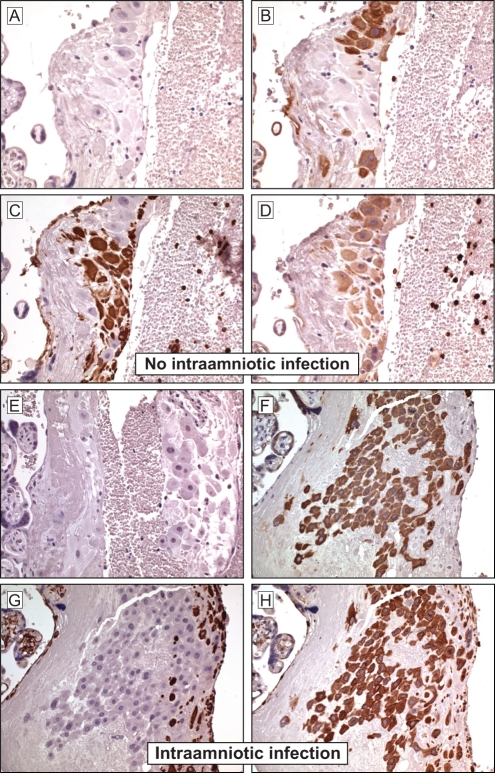
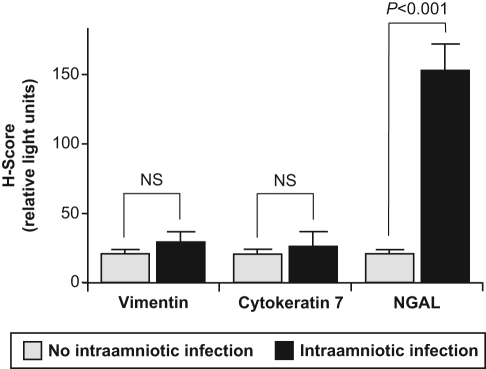
Serial sections of term placental tissues collected from women with and without IAI were immunostained for NGAL, cytokeratin-7 (to identify trophoblast cells) and vimentin (to identify decidual cells). Using immunohistochemical analysis, we demonstrate for the first time the presence of NGAL at the maternal-fetal interface (Figure 1D and H). Under basal conditions (in the absence of IAI), NGAL is consistently expressed in trophoblast cells, but only in occasional decidual cells; negative controls showed no nonspecific binding (Figure 1A–D). In placental tissues with evidence of IAI (Figure 1E–H), histological analysis showed a significant increase in NGAL expression within cytotrophoblast cells (H-SCORE [mean ± SEM] of 19.2 ± 7.6 vs 151.2 ± 17.4 in IAI and healthy controls, respectively; P < .001 [Figure 2 ]). This increase in NGAL expression in the setting of IAI was most striking in the cytotrophoblast cells, especially extravillous cytotrophoblast cells, with very little increase in syncytiotrophoblast and decidua (Figure 1E–H). In all tissues, NGAL expression is localized to the cytoplasm of the trophoblast cells and is not present in the nucleus.
Figure 1.
Immunohistochemical localization of neutrophil gelatinase-associated lipocalin (NGAL) at the maternal-fetal interface. Serial sections of placental tissues collected from women with or without clinical and histologic evidence of intra-amniotic infection (IAI) were stained for cytokeratin-7 (to identify trophoblast; B and F), vimentin (to identify decidual stromal cells [DSCs]; C and G), and NGAL (D and H) as described in the Materials and Methods section. Representative images are shown. In the absence of IAI (A–D), NGAL staining was seen consistently in cytokeratin-positive trophoblast cells, but only in occasional vimentin-positive decidual stromal cells (DSCs). In the presence of IAI (E–H), cytokeratin-positive trophoblast cells (especially extravillous cytotrophoblast cells) showed strong staining for NGAL with no apparent increase in staining in syncytiotrophoblast and decidual cells (H). Negative controls using second antibody only showed the absence of nonspecific binding (A and E). In all tissues, NGAL expression is localized to the cytoplasm of the trophoblast cells and is not present in the nucleus. Magnification is ×40.
Figure 2.
Quantitative analysis of immunohistochemical studies of neutrophil gelatinase-associated lipocalin (NGAL) expression at the maternal-fetal interface with or without intra-amniotic infection. As described in the Materials and Methods section, serial sections of placental tissues with or without clinical and histologic evidence of intra-amniotic infection (IAI) were stained for NGAL and analyzed using AxioVision (Carl Zeiss Microimaging, Inc., Thornwood, New York). A computer-generated H-score (in relative light units) was assigned for a representative tissue section and corrected for background light intensity. Results are reported as mean + SEM from at least 3 separate tissue sections with measurements performed in triplicate. Statistical differences are shown. NS indicates not significant.
Pro-inflammatory Mediators Upregulate NGAL Expression by Term Trophoblast Cells In Vitro
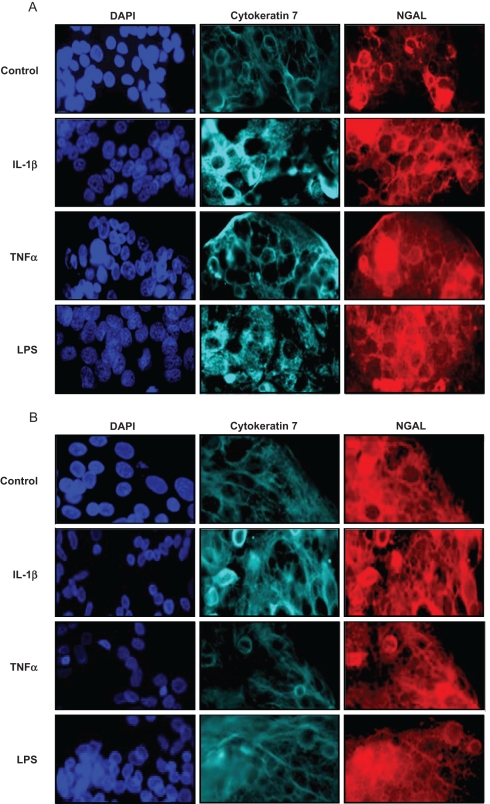
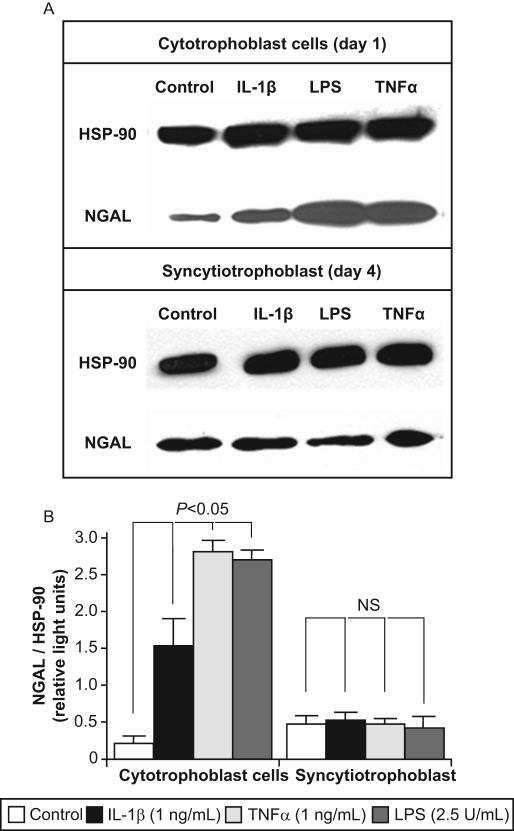
To mimic IAI, freshly isolated term cytotrophoblast cells were treated for 24 hours with or without pro-inflammatory mediators (IL-1β, TNF-α, or LPS), and NGAL expression investigated by immunocyto chemistry and Western blot as described. Similar studies were carried out in DSCs and in trophoblast cells allowed to syncytialize spontaneously for 72 hours (syncytiotrophoblast). Under both basal and stimulated conditions, NGAL expression was noted to colocalize with cytokeratin-7, and NGAL staining was localized exclusively to the cytoplasm with no nuclear expression (Figure 3 ). Qualitatively, all 3 pro-inflammatory mediators appeared to upregulate NGAL expression in day 1 (cytotrophoblast) cells (Figure 3A) but not in day 4 (syncytiotrophoblast) cells (Figure 3B). The increase in NGAL expression by day 1 (cytotrophoblast) cells—but not day 4 (syncytiotrophoblast) cells—in response to stimulation by all 3 pro-inflammatory mediators was confirmed quantitatively by Western blot analysis (Figure 4 ).
Figure 3.
Effects of pro-inflammatory mediators on neutrophil gelatinase-associated lipocalin (NGAL) expression by term trophoblast cells as determined by immunocytochemistry. Term trophoblast cells on day 1 (cytotrophoblast) and day 4 of culture (syncytiotrophoblast) were treated for 24 h with or without IL-1β (1 ng/mL), TNF-α (1 ng/mL), or LPS (1 μg/mL), and NGAL expression measured by immunocytochemistry as described in the Materials and Methods section. Representative images are shown. Trophoblast cells on days 1 (cytotrophoblast; A) and (syncytiotrophoblast; B) were fixed and labeled with cytokeratin 7 (a trophoblast-specific marker) and NGAL. DAPI staining was used to identify the cell nuclei. The images were analyzed using AxioVision (Carl Zeiss Microimaging, Inc., Thornwood, New York). A computer-generated H-score (in relative light units) was assigned for a representative section and corrected for background light intensity. Data are presented as fold change compared with no stimulation (control), and results are reported as mean + SEM from at least 3 separate sections with measurements performed in triplicate (C). Statistical differences are shown. Abbreviations: IL-1β indicates interleukin-1β; LPS, lipopolysaccharide; NS, not significant; TNF-α, tumor necrosis factor-α.
Figure 4.
Effects of pro-inflammatory mediators on NGAL expression by term trophoblast cells as measured by western blot analysis. Term trophoblast cells on day 1 (cytotrophoblast) and day 4 of culture (syncytiotrophoblast) were treated for 24 hours with or without IL-1β (1 ng/mL), TNFα (1 ng/mL), or LPS (2.5 U/mL), and NGAL expression determined by western blot analysis as described in the Materials and Methods section. A representative western blot is shown [A]. To quantify the results, the intensity of the NGAL bands was corrected for expression of the housekeeping protein, heat shock protein-90 (HSP-90). Data are presented as mean ± SEM from 4 separate experiments [B]. Statistical differences are shown. Abbreviations: IL-1b, interleukin-1b; LPS, lipopolysaccharide; NS, not significant; TNFα, tumor necrosis factor-α.
Effect of Pro-inflammatory Mediators on NGAL Gene Expression by Term Trophoblast Cells
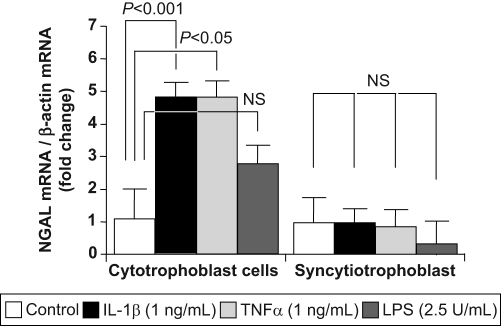
Term trophoblast cells on days 1 (cytotrophoblast) and 4 of culture (syncytiotrophoblast) were treated for 6 hours with or without IL-1β, TNF-α, or LPS. RNA was extracted and subjected to RT-qPCR using specific primers as described. IL-1β and TNF-α stimulation resulted in a significant 4.81 ± 0.38-fold (mean ± SEM; P < .001) and 4.77 ± 0.41-fold (P < .05) increase in NGAL gene expression in day 1 (cytotrophoblast) cells, respectively (Figure 5 ). A 2.81 ± 0.47-fold increase in NGAL gene expression was also seen following stimulation with LPS, but this failed to reach statistical significance (Figure 5). As expected, none of the pro-inflammatory mediators increased NGAL gene expression in day 4 (syncytiotrophoblast) cells (0.97 ± 0.40-fold, 0.79 ± 0.37-fold, and 0.29 ± 0.70-fold for IL-1β, TNF-α, and LPS stimulation, respectively; P > .05 for all; Figure 5).
Figure 5.
Effects of preeclampsia on neutrophil gelatinase-associated lipocalin (NGAL) gene expression. Term trophoblast cells on day 1 (cytotrophoblast) and day 4 of culture (syncytiotrophoblast) were treated for 6 hours with or without IL-1β (1 ng/mL), TNF-α (1 ng/mL), or LPS (1 μg/mL). RNA was extracted and subjected to RT-qPCR using specific primers as described in the Materials and Methods section. NGAL mRNA levels were corrected for expression of the housekeeping gene, β-actin. Data are expressed as mean ± SEM from 3 separate experiments performed in duplicate. Statistical differences are shown. Abbreviations: IL-1β indicates interleukin-1β; LPS, lipopolysaccharide; NS, not significant; TNF-α, tumor necrosis factor-α.
NGAL Expression by Term DSCs In Vitro
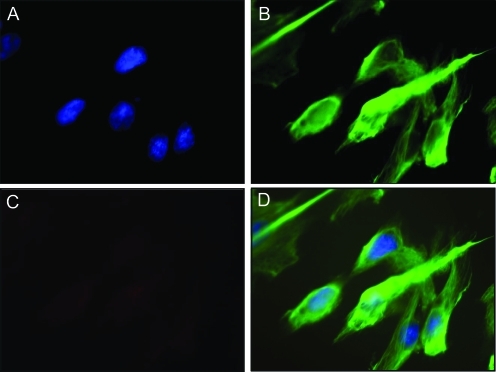
In keeping with the absence of NGAL expression in decidual tissues by immunohistochemistry (Figure 1), no NGAL expression was evident in term DSCs in vitro as measured by immunocytochemistry under basal conditions (Figure 6 ) or after stimulation with pro-inflammatory mediators (data not shown). Similarly, no NGAL gene expression was evident by RT-qPCR in term DSCs in vitro under basal conditions or after stimulation with pro-inflammatory mediators (data not shown).
Figure 6.
NGAL expression by term decidual stromal cells as determined by immunocytochemistry. Term decidual stromal cells (DSCs) were isolated, purified, and cultured as described in the Materials and Methods section. NGAL expression was measured by immunocytochemistry. Representative images are shown from cells without IL-1b or thrombin stimulation. Fixed DSCs were stained with DAPI to identify the cell nuclei [A], the stromal cell-specific marker vimentin [B], and NGAL [C]. A composite image is included [D]. The images were analyzed using AxioVision (Carl Zeiss Microimaging, Inc, Thornwood, NY).
Discussion
There is substantial evidence to suggest that the fetus—or more correctly, the fetoplacental unit—controls the timing of labor.27 Although the mechanisms by which the fetoplacental unit triggers labor is not well understood, it is clear that “decidual activation” plays a central role.28 Neutrophil gelatinase-associated lipocalin, a member of the lipocalin family, serves as a transport shuttle for small compounds, including a number which are known to be intimately involved in the process of rupture of the fetal membranes and parturition at term as well as preterm labor and delivery, including arachidonic acid, prostaglandins, and MMPs.7–9 Recent studies suggest that NGAL may be a reliable biomarker for cancer17 and for acute kidney injury in the nonpregnant patient.13–16,29 It has also been implicated in a number of pregnancy-related complications, including preeclampsia30 and gestational diabetes.31 In addition to its function as a transport shuttle, NGAL has also been shown to play a critical role in innate immunity to bacterial infection.11,12 Despite these observations, the expression of NGAL at the maternal-fetal interface and its role as a mediator of “decidual activation” and thereby parturition, both at term and preterm, has not previously been examined.
In the current study, we demonstrate that NGAL is expressed at the maternal-fetal interface in trophoblast cells, but not in decidua. Using immunohistochemical analysis of term placental tissues collected from women with and without IAI, we show that NGAL expression in trophoblast cells is significantly elevated in the setting of IAI in vivo (Figures 1 and 2). These observations were confirmed by both immunocytochemistry (Figure 3) and Western blot analysis (Figure 4) in freshly isolated term trophoblast cells in vitro, which further demonstrated that the effect was selective for cytotrophoblast cells and was not seen in syncytiotrophoblast (Figures 3 and 4). The use of immunomagnetic microsphere purification and flow cytometric analysis to confirm the purity of the cell cultures confirmed that these effects were localized to trophoblast cells and not to any contaminating cell populations. Interestingly, NGAL gene expression was significantly increased in cytotrophoblast (not syncytiotrophoblast) cells in vitro following IL-1β and TNF-α stimulation, but not following stimulation with LPS (Figure 5). These data suggest that IL-1β and TNF-α stimulate NGAL expression directly at the level of gene transcription, whereas LPS-mediated increase in NGAL expression likely occurs by a different mechanism. This may involve posttranscriptional events resulting, for example, in less NGAL degradation or may involve upregulation of NGAL gene expression via an indirect route, for example, by first stimulating IL-1β production. In keeping with the immunohistochemical data which failed to demonstrate NGAL immunoreactivity on the maternal (decidual) aspect of the placenta, we were unable to demonstrate NGAL mRNA or protein expression in cultured term DSCs in vitro.
The reason why NGAL is expressed in freshly isolated cytotrophoblast cells (and increases in response to stimulation by IL-1β, TNFα, and LPS), but not in these same cells once they have been allowed to syncytialize for 72 hours in culture, is not immediately apparent. It may be due to a generalized loss of transcriptional and translational activity within the nuclei of the syncytiotrophoblast as compared with the cytotrophoblast cells. Alternatively, it may reflect differential expression of the various forms of NGAL such as Holo-NGAL (bound to iron) and free (unbound) Apo-NGAL.10 Regardless of the mechanism, these findings are consistent with our current understanding that the syncytium is involved primarily in transport and hormone production, whereas it is the cytotrophoblast (especially extravillous cytotrophoblast cells) that invade the decidua and remodel the maternal vasculature, which involves inflammation and apoptosis.
In contrast to other tissues (such as liver and kidney) where NGAL expression is present only in disease states,12–16 the immunohistochemical, immunocytochemical, Western blot, and RT-qPCR data presented in this study all confirm that cytotrophoblast cells expressed NGAL mRNA and protein even under basal conditions (in the absence of IAI). The reason for this difference is not clear. It may be related to local hormonal or immunologic factors. Alternatively, it may reflect the fact that placental tissues exists in a largely hypoxic environment with a pO2 in the range of 18–20 mm Hg. Indeed, preliminary data suggest that NGAL expression in cytotrophoblast cells is regulated by culture under hypoxic conditions (Norwitz, unpublished data). Either way, the presence of large amounts of NGAL at the maternal-fetal interface would improve the ability of the body to mount an aggressive response to the presence of infectious organisms at this site which, in turn, would improve the chances for successful pregnancy outcome and thereby survival of the species.
An NGAL knockout has been developed.32 These animals exhibit an increased susceptibility to bacterial infection (especially gram-negative sepsis) in keeping with the proposed function of NGAL as a critical regulator of innate immunity. Neutrophils isolated from NGAL-/- mice showed significantly less bacteriostatic activity compared with wild type controls, and the bacteriostatic property of wild type neutrophils can be abolished by the addition of exogenous iron.32 Taken together, these data suggest that the main function of NGAL in the antibacterial innate immune response may be to regulate the availability of this essential element.32,33 It has long been known that increased production of Th1 cytokines (IL-1β, TNF-α) and release of LPS from gram negative bacteria, which are commonly isolated from women with IAI, have profound biochemical effects on gestational tissues and are associated with adverse perinatal outcome. The current study demonstrates that NGAL is significantly upregulated in placental tissues collected from women with clinical and histological evidence of IAI as compared with healthy controls, that it is produced locally, and that it is localized to the fetal (trophoblast) aspect of the placenta. Despite the fact that IAI is associated with the release of large amounts of chemokines and other chemotactic factors within the tissues of the uterus3,34 and that fetal immune cells are capable of invading the umbilical cord causing histologic funisitis, immune cell infiltration into the villous tissues of the placenta is rarely seen in the setting of IAI. Although teleologically advantageous (since the presence of large numbers of neutrophils at the maternal-fetal interface would almost certainly lead to widespread tissue damage, impairment of gas exchange, and fetal death), the mechanism by which this is achieved is not known. We posit that, analogous to its role in the diseased kidney,7,8,12 NGAL production within the infected placenta may limit leukocyte infiltration and trafficking by regulating the availability of iron and possibly by inducing neutrophil apoptosis.12
In summary, we demonstrate for the first time that NGAL is produced and expressed by trophoblast cells (not decidua) at the maternal-fetal interface at term. Moreover, its expression is increased in the setting of IAI in vivo and in response to exposure to pro-inflammatory cytokines (IL-1β and TNF-α) and LPS in vitro suggesting that it functions downstream in the inflammatory cascade. Substantial epidemiological, clinical, microbiological, and placental pathological studies support a link between IAI and preterm birth.1–3 Taken together, these data suggest that, in keeping with its function as a critical mediator of innate immunity to bacterial infection, NGAL may have a central role to play in IAI-induced preterm birth.
Footnotes
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Supported by the NIH/NICHD-sponsored Reproductive Scientist Development Program (to ERN) and March of Dimes (21-FY05-1250 to ERN).
References
- 1. Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360(9344):1489–1497 [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–584 [DOI] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Obstetricians and Gynecologists and American Academy of Pediatrics Newborn Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. 2003;Washington, DC: ACOG, [Google Scholar]
- 5. Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109(3):739–749 [DOI] [PubMed] [Google Scholar]
- 6. Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):547–550 [DOI] [PubMed] [Google Scholar]
- 7. Flower DR. The lipocalin family: a role in cell regulation. FEBS Lett. 1994;354(1):7–11 [DOI] [PubMed] [Google Scholar]
- 8. Schmidt-Ott KM, Mori K, Kalandadze A, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15(4):442–449 [DOI] [PubMed] [Google Scholar]
- 9. Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52(3):595–605 [DOI] [PubMed] [Google Scholar]
- 10. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921 [DOI] [PubMed] [Google Scholar]
- 11. Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482(1-2):272–83 [DOI] [PubMed] [Google Scholar]
- 12. Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413 [DOI] [PubMed] [Google Scholar]
- 13. Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212 [DOI] [PubMed] [Google Scholar]
- 14. Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(suppl 4):S159–S165 [DOI] [PubMed] [Google Scholar]
- 15. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024 [DOI] [PubMed] [Google Scholar]
- 16. Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolignano D, Donato V, Lacquaniti A, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288(1):10–16 [DOI] [PubMed] [Google Scholar]
- 18. Budwit-Novotny DA, McCarthy KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46(10):5419–5425 [PubMed] [Google Scholar]
- 19. Snegovskikh VV, Schatz F, Arcuri F, et al. Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and -2 expression: implications for infection-related preterm birth. Reprod Sci. 2009;16(8):767–780 [DOI] [PubMed] [Google Scholar]
- 20. Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582 [DOI] [PubMed] [Google Scholar]
- 21. Pötgens AJ, Gaus G, Frank HG, Kaufmann P. Characterization of trophoblast cell isolations by a modified flow cytometry assay. Placenta. 2001;22(2-3):251–255 [DOI] [PubMed] [Google Scholar]
- 22. Guller S, Buhimschi CS, Ma YY, et al. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88(10):1057–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med. 2002;11(1):11–17 [DOI] [PubMed] [Google Scholar]
- 24. Lockwood CJ, Arcuri F, Toti P, et al. Tumor necrosis factor-alpha and interleukin-1beta regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol. 2006;169(4):1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norwitz ER, Snegovskikh V, Schatz F, et al. Progestin inhibits and thrombin stimulates the plasminogen activator/inhibitor system in term decidual stromal cells: implications for parturition. Am J Obstet Gynecol. 2007;196(4):382.e1–382.e8 [DOI] [PubMed] [Google Scholar]
- 26. Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS. PPAR gamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One. 2009;4(11):e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norwitz ER, Robinson JN, Challis JRG. The control of labor. N Engl J Med. 1999;341(9):660–666 [DOI] [PubMed] [Google Scholar]
- 28. Casey ML, MacDonald PC. Biomolecular processes in the initiation of parturition: decidual activation. Clin Obstet Gynecol. 1988;31(3):533–552 [DOI] [PubMed] [Google Scholar]
- 29. Soni SS, Cruz D, Bobek I, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–150 [DOI] [PubMed] [Google Scholar]
- 30. D'Anna R, Baviera G, Giordano D, et al. Neutrophil gelatinase-associated lipocalin serum evaluation through normal pregnancy and in pregnancies complicated by preeclampsia. Acta Obstet Gynecol Scand. 2010;89(2):275–278 [DOI] [PubMed] [Google Scholar]
- 31. D'Anna R, Baviera G, Corrado F, Giordano D, Recupero S, Di Benedetto A. First trimester serum neutrophil gelatinase-associated lipocalin in gestational diabetes. Diabet Med. 2009;26(12):1293–1295 [DOI] [PubMed] [Google Scholar]
- 32. Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2006;103(6):1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bachman MA, Miller VL, Weiser JN. Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog. 2009;5(10):e1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esplin MS, Peltier MR, Hamblin S, et al. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta. 2005;26(8-9):661–671 [DOI] [PubMed] [Google Scholar]