Abstract
Epigenetic mechanisms have been ascribed important roles in endometriosis. Covalent histone modifications at lysine residues have been shown to regulate gene expression and thus contribute to pathological states in many diseases. In endometriosis, histone deacetylase inhibition (HDACi) resulted in reactivation of E-cadherin, attenuation of invasion, decreased proliferation of endometriotic cells, and caused lesion regression in an animal model. This study was conducted to assess basal and hormone-regulated gene expression levels of HDAC1 and HDAC2 (HDAC1/2) in cell lines and protein expression levels in tissues. Basal and steroid hormone-regulated HDAC1/2 gene expression levels were determined by quantitative polymerase chain reaction in cell lines and tissues. Protein levels were measured by immunohistochemistry (IHC) in tissues on an endometriosis tissue microarray (TMA). Basal HDAC1/2 gene expression levels were significantly higher in endometriotic versus endometrial stromal cells, which was confirmed by Western blot analysis. Estradiol (E2) and progesterone (P4) significantly downregulated HDAC1 expression in endometrial epithelial cells. Levels of HDAC2 were upregulated by E2 and downregulated by E2 + P4 in endometrial stromal cells. Hormone modulation of HDAC1/2 gene expression was lost in the endometriotic cell line. Immunohistochemistry showed that HDAC1/2 proteins were expressed in a substantial proportion of lesions and endometrium from patients, and their expression levels varied according to lesion localization. The highest proportion of strong HDAC1 immunostaining was seen in ovarian, skin, and gastrointestinal lesions, and of HDAC2 in skin lesions and endometrium from patients with endometriosis. These studies suggest that endometriosis etiology may be partially explained by epigenetic regulation of gene expression due to dysregulations in the expression of HDACs.
Keywords: endometriosis, endometrium, HDAC1, HDAC2, histone deacetylases, tissue microarray (TMA), ovarian steroid hormones, estrogen, progesterone
Introduction
Although the etiology of endometriosis is not completely understood, various theories attempt to explain the mechanisms whereby endometrial cells are able to survive and grow in the peritoneum and other ectopic sites, including genetic predisposition, immune system disturbances, and environmental exposures.1,2 Global gene expression studies in both human and animal models have shown that endometriotic lesions have aberrant gene expression profiles when compared to normal endometrium.3–7 The mechanisms at play have not been sufficiently studied, but epigenetic regulation of gene expression is emerging as a key player in endometriosis.8 Thus, the current knowledge on the pathophysiology of endometriosis supports the involvement of both genetic and epigenetic phenomena in inducing changes in gene expression that lead to disease.
Histone modification, including deacetylation of lysine residues by histone deacetylases (HDACs), is a key mechanism of gene expression regulation in cancer and other diseases.9–11 Histone deacetylases, involved in cell cycle regulation, proliferation, and cell differentiation, covalently acetylate key residues in histones, thus changing chromatin conformation and result in transcriptional repression.12,13 Histone deacetylation, like promoter methylation, generally results in gene silencing; thus HDACs act as transcriptional repressors. The HDAC-related mechanisms also appear to play a role in endometriosis. Recently, Wu et al showed that HDAC inhibition (HDACi) resulted in reactivation of E-cadherin, attenuation of invasion, and decreased proliferation of endometriotic cells.14,15 In vitro, HDACi induced cell cycle arrest by upregulating p21 levels and promoted apoptosis16,17; in vivo, HDACi reduced endometriotic lesion growth and hyperalgesia in a mouse model.18 These data indicate that methylation changes are not the only epigenetic mechanism involved in endometriosis and led to the proposal that HDACi could be a possible new treatment for this condition. However, the HDAC expression profile of endometriotic tissues is yet to be assessed.
We hypothesized that histone modification regulates gene expression in endometriosis and is therefore a potential target for therapy. Since endometriotic lesions are characterized by high endogenous estradiol (E2) levels and show abnormal responses to progesterone (P4),19 we also hypothesized that HDAC expression is differentially regulated in normal endometrium versus endometriosis. We specifically aimed to assess the expression levels of HDAC1/2—regulators of most of the reported changes in histone acetylation levels among Class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8)—in cell lines and human tissues, and their regulation by ovarian steroid hormones in vitro.20 These data will significantly contribute to the current understanding of endometriosis by dissecting the epigenetic mechanisms at play.
Materials and Methods
Cell Culture and Ovarian Steroid Hormone Treatments
Human endometrial stromal (HESCs) and epithelial endometrial (EEC) cell lines were cultured as described.21,22 The stromal endometriotic cell line Hs832cT (CRL-7566) was obtained from American Type Culture Collection (ATCC; Manassas, Virginia) and cultured following their recommendations. This cell line, derived from an ovarian cyst from a patient with endometriosis, has been partially characterized as stromal (expressing vimentin but not cytokeratin nor E-cadherin). Compared to EEC, Hs832cT cells have a lower ER1/ER2 ratio and lower PR protein levels (Bello et al, unpublished data). Steroid hormone treatments were conducted as described by Ruiz et al.23
Tissue Collection
Flash-frozen endometriotic lesions and endometrial biopsies were collected from volunteers (19-47 years old) undergoing surgery for benign gynecologic conditions, not on hormonal medication for at least 3 months prior. Average age of patients and controls were 32.9 years (range: 19-47 years) and 42.7 years (range: 40-47 years), respectively (P < .05). Disease severity was determined by collaborating gynecologists using the revised American Fertility Society criteria.24 Frozen tissues (n = 49) were analyzed for pathological diagnosis and macrodissection to exclude nonendometriotic tissue. Tissues analyzed included 15 endometriotic lesions, 11 endometrial biopsies from endometriosis patients, and 23 endometrial biopsies from women without endometriosis. Protocols involving tissue collection were approved by the PSMHS IRB Committee. All participants signed a consent form and completed a demographic and clinical questionnaire.
HDAC1/2 Gene Expression Levels in Cells and Tissues
RNA was extracted following standard procedures (RNAeasy Qiagen, Valencia, California). After deoxyribonuclease treatment, total RNA was reverse transcribed (iScript cDNA synthesis kit, Bio-Rad, Hercules, California), and gene expression levels were determined using TaqMan Gene Expression Assays (HDAC1: Hs00605262_g1; HDAC2: Hs00231032_m1; Applied Biosystems, Foster City, California). Quantitative polymerase chain reaction was performed as described using glyceraldehyde 3-phosphate dehydrogenase (Hs99999905_m1) and beta2M (Hs99999907_m1) for normalization in cells and tissues, respectively.23 Basal gene expression was reported as relative units (RUs; 2−▵Ct).25
HDAC1/2 Protein Expression Levels in Cells
Total protein extraction, quantification, and Western blot analysis were conducted as described in detail by Ruiz et al (2010).23 Polyclonal primary antibodies to HDAC1 and HDAC2 were used at dilutions of 1:500 and 1:5000, respectively. Enhanced chemiluminescence was analyzed on a ChemiDoc XRS+ system (Bio-Rad, Hercules, California).
Immunohistochemistry of HDAC1/2 in Human Endometrium and Endometriotic Samples on a Tissue Microarray (TMA)
De-identified formalin-fixed paraffin-embedded endometrium and endometriosis tissues were obtained from a collaborating pathology laboratory after protocol approval by the PSMHS IRB Committee. Average age of patients and controls represented in the TMA were 35 and 42 years, respectively. All biopsies were evaluated by a pathologist (M.G.) who confirmed the diagnosis and stage of the menstrual cycle of eutopic endometrial samples using Noyes criteria.26 A total of 164 core biopsies obtained from 83 tissue blocks were used to construct a TMA at the Moffitt Cancer Center Pathology Department. From most blocks, 2 different core biopsies were included in the TMA. The total number of cores on the TMA were 29 ovarian endometriosis, 16 fallopian tube endometriosis, 34 peritoneal endometriosis, 4 skin (umbilical) endometriosis, 7 gastrointestinal (GI), 22 eutopic endometrium of patients with endometriosis (EE), 14 control proliferative phase endometrium (PE), and 38 control secretory phase endometrium (SE). Control endometrium were obtained from women with uterine myomas, abnormal uterine bleeding, or enlarged/prolapsed uterus.
Immunostaining of HDAC1/2 in Human Endometrium and Endometriotic Samples on a TMA
Ten-micrometer sections of the TMA block were cut for immunostaining. The endometriotic and eutopic endometrial tissue sections on the TMA were stained using the LSAB+ Kit (Dako, Carpinteria, California) following the manufacturer’s recommendations. After dewaxing and rehydration, antigen retrieval was conducted with target retrieval solution (pH = 9; Dako) followed by the removal of endogenous peroxidase activity using standard methods. Primary antibodies against HDAC1 (1:250) and HDAC2 (1:100) were incubated in antibody diluents (Dako) for 1 hour at room temperature (RT). Sections were incubated for 30 minutes at RT with the secondary biotinylated antibody developed with diaminobenzidine and counterstained with Meyer hematoxylin solution. Positive controls were sections of tonsils. The slides were mounted and visualized at 10, 20, and ×40 magnification on an inverted microscope with an Olympus 35 mm camera (Nikon, Japan). Immunostaining intensity was blindly scored in nuclei of epithelium, glands, and stroma using a 0 to 3 score system: 0 = no staining, 1 = weak, 2 = moderate, and 3 = strong staining.
Statistical Analyses
Gene expression data are presented as mean RU ± standard deviation (SD). Group variables were compared by Kruskal-Wallis nonparametric analysis of variance (ANOVA). Independent sample Wilcoxon tests were done to assess statistical significance of selected differences between groups. Nonparametric Spearman correlations between gene expression levels of HDAC1/2 were analyzed using GraphPad Prism 4.0 (GraphPad Software Inc, La Jolla, California). Statistical significance of the differences in proportions of tissues on the TMA with strong immunostaining was assessed by 2-sided Fisher Exact test. Statistical significance level was set at P < .05. SPSS15 (SPSS Inc, Chicago, Illinois) was used for the statistical analysis.
Results
Basal and Ovarian Steroid Hormone Regulated Expression of Class HDAC1/2 in Human Endometrial and Endometriotic Cell Lines
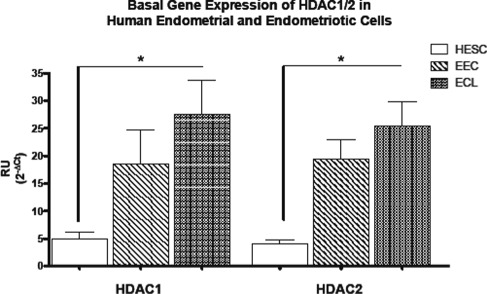
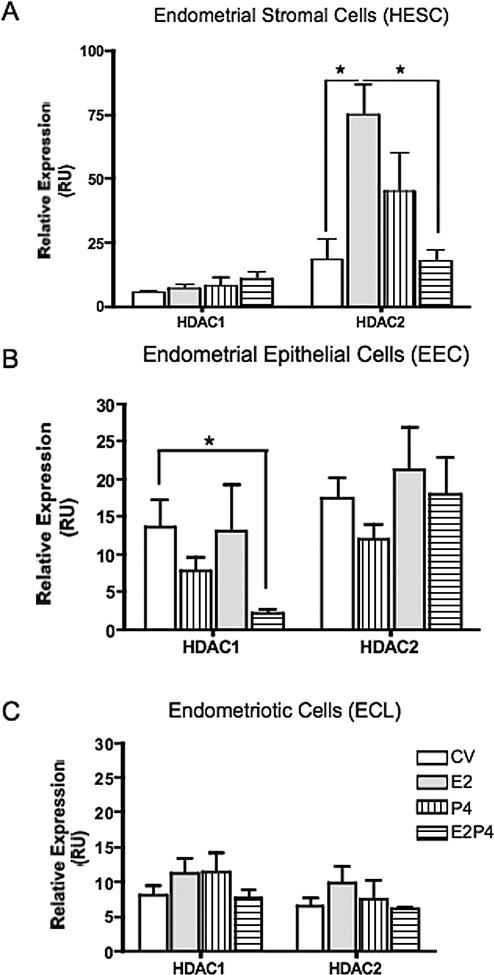
Hs832cT cells express higher levels of HDAC1/2 messenger RNA (mRNA) than endometrial cell lines, but these differences were only significant between the endometriotic cells (which are of stromal characteristic) and the endometrial stromal cells (Figure 1). Western blot experiments confirmed that endometriotic cells express higher protein levels of these proteins when compared only to endometrial stromal cells (data not shown). Compared to control (media plus vehicle or CV), E2 + P4 significantly downregulated HDAC1 expression in EEC but not in HESC (Figure 2). Histone deacetylase 2 was significantly upregulated by E2 only in HESC, while E2 + P4 brought levels to those of control. No statistically significant changes in gene expression were observed for Hs832cT.
Figure 1.
Basal gene expression of class I histone deacetylases (HDACs) in human endometrial and endometriotic cell lines. Quantitative polymerase chain reaction (qPCR) quantification of class I HDACs (HDAC1 and HDAC2) was conducted using TaqMan gene expression assays. Results are expressed as mean ± standard deviation (SD) of at least 3 experiments in duplicate. Both HDAC1 and HDAC2 are differentially expressed in human endometrial cell lines. Endometriotic cells express significantly higher levels of HDAC1/2. HESC, endometrial stromal; EEC, endometrial epithelial; ECL, endometriotic cell line. *P < .05.
Figure 2.
Effects of ovarian steroid hormone treatment on histone deacetylase (HDAC) 1/2 gene expression in human endometrial and endometriotic cell lines. Cells were treated for 24 hours with 10−8 mol/L estradiol (E2), 10−7 mol/L progesterone (P4), or E2/P4 for 24 hours. Total RNA was isolated from treated and untreated (medium with vehicle, CV) cells. Quantitative polymerase chain reaction (qPCR) of HDAC1/2 was conducted using TaqMan gene expression assays. Results are expressed as relative expression (relative units, RU) after normalization with the reference gene. The HDAC1 gene expression was significantly downregulated by E2/P4 in endometrial epithelial cells (B) but not in endometrial stromal nor in the endometriotic cells (C). The HDAC2 gene expression levels were significantly increased by E2 and dowregulated by E2 + P4 in endometrial stromal cells (A). **P < .01.
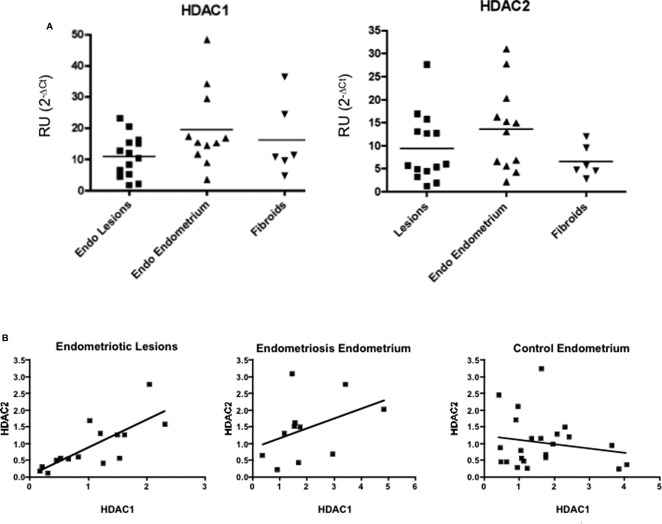
Gene Expression Levels of HDAC1/2 in Human Endometriotic and Endometrial Fresh Tissues
Mean HDAC1 expression was 10.95 RU (SD ± 6.61; median = 11.14) in lesions, 19.55 RU (SD ± 12.85; median = 15.41) in eutopic endometrium from patients with EE, and 16.18 RU (SD ± 11.87; median = 11.04) in endometrium of women with fibroids (EF; Figure 3). For HDAC2, mean expression was 9.40 RU (SD ± 7.41; median = 5.85) in lesions, 13.69 RU (SD ± 9.23; median = 14.01) in EE, and 6.58 RU (SD ± 3.48; median = 5.23) in EF. Endometriotic lesions expressed the highest mean levels of both HDAC1/2. Across tissues, HDAC1 expression was higher than that of HDAC2. Levels of HDAC1/2 expression were significantly correlated in lesions (Spearman r = .80; 95% CI: 0.41-0.95; P = .002) but not in EE (Spearman r = .43; 95% CI: −0.24 to 0.83; P = .18) nor in endometria from controls (Spearman r = −0.06; 95% CI: −0.47 to 0.38; P = .80).
Figure 3.
Gene expression levels of histone deacetylase (HDAC) 1/2 in endometrial and endometriotic tissues. A, Relative gene expression levels in tissues: RNA was isolated from flash-frozen tissues and quantitative polymerase chain reaction (qPCR) was conducted using TaqMan assays. Results are expressed as relative units (RU) after normalization with the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The HDAC1 was expressed in tissues of patients with endometriosis (endometriotic and eutopic endometrium), and in the endometria of patients with fibroids. The highest mean expression of HDAC1 was observed in the endometria of patients with endometriosis. Similar results were observed for HDAC2, which was expressed in a substantial proportion of tissues from patients with endometriosis and fibroids. The highest expression of HDAC2 was in endometria from patients with endometriosis. B, Correlations of HDAC1/2 gene expression levels in tissues: a positive and significant correlation was observed in the levels of HDAC1/2 only in endometriotic lesions.
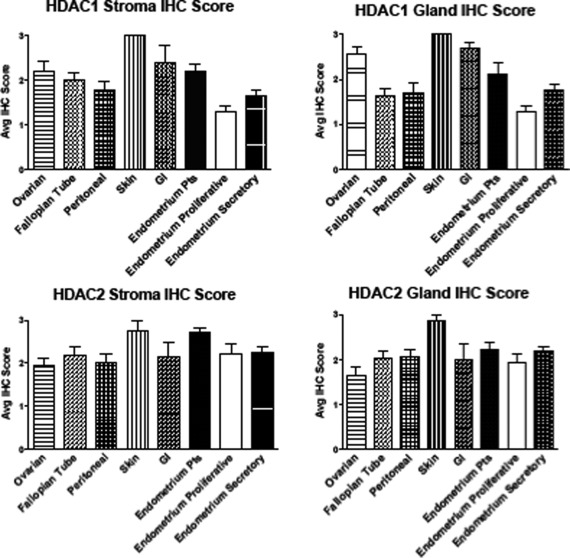
Immunostaining Intensity of HDAC1/2 Proteins in Human Endometriotic and Endometrial Tissues on a TMA
Nuclear immunostaining for HDAC1/2 was detected in stroma and glands in both diseased and control tissues; however, the intensity of HDAC1/2 protein staining was generally higher in endometriotic lesions compared to eutopic endometrium of controls, with noticeable differences depending on lesion localization (Figure 4). There were no statistically significant differences in mean protein expression of HDAC1/2 in PE versus SE from controls. Expression levels of HDAC1 protein were higher among the endometriotic tissues when compared to PE and SE. In stroma and glands, average HDAC1 immunostaining was lowest in PE, followed by peritoneal lesions and SE. The highest mean expression of HDAC1 was observed in skin, GI, ovarian, and EE. Histone deacetylase 1 staining was significantly stronger in skin lesions in stroma (P = .006) and gland (P = .034) compared to all tissues except for GI lesions. Histone deacetylase 2 protein levels, on the other hand, were lowest in ovarian and highest in skin lesions, followed by EE and GI. In stroma, HDAC2 expression was significantly higher in EE compared to SE (P = .046). Skin lesions expressed higher levels of HDAC2 in both glands and stromal than peritoneal, ovarian, fallopian tubes, and PE (P = .07). Both PE and SE expressed lower levels of HDAC1 than most lesion types, while differences in HDAC2 between diseased and control tissues were subtler.
Figure 4.
Immunostaining intensity of histone deacetylase (HDAC)1/2 proteins in endometrial and endometriotic tissues. A total of 164 formalin-fixed paraffin-embedded endometrial and endometriotic tissues on a tissue microarray (TMA) were analyzed by immunohistochemistry. Immunostaining intensity of HDAC1 and HDAC2 was evaluated and scored blindly in glands (A and C) and stroma (B and D) by 2 independent observers using the intensity scale (3 = strong, 2 = moderate, 1 = weak, and 0 = no staining). Staining was reported as mean immunohistochemistry (IHC) score ± standard deviation (SD). The highest mean expression of HDAC1 was seen in skin lesions followed by gastrointestinal (GI), ovarian, and endometrium from patients with endometriosis (EE). Differences in mean HDAC2 immunostaining intensity were subtler, with the highest mean expression seen in skin, EE, and GI lesions. The level of expression of HDAC2 in EE was significantly higher than secretory phase endometrium (SE).
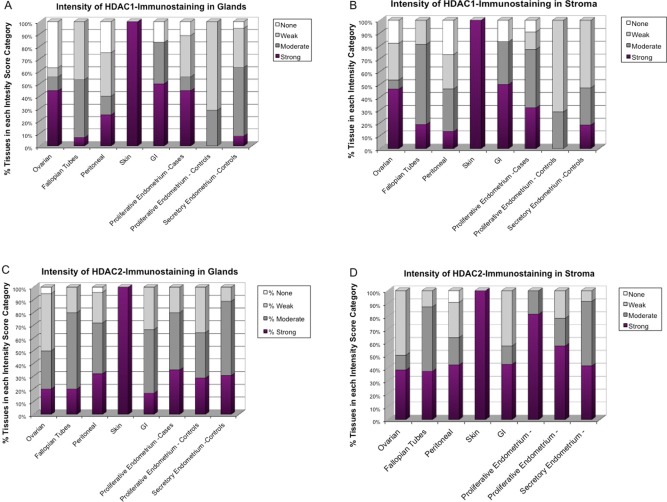
Proportion of Tissues in Each HDAC1/2 Immunostaining Intensity in Human Endometrial and Endometriotic Tissues
In skin endometriosis, 100% (4 of 4) of tissues stained strongly for HDAC1 in both stroma and glands. Significantly higher proportions of tissues with strong HDAC1 immunostaining were observed in GI lesions (50% in glands and 50% in stroma) and ovarian lesions (44% in glands and 46% in stroma). Almost half of EE tissues stained strongly for HDAC1 in glands (44%; P = .056) and less in stroma (32%), but this was higher than strongly staining PE (0% in glands and 0% in stroma) and SE (10% in glands and 18% in stroma). The proportion of tissues with strong HDAC2 immunostaining was higher in skin (100% in glands and stroma), followed by peritoneum (32% in glands and 42% in stroma), GI (17% in glands and 43% in stroma), and ovarian (20% in glands and 39% in stroma). A higher proportion of EE had strong expression of HDAC2 in stroma (82%), but not in glands (35%), compared to PE (29% in glands and 57% in stroma) and SE (27% in glands and 40% in stroma; Figures 5 and 6).
Figure 5.
Proportion of tissues in each histone deacetylase (HDAC)1/2 immunostaining intensity category in endometrial and endometriotic tissues. The proportion of tissues in each tissue type categorized as having strong (3), moderate (2), weak (1), or no (0) immunostaining for HDAC1 and HDAC2 was determined. At the protein level, HDAC1/2 were differentially expressed according to lesion localization, with the highest proportion of positive HDAC1 immunostaining seen in ovarian, skin, and gastrointestinal (GI) lesions, and of HDAC2 in skin and endometria of patients with endometriosis.
Figure 6.
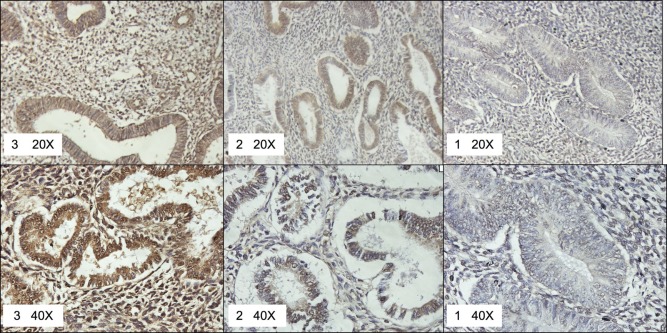
Representative pictures of histone deacetylase (HDAC)1-specific intensities scored as strong (3), moderate (2), or weak (1) on the tissue microarray (TMA). ×20 and ×40 pictures are provided for detail.
Discussion
Epigenetic mechanisms are emerging as important regulators of gene expression in endometriosis and other inflammatory diseases. This study shows for the first time that (i) levels of gene and protein expression of HDAC1/2 are higher in an endometriotic stromal cell line compared to a normal endometrial stromal cell line, (ii) a substantial proportion of tissues from patients express high mRNA levels of HDAC1/2, (iii) their genes are regulated by ovarian steroid hormones in endometrial cells; and (iv) HDAC1/2 proteins are differentially expressed in endometriotic tissues depending on lesion type/localization.
Histone acetylation and deacetylation, regulated by the counterbalanced action of histone acetyl transferases and HDACs, act in concert to regulate gene expression by changing the conformation of the chromatin.13 Histones in transcriptionally active regions of the genome are generally hyperacetylated. Aberrant HDAC levels or sustained HDAC activity would therefore result in histone deacetylation, tightly packaged chromatin, and downregulation of gene transcription. It has been demonstrated that HDACi can reduce the size of lesions and relieve pain symptoms in mice with experimentally induced endometriosis, suggesting that HDACi could potentially be used as a novel alternative for endometriosis treatment.18 However, there is a need to identify the HDAC isoforms that are relevant in this context and to dissect the specific mechanisms activated in the endometriotic lesions via HDAC expression and function. This understanding will also help explain why endometriotic cells share some molecular mechanisms with cancer cells but do not frequently transform and rarely metastasize.
We also assessed whether gene expression levels of HDAC1/2 are regulated by E2 and/or P4. Histone deacetylase 1 gene expression was significantly downregulated by E2 + P4 in endometrial epithelial cells. In contrast, HDAC2 was upregulated by E2 in stromal cells, while E2 + P4 brought levels back to baseline. Thus, HDAC1/2 expression appears to be downregulated during the secretory phase of the menstrual cycle, when both E2 and P4 levels are elevated, which is consistent with the increase in levels of acetylated H3 and H4 by E2 + P4 in HESCs and EECs.27,28 This pattern of hormonal regulation was lost in the endometriotic cell line. Taken together, these data suggest that due to the known resistance to P4 of endometriotic lesions, downregulation of HDAC1/2 expression is lost leading to increased levels in endometriosis.29 This, in turn, would result in a hypoacetylated state and aberrant gene expression profiles. Ongoing studies in our laboratory are designed to identity genes that are regulated by this mechanism.
Expectedly, HDAC1/2 gene expression levels in fresh frozen human endometriotic tissues were highly heterogeneous. Also, both genes were expressed in a substantial proportion of eutopic endometria from women with endometriosis and of women with uterine fibroids, indicating that aberrant histone deacetylation activity could also play a role in other gynecological diseases. There were no statistically significant differences in the mean expression levels of HDAC1/2 according to menstrual cycle phase, but this study is limited in that normal endometrium samples from healthy volunteers were unavailable for analysis. Interestingly, expression levels of HDAC1/2 were significantly correlated in endometriosis lesions but not in the endometria of women with endometriosis or with other gynecologic diseases. We speculate that HDAC1/2 are co-regulated within the lesions due to their particular inflammatory and hormonal microenvironment.
The observed coexpression and apparent coregulation of HDAC1/2 has important implications in regard to their function as regulators of gene expression.30,31 Histone deacetylase 1/2 are conserved enzymes that are part of the Sin3a repressor complex. Sin3a transcriptionally regulates steroidogenic acute regulatory protein gene, glucocorticoid receptor-mediated transcription, and Snail-mediated E-cadherin repression.32 ,33 Also, HDAC1/2 have overlapping functions in cell cycle regulation and their expression has been correlated with Ki-67 levels.34,35 Their silencing leads to cell cycle arrest and upregulation of the cyclin-dependent kinase inhibitor p21. 36 This correlates with the work by Wu and Guo, showing that HDACi decreased proliferation of an endometriotic cell line by upregulating p21, first uncovering an important role of histone modifications in the pathophysiology of endometriosis.
Eutopic, cycling endometrium has been shown to express Class I HDACs constitutively throughout the menstrual cycle. At the protein level, HDAC2 was found to be slightly elevated during the secretory phase.37 Recently, the profile of global histone acetylation in normal endometrium was assessed.38 In this study, global acetylation was shown to increase when transcription activity is likely to be higher. These data indicate that histone acetylation/deacetylation is hormonally regulated and that abnormal epigenetic modifications may be associated with endometrial pathologies.
We also assessed protein expression levels and cellular localization of HDAC1/2 using an endometriosis TMA. To our knowledge, ours is just the second study of protein expression using this powerful technology. We observed that HDAC1 was highly expressed in skin, GI, and ovarian endometriosis, and less in lesions localized in fallopian tubes or peritoneum; HDAC1 protein expression was also higher in the endometria of patients than controls. Histone deacetylase 2 showed a similar pattern of expression in peritoneal versus ovarian lesions, and it was expressed in a higher proportion of control endometria than HDAC1. It is of great interest to document that not all the lesion types and/or localizations express high levels of HDACs, and that there are statistically significant differences in its expression in glands versus stroma. The relevance of the observed differences in expression related to lesion localization is currently unknown, but we speculate that they may be due to the distinctive etiopathologies of the different lesion types as has been proposed before.39,40
A high degree of heterogeneity in HDAC expression levels has also been described in cancer.41 ,42 Within cancer types the proportion of samples expressing high levels of HDACs ranges from 30% to 95%.41,43 Elevated expression of HDAC1-3 was shown in 61%, 95%, and 83% of endometrial carcinomas and in 61%, 93%, and 84% of ovarian carcinomas, respectively. In ovarian carcinoma, the protein levels of HDAC1-3 increased as lesions progressed from benign to borderline to malignant. Also, levels of expression were stronger in highly proliferative and dedifferentiated colorectal tumors.44 Thus, our results are in agreement with the cancer data in that HDACs expression levels varied considerably among tissue type and localization, and immunostaining was not observed in all tissues studied. Based on our observation that ovarian endometriosis lesions express aberrant HDAC levels compared to peritoneal lesions, together with the knowledge that ovarian endometriosis has been shown to be a risk factor for ovarian cancer,45,46 it can be speculated that epigenetic mechanisms involving histone modifications play an important role in the observed dysregulations of endometriotic cells that, if untreated, may in the long term result in cancer. It would be interesting to correlate the levels of HDAC isoform expression in ovarian lesions with an increased potential for transformation in order to better understand the underlying mechanisms.
This study documents for the first time the expression profile of HDAC1/2 in endometriosis. We showed that HDAC1/2 protein levels vary among lesions, and that a substantial proportion of tissues show aberrant nuclear expression of these enzymes. We speculate that dysregulations in HDAC expression lead to a diseased phenotype characterized by increased cell proliferation and invasion.34 The major limitation of this study is that our comparison group is composed of endometrial tissues from women with uterine fibroids and not women free of any disease. The disadvantage of using such tissues as referent is that we cannot conclusively establish whether healthy endometrium is hypo- or hyper-acetylated as compared to endometriotic tissues. We can only confirm that there are differences in the expression of these genes in 2 different pathological states (a finding that, if validated, could constitute the basis for a diagnostic biomarker). This limitation is due to the fact that our inclusion criteria included laparoscopic confirmation of absence of peritoneal lesions. Since endometriosis requires laparoscopy for diagnosis, women having this type of intervention are unlikely to be free of any other gynecological disease. Despite the limitations of the study, we showed that endometriosis has an epigenetic component that needs to be addressed. Our results should encourage follow-up investigations with tissue free of disease to confirm the role of HDACs in endometriosis.
In summary, these data provide additional evidence in support of a possible role of histone deacetylation in the pathophysiology of endometriosis and other gynecologic diseases and suggest that the use of HDACi as therapeutic agents for endometriosis should take into consideration the localization of lesions and the specific profile of expression of HDAC isoforms, as it is being done for cancer.
Acknowledgments
MC is sponsored by 1F31 HD065431-01A1, PB by 1F31 HD056964-01A1, and AR by PSM RISE Program (R25 GM082406). We thank Dr Asgerally Fazleabas for kindly providing the human endometrial cell lines (HESC and EEC) and Dr Ed Seto for the Class I HDACs antibodies. We acknowledge the technical support of Lynnette Ruiz, Jessica Castellanos, and Elvin Estrada.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the National Institutes of Health [grants numbers: NIH-NICHD R01-HD050559; ARRA Grant # 3 R01 HD050559-04S1; NIH-MBRS S06-GM08239; NIH-NCI 1U56-CA126379-01; NIH-NIGMS R25 GM082406].
References
- 1. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279 [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799 [DOI] [PubMed] [Google Scholar]
- 3. Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril. 2007;87(5):1180–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol. 2007;58(4):330–343 [DOI] [PubMed] [Google Scholar]
- 5. Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril. 2002;77(1):38–42 [DOI] [PubMed] [Google Scholar]
- 6. Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Dechelotte PJ, Mage G. Differential expression of genes in eutopic and ectopic endometrium from patients with ovarian endometriosis. Fertil Steril. 2006;86(3):548–553 [DOI] [PubMed] [Google Scholar]
- 7. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881 [DOI] [PubMed] [Google Scholar]
- 8. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607 [DOI] [PubMed] [Google Scholar]
- 9. Shabason JE, Tofilon PJ, Camphausen K. HDAC inhibitors in cancer care. Oncology (Williston Park). 2010;24(2):180–185 [PMC free article] [PubMed] [Google Scholar]
- 10. Marks PA. HDAC inhibitors: much to learn about effective therapy. Oncology (Williston Park). 2010;24(2):185, 188 [PubMed] [Google Scholar]
- 11. Sleiman SF, Basso M, Mahishi L, et al. Putting the ‘HAT’ back on survival signalling: the promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin Investig Drugs. 2009;18(5):573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bertrand P. Inside HDAC with HDAC inhibitors. Eur J Med Chem. 2010;45(6):2095–2116 [DOI] [PubMed] [Google Scholar]
- 13. Cress WD, Seto E. Histone deacetylases, transcriptional control, and cancer. J Cell Physiol. 2000;184(1):1–16 [DOI] [PubMed] [Google Scholar]
- 14. Wu Y, Starzinski-Powitz A, Guo SW. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod Sci. 2007;14(4):374–382 [DOI] [PubMed] [Google Scholar]
- 15. Wu Y, Starzinski-Powitz A, Guo SW. Capsaicin inhibits proliferation of endometriotic cells in vitro. Gynecol Obstet Invest. 2008;66(1):59–62 [DOI] [PubMed] [Google Scholar]
- 16. Wu Y, Guo SW. Histone deacetylase inhibitors trichostatin A and valproic acid induce cell cycle arrest and p21 expression in immortalized human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):198–203 [DOI] [PubMed] [Google Scholar]
- 17. Imesch P, Fink D, Fedier A. Romidepsin reduces histone deacetylase activity, induces acetylation of histones, inhibits proliferation, and activates apoptosis in immortalized epithelial endometriotic cells. Fertil Steril. 2010;94(7):2838–2842 [DOI] [PubMed] [Google Scholar]
- 18. Lu Y, Nie J, Liu X, Zheng Y, Guo SW. Trichostatin A, a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum Reprod. 2010;25(4):1014–1025 [DOI] [PubMed] [Google Scholar]
- 19. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunliffe VT. Eloquent silence: developmental functions of Class I histone deacetylases. Curr Opin Genet Dev. 2008;18(5):404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hombach-Klonisch S, Kehlen A, Fowler PA, et al. Regulation of functional steroid receptors and ligand-induced responses in telomerase-immortalized human endometrial epithelial cells. J Mol Endocrinol. 2005;34(2):517–534 [DOI] [PubMed] [Google Scholar]
- 22. Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296 [DOI] [PubMed] [Google Scholar]
- 23. Ruiz A, Salvo VA, Ruiz LA, Baez P, Garcia M, Flores I. Basal and steroid hormone-regulated expression of CXCR4 in human endometrium and endometriosis. Reprod Sci. 2010;17(10):894–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821 [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 26. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263 [DOI] [PubMed] [Google Scholar]
- 27. Sakai N, Maruyama T, Sakurai R, et al. Involvement of histone acetylation in ovarian steroid-induced decidualization of human endometrial stromal cells. J Biol Chem. 2003;278(19):16675–16682 [DOI] [PubMed] [Google Scholar]
- 28. Uchida H, Maruyama T, Nagashima T, Asada H, Yoshimura Y. Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2005;146(12):5365–5373 [DOI] [PubMed] [Google Scholar]
- 29. Bulun SE, Cheng YH, Pavone ME, et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo Y, Jian W, Stavreva D, et al. Trans-regulation of histone deacetylase activities through acetylation. J Biol Chem. 2009;284(50):34901–34910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev. 2005;19(13):1581–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24(1):306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grzenda A, Lomberk G, Zhang JS, Urrutia R. Sin3: master scaffold and transcriptional corepressor. Biochim Biophys Acta. 2009;1789(6-8):443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayashi A, Horiuchi A, Kikuchi N, et al. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int J Cancer. 2010;127(6):1332–1346 [DOI] [PubMed] [Google Scholar]
- 35. Wilting RH, Yanover E, Heideman MR, et al. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J. 2010;29(15):2586–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamaguchi T, Cubizolles F, Zhang Y, et al. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010;24(5):455–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krusche CA, Vloet AJ, Classen-Linke I, von Rango U, Beier HM, Alfer J. Class I histone deacetylase expression in the human cyclic endometrium and endometrial adenocarcinomas. Hum Reprod. 2007;22(11):2956–2966 [DOI] [PubMed] [Google Scholar]
- 38. Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310 [DOI] [PubMed] [Google Scholar]
- 39. Nisolle M. Ovarian endometriosis and peritoneal endometriosis: are they different entities from a fertility perspective? Curr Opin Obstet Gynecol. 2002;14(3):283–288 [DOI] [PubMed] [Google Scholar]
- 40. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–596 [DOI] [PubMed] [Google Scholar]
- 41. Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280(2):168–176 [DOI] [PubMed] [Google Scholar]
- 42. Fakhry H, Miyamoto T, Kashima H, et al. Immunohistochemical detection of histone deacetylases in endometrial carcinoma: involvement of histone deacetylase 2 in the proliferation of endometrial carcinoma cells. Hum Pathol. 2010;41(6):848–858 [DOI] [PubMed] [Google Scholar]
- 43. Weichert W, Denkert C, Noske A, et al. Expression of class I histone deacetylases indicates poor prognosis in endometrioid subtypes of ovarian and endometrial carcinomas. Neoplasia. 2008;10(9):1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weichert W, Roske A, Niesporek S, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res. 2008;14(6):1669–1677 [DOI] [PubMed] [Google Scholar]
- 45. Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril. 2008;90(5):1559–1570 [DOI] [PubMed] [Google Scholar]
- 46. Ness RB. Endometriosis and ovarian cancer: thoughts on shared pathophysiology. Am J Obstet Gynecol. 2003;189(1):280–294 [DOI] [PubMed] [Google Scholar]