Abstract
Antenatal maternal long-term hypoxia (LTH) can alter serotonin (5-HT) and calcium (Ca2+) signaling in fetal pulmonary arteries (PAs) and is associated with persistent pulmonary hypertension of the newborn (PPHN). In humans, the antenatal maternal hypoxia can be secondary to smoking, anemia, and chronic obstructive pulmonary disorders. However, the mechanisms of antenatal maternal hypoxia-related PPHN are unresolved. Because both LTH and 5-HT are associated with PPHN, we tested the hypothesis that antenatal maternal LTH can increase 5-HT-mediated PA contraction and associated extracellular Ca2+ influx through L-type Ca2+ channels (CaL), nonselective cation channels (NSCCs), and reverse-mode sodium–calcium exchanger (NCX) in the near-term fetus. We performed wire myography and confocal-Ca2+ imaging approaches on fetal lamb PA (∼140 days of gestation) from normoxic ewes or those acclimatized to high-altitude LTH (3801 m) for ∼110 days. Long-term hypoxia reduced the potency but not the efficacy of 5-HT-induced PA contraction. Ketanserin (100 nmol/L), a 5-HT2A antagonist, shifted 5-HT potency irrespective of LTH, while GR-55562 (1 µmol/L), a 5-HT1B/D inhibitor, antagonized 5-HT-induced contraction in normoxic fetuses only. Various inhibitors for CaL, NSCC, and reverse-mode NCX were used in contraction studies. Contraction was reliant on extracellular Ca2+ regardless of maternal hypoxia, NSCC was more important to contraction than CaL, and reverse-mode NCX had little or no role in contraction. Long-term hypoxia also attenuated the effects of 2-APB and flufenamic acid and reduced Ca2+ responses observed by imaging studies. Overall, LTH reduced 5HT1B/D function and increased NSCC-related Ca2+-dependent contraction in ovine fetuses, which may compromise pulmonary vascular function in the newborn.
Keywords: ion channels, L-type calcium channels, nonselective cation channels, sheep, transient receptor potential channels
Introduction
Antenatal maternal long-term hypoxia (LTH) can lead to pulmonary vascular dysfunction in the fetus and is a risk factor for the development of persistent pulmonary hypertension of the newborn (PPHN). Persistent pulmonary hypertension of the newborn is associated with several conditions including long-term high-altitude exposure, placental insufficiency, and severe maternal anemia.1,2 A recent study suggests that long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension.3 In studies on our near-term fetal sheep that experienced high-altitude gestational LTH, the pulmonary vascular reactivity was markedly altered as a consequence of changes in Rho kinase and cGMP-mediated relaxation.4,5 In addition, gestational LTH accentuated the role of the epidermal growth factor receptor to arterial remodeling.6 These and other aspects of fetal pulmonary vasculature responses to LTH have recently been reviewed.7 Moreover, LTH can modulate the release of several substances from the endothelium and platelets including serotonin (5-hydroxytryptamine [5-HT]), platelet-derived growth factor, and platelet-activating factor, which are pivotal in altered pulmonary arterial (PA) contractility and in development of PPHN.8,9
Direct evidence of 5-HT as a causative agent for PPHN comes from recent studies demonstrating an increased risk of PPHN in infants born to mothers treated with selective serotonin reuptake inhibitors (SSRIs) during pregnancy.10 Long-term hypoxia also enhances 5-HT-induced PA contraction in rats and mice11–13; however, the mechanistic base of these changes is unclear. Nevertheless, 5-HT signaling is important for PA contraction and enhanced reactivity to this inflammatory substance is a feature of pulmonary hypertension.
5-Hydroxytryptamine is one of the most potent PA vasoconstrictors.14,15 Of note, ketanserin inhibits 5-HT-induced contraction of canine PAs, indicating that 5-HT2A-receptor activation is important to arterial reactivity.16 5-HT2A receptors are coupled with Gq proteins and when activated cause inositol 1,4,5-triphosphate-mediated calcium (Ca2+) release from sarcoplasmic reticulum stores and depolarization of the plasma membrane.16 Membrane depolarization activates L-type Ca2+ channels (CaL) and alters the electrochemical gradient acting on the sodium–calcium exchanger (NCX), which shifts the latter into a “reverse mode” providing for additional extracellular Ca2+ entry.17–20 Furthermore, the receptor-coupling processes can also activate nonselective cation channels (NSCCs), allowing for voltage-independent Ca2+ entry.21 Together, these Ca2+ release and entry pathways increase the cytosolic Ca2+ concentration ([Ca2+]i) and stimulate contraction.16
In addition to changes in 5-HT receptor signaling, LTH stress results in multifaceted changes in Ca2+-dependent signaling and contraction in the pulmonary vasculature. Hypoxic stress depresses CaL activity in adult rat PA smooth muscle cells22 but increases activity in myocytes from newborn pigs.23 Long-term hypoxia also augments NSCC activity in PA smooth muscle cells of adult rat.24,25 Reports also show that NCX20 and transient receptor potential (TRP) channels, which encode for NSCCs, contribute to the development of PA hypertension in humans.26–31 Long-term hypoxia also increases platelet-activated factor-induced Ca2+ responses in pulmonary venous smooth muscle cells of fetal sheep,32 which may be a consequence of increased platelet-activated factor receptor expression and resultant inositol 1,4,5-triphosphate generation.32 Although it is well established that chronic hypoxia alters 5-HT-induced vascular reactivity and Ca2+ signaling in mature animals, it is unknown whether antenatal in utero maternal stress also alters PA reactivity and underlying calcium signaling processes in the developing fetus. Thus, we tested the hypothesis that antenatal maternal LTH can increase 5-HT-mediated PA contraction and associated extracellular Ca2+ influx through the CaL, NSCC, and reverse-mode NCX in the near-term fetus.
Materials and Methods
Experimental Animals
All experimental procedures were performed within the regulations of the Animal Welfare Act and the Animal Care and Use Committee of the University of Mississippi and Loma Linda University (LLU). We conducted studies on third- and fourth-order PAs with internal diameters of roughly 500 to 700 μm, from near-term fetal lambs (∼140 days of gestation). Pregnant ewes were obtained from Nebeker Ranch (720 m, Lancaster, California) and, as previously described, were maintained either near sea level (Pao 2 = 95 ± 5 and 25 ± 2 Torr for ewe and fetus, respectively, at LLU, Loma Linda, California [353 m]) or were acclimatized to high altitude (Pao 2 = 60 ± 5 and 19 ± 3 Torr for ewe and fetus, respectively, at Barcroft Laboratory, White Mountain Research Station, Bishop, California [3801 m]) for ~110 days.33 The animals maintained at high altitude were transported to the Center for Perinatal Biology at LLU. Shortly after arrival at the center, a tracheal catheter was placed in the ewe, through which N2 flowed at a rate adjusted to maintain Pao 2 at ∼60 Torr,34 and this was maintained until the time of the experimental study. The pregnant ewes were anesthetized, and the fetus was delivered by hysterectomy, after which the fetuses and ewes were sacrificed as previously described.33 Lungs were removed from the fetal lambs and transported on ice (4°C-5°C) to the University of Mississippi via overnight express or were used for contraction experiments at LLU. To avoid complication from endothelium-mediated effects, the endothelium was removed by carefully inserting a small roughened hypodermic needle or rotating the artery on the mounting wire.16 Endothelium removal was confirmed by adding 100 µmol/L acetylcholine (Ach), while the artery was constricted by 125 mmol/L KCl (high K). Denudation was also confirmed by visual inspection of Fluo-4-loaded arterial strips during confocal Ca2+ imaging studies.
Tissue Preparation
Pulmonary arteries for contraction and Ca2+ imaging studies were dissected free of parenchyma and cut into 5-mm-long rings in ice-cold modified Krebs–Henseleit (K-H) solution at 4°C to 5°C containing in millimoles per liter: 120 NaCl, 4.8 KCl, 1.2 K2HPO4, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, and 10 glucose; or in a phosphate-free balanced salt solution (BSS) of the following composition (mmol/L): 126 NaCl, 5 KCl, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 MgCl2, 2 CaCl2, 10 glucose, pH 7.4 (adjusted with NaOH).
Contraction Studies
Pulmonary arterial rings were suspended in organ baths (Radnoti Glass Instruments, Inc, Monrovia, California) that contained 5 or 10 mL of modified K–H buffer maintained at 37°C and aerated with 95% O2 and 5% CO2 (pH = 7.4) as described in our previous studies.16,35,36 Arterial rings treated with La3+, Gd3+, or Ni2+ were bathed in nonaerated phosphate-free BSS to prevent metal chelation.37 Isometric force was recorded using low-compliance force transducers (Radnoti Glass Instruments, Inc) and analogue to digital data collection systems and software (Digidata 1200/pClamp 8.1; Molecular Devices, Sunnyvale, California; MIO-16/Labview; National Instruments, Austin, Texas; Powerlab 16/30/Chart 5.5; A/D Instruments, Colorado Springs, Colorado; or MP100/AcqKnowledge 3.9; Biopac, Goleta, California) as previously described.16 At the beginning of each experiment, vessels were equilibrated without tension for 30 minutes to 1 hour. In an initial series of studies, vessel rings were stretched progressively to obtain a maximum contraction response with 125 mmol/L KCl stimulation. Vessels were then allowed to stabilize for 30 to 45 minutes and optimize to their own basal tension, which was 588 ± 20 dynes in 18 arteries from 5 normoxic fetuses and 666 ± 29 dynes in 16 arteries from 5 hypoxic fetuses. In subsequent experiments, vessel rings were stretched progressively and allowed to attain their own optimal basal tension, which was 510 ± 15 dynes in 228 vessels from 28 normoxic fetuses and 463 ± 9 dynes in 278 vessels from 29 LTH fetuses.16 In most experiments, the tension was normalized for comparison to either an initial response obtained with 10 µmol/L 5-HT (%T5-HT control) or due to high K (%TKmax). The internal lumen diameter was calculated using geometric principles, as the width of longitudinally cut arteries is equivalent to circumference (diameter = circumference/π). The calculated lumen diameters were 537 ± 117 µm in 193 vessels from normoxic fetuses and 541 ± 88 µm in 276 vessels from LTH fetuses. For evaluating dose–response characteristics, arteries were stimulated by cumulatively applying 1 nmol/L to 100 µmol/L 5-HT in log increments without washing in between each 5-HT concentration increase.
Confocal Ca2 +-Imaging Studies
The [Ca2+]i was measured in PA myocytes in situ, with the Ca2+-sensitive dye Fluo-4 AM (Invitrogen, Carlsbad, California) using a Zeiss 510 META laser scanning confocal imaging workstation (Zeiss Microimaging, Thornwood, New York) with an inverted microscope (Axiovert 200). Fluo-4 AM was dissolved in dimethyl sulfoxide (DMSO) with 0.1% pluronic F127 and added at a final concentration of 10 μmol/L to denuded arteries for 1 to 1.5 hours at room temperature in the dark. Arterial segments were then washed for 30 minutes to allow for dye esterification and cut into linear strips. The arterial segments were pinned to Sylgard blocks (Ellsworth Adhesives, Germantown, Wisconsin) and placed in an open bath imaging chamber (Warner Instruments, Hamden, Connecticut), mounted on the confocal imaging stage. In some cases, the arteries were perfused at ∼1 mL/minute using a peristaltic pump (Rainin, Oakland, California) with an electronic pinch valve system (Automate Scientific, Berkeley, California), while in others, the arteries were maintained in a static bath. Cells were illuminated at 488 nm with a krypton argon laser and the emitted light was collected using a photomultiplier tube (PMT) for frames of 512 × 512 pixels with a long-pass filter >505 nm. Full-frame images were acquired every 700 to 900 ms at an imaging depth of ∼10 μm. This depth is roughly equivalent to the width of 2 cells based on morphological examination of fixed and live preparations (data not shown). Generally, images were acquired at a 12-bit sampling depth, but an 8-bit depth was used occasionally. Most recordings were made using a water immersion ×40 c-Apochromat (numerical aperture, NA 1.2) objective, but a ×20 nonimmersion (NA 0.8) or ×63 oil immersion Plan Apochromat (NA 1.4) was also used. The acquisition depth and objective used did not influence the spatial–temporal characteristics presented in this study. Regions of interest (ROIs) were examined post hoc and analyzed using Zeiss LSM image examiner. For presentation purposes, the fractional fluorescence intensity was calculated:
where baseline is the intensity from an ROI with no cells, F is the fluorescence intensity for ROI, and F o is the fluorescence intensity during a period from the beginning of the recording when there was no Ca2+ activity.
In a separate control study, the numbers of nuclei were recorded to estimate the percentage of myocytes with Ca2+ oscillations. These recordings were made in parallel arteries from the same groups of animals, which were labeled with Cy3-conjugated anti-α-SM actin (Sigma-Aldrich, Saint Louis, Missouri) and sealed with Vectashield mounting medium containing 4',6-diamidino-2-phenylindole ([DAPI]; H-1200; Vector Labs, Burlingame, California). Images were recorded serially through the whole arterial wall; from the endothelial through the adventitial layers at ∼0.3 μm increments 1024 × 1024, 12 bit using a ×63 oil immersion Plan Apochromat (NA 1.4) objective. A pinhole size corresponding to an imaging depth of ∼0.6 μm was used, which resulted in overlapping images through the z-axis. Once mounted, preparations were imaged by exciting Cy3 (543 nm excitation, >560 emission) and DAPI (405 nm excitation, 420-480 nm band-pass emission) on a Zeiss 510 META confocal microscope. Regions staining positive for Cy3 and DAPI ranged between 10 and 13 per 1000 μm2, and there were no significant differences between vessels obtained from normoxic and LTH fetal lambs. The density of cells firing was determined in live cell preparations by counting the number of myocytes with Ca2 + oscillations, which was compared against the number of nuclei in the fixed preparations. These Ca2 + recordings were made in 33 ROIs in control and 30 ROIs in the presence of 10 µmol/L 5-HT from 4 normoxic and 15 ROIs in each condition from 5 LTH fetal lambs.
Chemicals and Drugs
Most reagents and chemicals were purchased from Sigma-Aldrich. Fluo-4 AM and Pluronic F127 were purchased from Invitrogen, KB-R7943, LOE 908, and SN-6 from Tocris (Ellisville, Missouri), and SKF 96365 from Tocris or Sigma-Aldrich.
Statistical Methods
All time series recordings were graphed with IGOR pro 6.0 (Wavemetrics, Lake Oswego, Oregon), and the data were presented as mean ± standard error of the mean (SEM). Statistical analyses were made using GraphPad Prism 5.0 (La Jolla, California). For most studies, comparisons were made within and among groups using a 2-way analysis of variance (ANOVA) and a Bonferroni post hoc analysis. In some cases, comparisons were made using an unpaired Student t test. Dose–response curves were fitted in Prism 5.0 using a Hill equation.16 The N values reported reflect the total number of arterial segments and total number of sheep tested. A P value of <.05 was accepted as statistically significant.
Results
We stimulated isolated PA rings with high K, which depolarizes the membrane and activates CaL channels. This procedure allows for normalization of arterial contraction to the relative quantity of contractile smooth muscle and CaL channel activation.35,38,39 Stimulation with high K caused a contraction of 493 ± 73 dynes in vessels from 17 normoxic fetuses and a contraction of 452 ± 47 dynes in vessels from 22 LTH fetuses. These values were not statistically different (unpaired t test), demonstrating that depolarization-induced contraction is well conserved during maternal LTH acclimatization to high altitude.
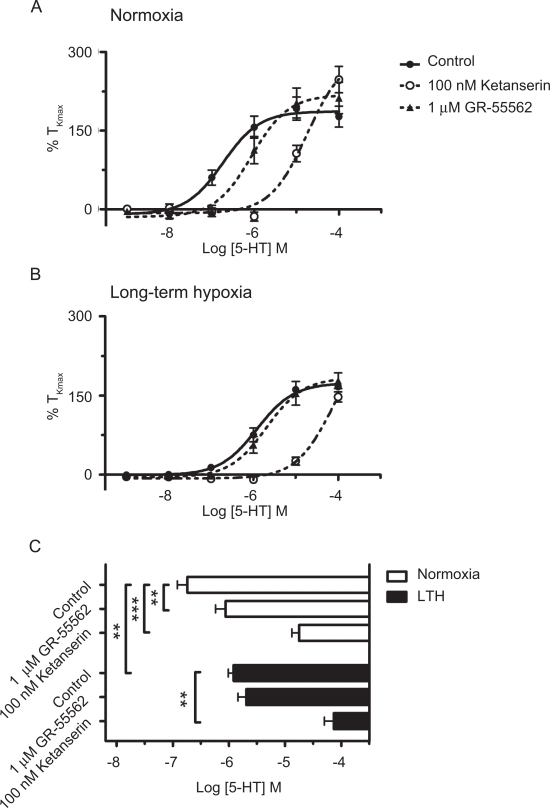
We applied cumulative doses of 5-HT to PA segments from normoxic and LTH animals to assess the general ability of 5-HT to bind to its receptor and cause a contraction response.16,40 Using Hill’s equation, the data provide estimates of potential changes in the sensitivity of arterial contraction measured as the half-maximum Ca2 + contractile response (EC50). Data from these experiments also provide a determination of the contraction response to different 5-HT concentrations. The dose–response data in Figure 1A and B and summarized EC50 data in Figure 1C show that antenatal maternal LTH decreased the sensitivity to 5-HT as there was a rightward shift of the dose–response curves for 5-HT, from the log value of −6.74 ± 0.18 to −5.92 ± 0.09 mol/L. The effectiveness for 5-HT was then determined by comparing the tensions recorded near to the EC50 (100 nmol/L) and at the maximum response (Emax) for the agonist (10 µmol/L). As shown in Figure 1A and B, the contraction due to 100 nmol/L 5-HT was significantly reduced from 60% ± 14% TKmax in normoxic fetuses (N = 19/6) to 14% ± 6% TKmax in LTH fetuses (N = 11/3; P < .05, unpaired t test). In comparison, at 10 μmol/L 5-HT, the contraction was unchanged by LTH, being 663 ± 50 dynes in vessels from 22 normoxic fetuses and 707 ± 69 dynes in vessels from 20 LTH fetuses (unpaired t test). Thus, these experiments demonstrate that antenatal maternal LTH desensitized the PAs to 5-HT at lower 5-HT contractions. However, the maximum contraction attained by 5-HT was maintained.
Figure 1.
Serotonin potency is diminished by maternal LTH. Isometric tension recordings of pulmonary arterial rings exposed to 1 nmol/L to 100 μmol/L of 5-hydroxytryptamine (5-HT) in an additive manner and corresponding dose–response relationship. Data were fitted using Hill equation to the mean values normalized to %TKmax (percentage contraction compared to initial stimulation with high K) for (A) normoxic and (B) LTH vessels from fetal lambs, in the presence or absence of 100 nmol/L ketanserin or 1 μmol/L GR-55562. (C) Comparison of mean ± SEM for EC50 values between normoxic (open) and LTH (solid) in pulmonary arteries of fetal sheep in the presence or absence of 100 nmol/L ketanserin or 1 μmol/L GR-55562. **P < .01 and ***P < .001 denote significant difference between the EC50 based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; SEM, standard error of the mean; ANOVA, analysis of variance.
To delineate further LTH-mediated changes in 5-HT2A and 5-HT1B/D receptor subtypes, we conducted experiments in the presence and absence of selective antagonists for 5-HT1B/D receptors (1 μmol/L GR-55562) and 5-HT2A receptors (100 nmol/L ketanserin). Figure 1A and B shows the dose–response curves for 5-HT and the inhibitory influence of 5HT1B/D and 5HT2A receptor antagonists. Figure 1C compares the EC50 values for 5-HT and the actions of the receptor inhibitors. 5-HT1B/D receptors were inhibited with 1 μmol/L GR-55562 because it has pKB values of 7.3 and 6.3 for human-cloned 5-HT1B and 5-HT1D receptors, respectively, and only weak binding to a number of other 5-HT subtypes.41 The EC50 for 5-HT was significantly shifted from −6.74 ± 0.18 mol/L (N = 19/6) to −6.06 ± 0.18 mol/L (N = 17/5) in normoxic animals by 1 μmol/L GR-55562; however, in LTH animals (N = 15/3), the EC50 was not shifted. Because 5-HT2A receptors are also important for PA reactivity, we inhibited these with 100 nmol/L ketanserin, a concentration that preferentially inhibits 5-HT2A receptors.16,42,43 The EC50 for 5-HT was shifted approximately 2 log units by 100 nmol/L ketanserin in vessels from both normoxic and LTH fetuses, being −4.74 ± 0.09 mol/L (N = 11/3) and −4.13 ± 0.17 mol/L (N = 15/3), respectively. These experiments demonstrate that 5-HT2A receptors are predominantly involved in 5-HT-induced contractile responses in both normoxic and LTH fetuses, and LTH decreases the role of 5HT1B/D receptors.
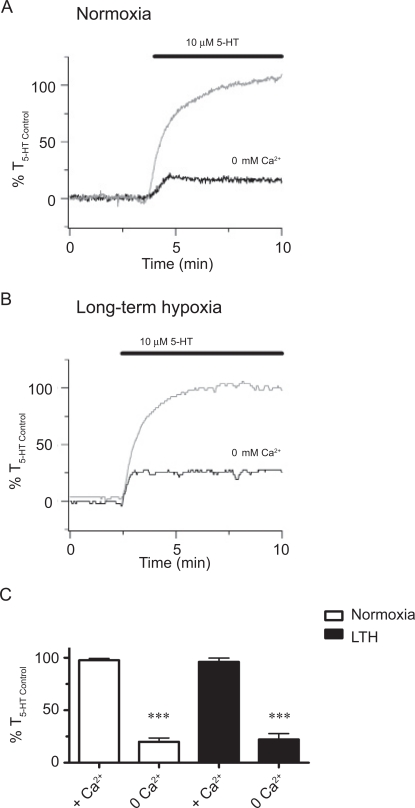
To examine the extent to which LTH alters the role of extracellular Ca2+ entry with 5-HT-elicited PA contraction, we conducted studies in the presence and absence of extracellular Ca2+. Figure 2A and B shows representative traces of 5-HT-elicited contraction in the presence as well as in the absence of extracellular Ca2 + for arteries from normoxic and LTH fetuses, respectively. These figures show that 5-HT elicited repeatable contractions in the continued presence of extracellular Ca2 +. Removal of extracellular Ca2 + substantially reduced arterial contraction whether or not the vessels were from LTH fetuses. Figure 2C provides averaged data showing that removal of extracellular Ca2 + reduced 5-HT-induced contraction to 20% ± 4% (N = 9/3) and 22% ± 6% (N = 11/4) in PAs from normoxic and LTH fetuses, respectively. Reintroduction of Ca2 + to the bathing solution recovered 5-HT-elicited contraction to control levels in several experiments (data not shown). These experiments demonstrate that extracellular Ca2 + is important in 5-HT-induced contraction during normoxia and LTH.
Figure 2.
Extracellular Ca2+ is vital for 5-hydroxytryptamine (5-HT)-elicited pulmonary arterial contraction. Isometric tension recording of pulmonary arterial rings, constricted in the absence of 2 mmol/L extracellular Ca2+ (black), or in the presence of extracellular Ca2+ (gray) from (A) normoxic and (B) LTH fetal lambs. Tracings are plotted in relation to maximal contraction from an initial 10 μmol/L 5-HT stimulation (%T5-HT Control). (C) Bars indicate mean ± SEM of %T5-HT Control in the presence or absence of extracellular Ca2+ for pulmonary arteries from normoxic (open) and LTH (solid) fetuses. ***Denotes significant difference between the presence and absence of extracellular Ca2+ by an unpaired Student t test (P < .001). LTH indicates long-term hypoxia; SEM, standard error of the mean.
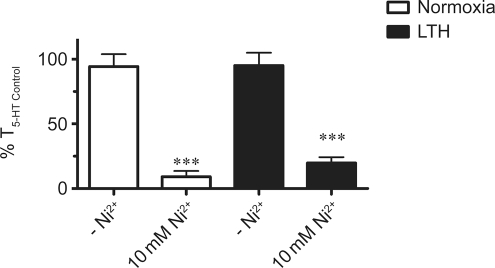
We conducted the next series of experiments to limit extracellular Ca2+ influx independent of any potential depletion of the intracellular Ca2+ stores.16,44 This is important as removal of extracellular Ca2+ can reduce the Ca2+ concentration in the sarcoplasmic reticulum and thereby limit the Ca2+ responses due to 5-HT. Extracellular Ca2+ entry was selectively reduced by treating arteries with 10 mmol/L Ni2+ because at a high concentration, Ni2+ blocks plasma membrane Ca2+ entry without inhibiting intracellular Ca2+ release.16 Figure 3 demonstrates the influence of 10 mmol/L Ni2+ on 10 μmol/L 5-HT-induced contractions in PAs from normoxic and LTH fetuses. Exposure to Ni2+ reduced 5-HT-elicited contraction to a similar extent as extracellular Ca2+ removal. Figure 3 shows that 10 mmol/L Ni2+ reduced 5-HT-dependent contraction to 9% ± 4% (N = 10/3) and 20% ± 5% (N = 12/3) as compared to the initial 5-HT contractile response (T5-HT Control) in PAs from normoxic and LTH fetuses, respectively. Together, Figures 2 and 3 demonstrate that extracellular Ca2+ is important to 5-HT-induced contraction in fetal PAs and its role is preserved following LTH.
Figure 3.
Ni2+ blocks 5-hydroxytryptamine (5-HT)-mediated pulmonary arterial contraction. Bars indicate mean ± SEM of %T5-HT Control in the absence or presence of 10 mmol/L Ni2+ for arteries from normoxic (open) and LTH (solid) fetuses.**P < .01 denotes significant difference between the absence and presence of 10 mmol/L Ni2+ by an unpaired Student t test. LTH indicates long-term hypoxia; SEM, standard error of the mean.
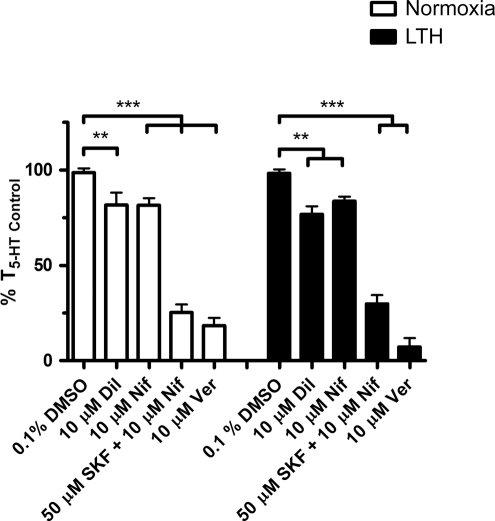
Extracellular Ca2 + enters across the plasma membrane chiefly through CaL channels, NSCC, and reverse-mode NCX. We designed subsequent studies to assess the extent to which LTH limits the role of each pathway in 5-HT-mediated PA contraction. For CaL, this was achieved by stimulating arteries with 10 μmol/L 5-HT in the presence and absence of 3 major classes of CaL channel blockers, including dihydropyridines (10 μmol/L nifedipine), benzothiazepines (10 μmol/L diltiazem), and phenylalkylamines (10 μmol/L verapamil). Of the 3 groups of calcium channel blockers, dihydropyridines are most specific for systemic arteries (Figure 4). Phenylalkylamines in comparison are more cardiospecific and benzothiazepines are in between with regard to their activity against systemic arteries and cardiac L-type channels.45 Thus, nifedipine was our first choice for blocking PA L-type calcium channels. The summarized data shows that contraction was unaffected by vehicle (0.1% DMSO), being 99% ± 2% (N = 40/11) and 98% ± 2% (N = 22/7) of T5-HT Control in PAs from normoxic and LTH fetuses, respectively. However, 10 μmol/L nifedipine reduced the 5-HT-induced contractile response to 82% ± 4% (N = 23/7) and 84% ± 2% (N = 23/9) of T5-HT Control in PAs from normoxic and LTH fetuses, respectively. Similarly, diltiazem (10 μmol/L) reduced 5-HT-induced contraction in vessels from normoxic (82% ± 6%; N = 17/5) and LTH (77% ± 4%; N = 10/3) fetuses compared to T5-HT Control. Interestingly, 10 μmol/L verapamil, a widely used CaL antagonist, reduced 5-HT-elicited tension to 18% ± 4% (N = 19/5) in normoxic and 7% ± 5% (N = 10/3) in LTH fetal PAs compared to T5-HT Control.
Figure 4.
L-type Ca2 + channels are important to 5-hydroxytryptamine (5-HT)-mediated pulmonary arterial contraction. Bars indicate mean ± SEM of %T5-HT Control in the presence or absence of the diltiazem (Dil), nifedipine (Nif), or verapamil (Ver) in pulmonary arteries from normoxic (open) and LTH (solid) fetal sheep. **P < .01 and ***P < .001 denote significant difference based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; SEM, standard error of the mean; ANOVA, analysis of variance.
To examine the role of NSCC during 5-HT-induced contractility, we treated PAs with 50 μmol/L SKF-96365 in the presence of 10 μmol/L nifedipine.46 ,47 The antagonists were applied simultaneously because SKF-96365 blocks CaL in the same concentration range in which it inhibits NSCC.46 The combination of 10 μmol/L nifedipine and 50 μmol/L SKF-96365 reduced 5-HT-induced contraction to 25% ± 4% (N = 21/6) and 30% ± 4% (N = 18/6) of T5-HT Control in PAs from normoxic and LTH fetuses, respectively. Thus, these experiments demonstrate that both voltage-dependent and -independent channels are important to 5-HT-induced contraction and their overall function is preserved following LTH.
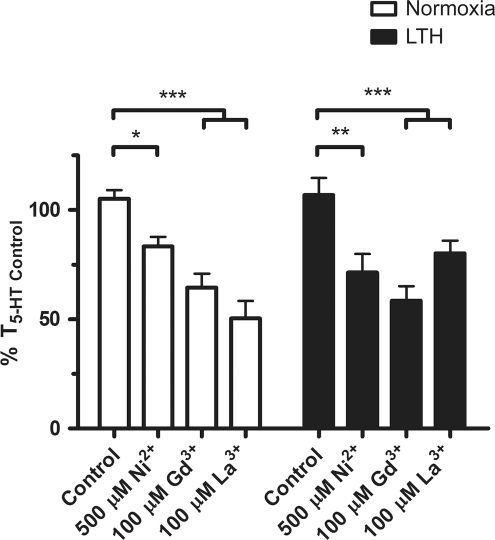
Because the combination of SKF-96365 and nifedipine relaxed PAs to a similar extent as extracellular Ca2 + removal, 10 mmol/L Ni2+ or 10 μmol/L verapamil, we conducted additional experiments to discriminate pharmacologically the various canonical TRP (TRPC) channels inhibited by SKF-96365, as well as melanostatin TRP (TRPM), vanilloid TRP (TRPV), and Stromal interaction molecule/Calcium release-activated calcium modulator 1 (STIM/ORAI) channels that may be important to pulmonary vascular tone. To elucidate the role of Ca2 + entry through TRP channels in 5-HT-induced contraction, we inhibited 5-HT-elicited contraction with various inorganic inhibitors (Figure 5). We used 500 μmol/L Ni2 +, 100 μmol/L Gd3 +, and 100 μmol/L La3 + as they are ubiquitous TRP channel antagonists.48 The summarized data show that 500 μmol/L Ni2 + reduced the 5-HT-induced PA tension to 84% ± 4% (N = 14/5) and 71% ± 9% (N = 9/3) of T5-HT Control, from normoxic and LTH fetuses, respectively. The actions of 100 μmol/L Gd3 + were more pronounced than 500 μmol/L Ni2 +, with the tension being reduced to 65% ± 6% (N = 11/4) in normoxic and 58% ± 7% (N = 9/3) in LTH fetal PAs. 5-HT-induced tension was reduced more substantially by 100 μmol/L La3 + in PAs from normoxic (51% ± 8%; N = 6/3) as compared to those from LTH (80% ± 6%; N = 10/3) fetuses.
Figure 5.
Gd3+, La3+, and low-dose Ni2+ inhibit 5-hydroxytryptamine (5-HT)-elicited contraction. Bars indicate mean ± SEM of %T5-HT Control for the noted condition in pulmonary arterial rings from normoxic (open) or LTH (solid) fetal sheep. *P < .05, **P < .01, and ***P < .001 denote significant difference based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; SEM, standard error of the mean; ANOVA, analysis of variance.
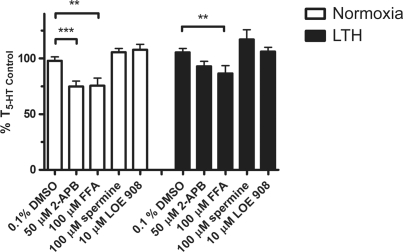
5-HT-induced contraction was then challenged with organic inhibitors of NSCC that have greater selectivity toward various TRP channels. This included 2-APB, flufenamic acid (FFA), LOE 908, and spermine, which block a variety of different TRP channel isoforms. 2-APB blocks TRPC1, 3, 4, 5, 6 and TRPM3, 7, 8 channels.48,49 Figure 6 shows 2-APB (50 μmol/L) reduced 5-HT-induced contraction to 73% ± 5% (N = 24/9) of T5-HT Control in vessels from normoxic fetuses. However, 2-APB failed to inhibit 5-HT-induced contraction in PAs from LTH fetuses, with tension being 93% ± 4% (N = 13/7) of T5-HT Control. Flufenamic acid, which blocks48,49 TRPC3, 5, and 7 and TRPM2, 4, 5 but may activate TRPC6, also had a lesser effect on 5-HT-induced contraction in LTH compared to normoxic fetuses. Contractile force was reduced to 76% ± 7% (N = 15/5) in vessels from normoxic fetuses and 87% ± 7% (N = 21/7) in those from LTH animals. LOE 908 inhibits native NSCC in A7R5 myocytes that are activated in response to arginine vasopressin but not store depletion.50,51 Spermine48,49 in comparison preferentially blocks TRPM4, 5, and 7. The influence of these TRP channel inhibitors on contraction shows that LTH causes subtle changes in the pharmacology that implicate potential changes in the expression of TRPC or TRPM gene products. However, these pharmacological antagonists cannot resolve the involvement of specific TRP gene product/products.
Figure 6.
The actions of 2-APB and flufenamic acid (FFA) on 5-hydroxytryptamine (5-HT)-elicited pulmonary arterial contraction are reduced by antenatal maternal LTH. Bars indicate mean ± SEM of %T5-HT Control for the noted condition in pulmonary arterial rings from normoxic (open) or LTH (solid) fetal sheep. **P < .01 and ***P < .001 denote significant difference from control based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; SEM, standard error of the mean; ANOVA, analysis of variance.
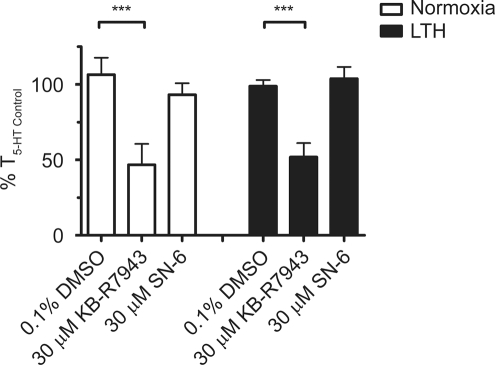
In addition to activation of extracellular Ca2+ influx through CaL and NSCC pathways, membrane depolarization may also accentuate Ca2+ entry through the NCX. Under basal conditions, the cell membrane is at a negative resting potential, and the NCX plays a role in extrusion of Ca2+ from the cytosol counterbalancing inward Na+ movement along its electrochemical gradient. During cell stimulation, NSCC increases Na+ entry and depolarizes the plasma membrane. The combination of increased cytosolic Na+ and reduced electrochemical driving force on Na+ reverses the electrochemical forces on the exchanger, thereby allowing for Ca2+ influx.52 Figure 7 shows the reduction in 5-HT-mediated contraction by KB-R7943 and SN-6. KB-R7943 inhibits the activity19,48,53 of NCX1, 2, 3, and TRPC3, 5, and 6. In comparison, SN-6 blocks NCX1, 2, and 3 without any known effect on TRPC channels.54 This figure shows that 30 μmol/L KB-R7943 reduced 5-HT-induced contraction to 47% ± 14% (N = 10/4) of T5-HT Control in normoxic and 52% ± 9% (N = 16/5) of T5-HT Control in LTH fetal pulmonary vessels. Of interest, SN-6 did not reduce 5-HT-induced contraction in either group. The results of these experiments suggest that reverse-mode NCX is not vital to 5-HT-induced contraction in ovine fetal PAs.
Figure 7.
The effect of NCX antagonists on pulmonary arterial ring contraction is not influenced by antenatal maternal LTH in fetal sheep. Bars indicate mean ± SEM of %T5-HT Control for the noted condition in pulmonary arterial rings from normoxic (open) or LTH (solid) fetal sheep. ***P < .001 denotes significant difference based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; 5-HT, 5-hydroxytryptamine; SEM, standard error of the mean; ANOVA, analysis of variance.
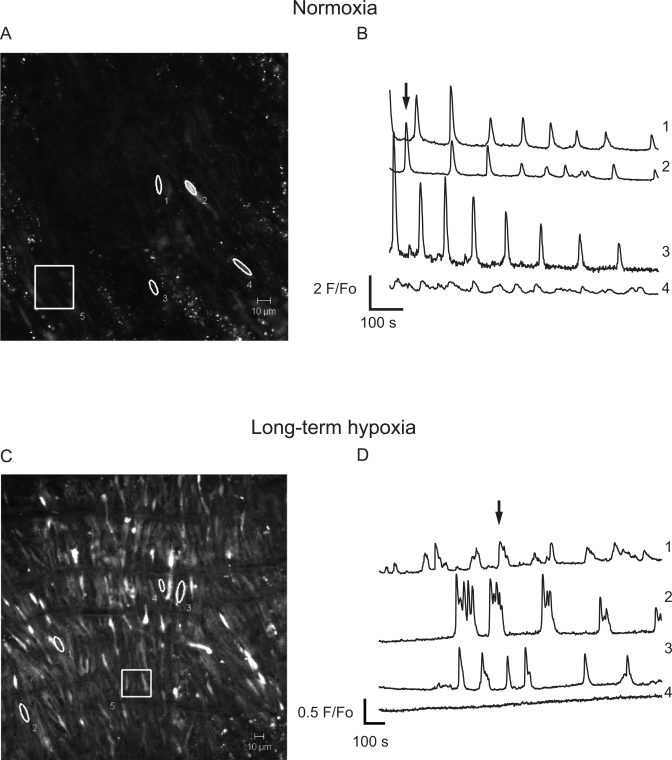
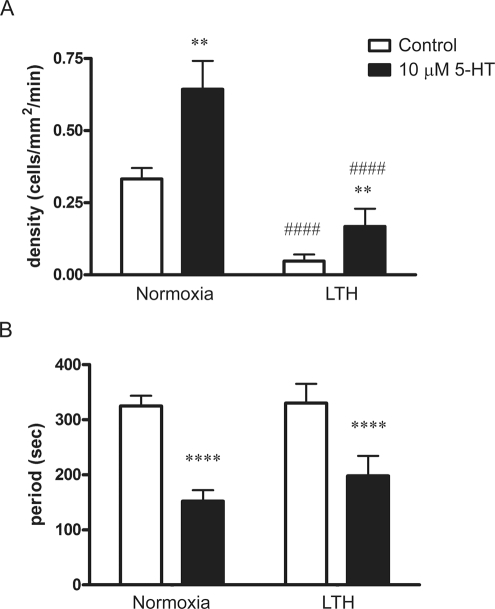
We conducted a series of confocal imaging studies on fetal ovine PA segments to directly examine the potential alterations in intracellular Ca2+ responses to 5-HT. These studies included measurements of the Ca2+-signaling activity of individual myocytes and their oscillatory signaling behavior. The Ca2+ imaging studies were conducted because our previous work indicates that Ca2+ signaling is central to 5-HT contraction of PAs.16,55 In these experiments, arterial segments were stimulated with 10 µmol/L 5-HT and cytosolic Ca2+ was monitored in single myocytes within the intact arterial wall. This in situ approach was achieved using the Ca2+ reporter Fluo-4 and laser scanning confocal microscopy techniques, as described in the methods. Figure 8 shows micrographs of the Fluo-4 signal and associated time series traces for PA myocytes recorded in the intact arterial wall, from normoxic and LTH fetuses. Figure 8A (normoxia) and C (LTH) provides micrographs of ROIs at the time points shown by the arrows on their respective time series traces in Figure 8B (normoxia) and D (LTH). Each ROI numbered 1 through 4 corresponds to an individual cell in the arterial wall and is the baseline subtracted and fractional fluorescence intensity tracing. The cell–cell variability in frequency and fluorescence-amplitude indicates the complexity of the Ca2+-signaling events. In Figure 8A and C, region 5 is a ∼33- × 33-μm box (1000 μm2) and is representative of the area used to measure the number of responsive cells in live preparations.
Figure 8.
Effect of antenatal maternal LTH on 5-hydroxytryptamine (5-HT)-elicited Ca2+ signaling recorded in situ. Micrographs of the fluo-4 fluorescence from pulmonary arteries from (A) normoxic and (C) LTH fetal lambs. Regions 1 to 4 indicates individual pulmonary arterial myocytes and region 5 represents a 1000 μm2 area used to calculate the number of responsive cells in each arterial segment. Average baseline-subtracted fractional fluorescence (F/F o) traces are shown for regions 1 to 4 for pulmonary arteries from (B) normoxic and (D) LTH fetuses. Arrows in panels B and D indicate the time corresponding to the associated fluorescence micrographs shown in panels A and C. LTH indicates long-term hypoxia.
Figure 9 provides averaged data, based on the analysis of the micrographs and data from individual myocytes, as presented in Figure 8. Figure 9A shows the number of responsive myocytes in each one-thousand micrometer square of the PA wall to be approximately 3-fold greater in normoxic relative to LTH fetal lambs. The figure also shows that 10 µmol/L 5-HT dramatically increased the number of cells with cytosolic Ca2+ oscillations whether or not the animals were exposed to maternal LTH. Nonetheless, maternal LTH blunted 5-HT-elicited myocyte activation. Figure 9B shows the oscillatory period for responsive cells. Stimulation with 10 µmol/L 5-HT increased the firing rate in myocytes of both normoxic and hypoxic sheep. For normoxic fetal lambs, recordings were made in 89 control cells and 72 stimulated with 5-HT. For hypoxic fetuses, recordings were made in 49 myocytes in each condition (control and stimulated). Thus, the results of these experiments indicate that with maternal LTH, there is a reduction in the number of myocytes responsive to 5-HT. However, the increase in firing frequency to 5-HT was equivalent in PA myocytes of normoxic and LTH acclimatized fetuses (Figure 9B).
Figure 9.
5-Hydroxytryptamine (5-HT)-dependent Ca2+ signals recorded in situ are reduced by antenatal maternal LTH in pulmonary arterial myocytes of fetal sheep. A, Bars indicate the average number of myocytes with Ca2+ responses each minute in the absence (open) and presence (solid) of 10 μmol/L 5-HT. B, Bars indicate the average time period in between Ca2+ events in the absence (open) and presence (solid) of 10 μmol/L 5-HT. Error bars represent ± SEM. ####P < .0001 denotes significant difference from normoxic counterparts, while **P < .01 or ****P < .0001 denotes significant difference from control based on a 2-way ANOVA and a Bonferroni post hoc analysis. LTH indicates long-term hypoxia; SEM, standard error of the mean; ANOVA, analysis of variance.
Discussion
Our studies demonstrate several novel mechanisms, which are either preserved or altered because of antenatal maternal LTH-induced acclimatization, due to high-altitude living. (1) Depolarization-induced contraction is well conserved during high-altitude LTH acclimatization. (2) Antenatal maternal LTH desensitizes PAs to 5-HT, although the maximum response attained by 5-HT is maintained. (3) 5-HT2A receptors are predominantly involved in 5-HT-induced contractile responses in both normoxic and LTH fetuses, and the role for 5HT1B/D receptors decreases with LTH. (4) Extracellular Ca2+ is equally important in 5-HT-induced contraction, irrespective of maternal oxygen status. Furthermore, voltage-dependent and -independent Ca2+ channels are important in 5-HT-mediated contraction, and their overall roles are preserved during acclimatization to LTH. However, pharmacological evidence indicates that the roles of select TRP channels change with LTH acclimatization, requiring further investigation. (5) Reverse-mode NCX is not involved in 5-HT-induced contraction in vessels from normoxic and LTH fetuses. (6) Long-term hypoxia reduces the number of myocytes with Ca2+ responses to 5-HT even though the firing rate is maintained. These findings are a logical extension of our previous work, showing that Ca2+ homeostasis and 5-HT-induced Ca2+ signaling are significantly altered by postnatal maturation in PA myocytes.55
Evidence indicates that 5-HT is important to pulmonary vascular function and pathology.11,13,56–59 Our studies add to this, as they demonstrate that antenatal LTH reduced the potency of 5-HT and the role for 5-HT1B/D-induced contraction in PAs from fetal sheep. These reductions can be attributed to changes in receptor-sensitization, -density, -isoform expression, -function, and/or even phenotypic alterations in the arterial myocytes. Recent reports support such occurrences. For instance, gestational hypoxia in fetal sheep from our laboratories increases norepinephrine-induced arterial contraction.36 Gestational hypoxia also increases Rho kinase expression,5 accentuates endothelin and platelet-activating factor-dependent vascular remodeling, and causes pulmonary vascular remodeling and growth.6,9 Our findings of reduced serotonin function are distinct, however, from the LTH-induced enhancement of 5-HT-dependent PA contraction in adult rat.13,59,60 More precisely, related studies in rats report that hypoxia increased 5-HT1B/D activity and promoted pharmacologic synergism with 5-HT2A in PAs.12,61 Related to this, the 5HT1B/D receptor also is thought to be important to the development of pulmonary hypertension in rats and mice.13,58 This leads to the possibility that the change in 5-HT potency and restriction of 5-HT1B/D-mediated contraction observed in sheep may be beneficial adaptations to maternal LTH that restrain the development of pulmonary hypertension in the newborn.
The reduction in Ca2+-signaling behavior in myocytes of LTH fetuses, despite the preservation of 5-HT-elicited force generation, suggests an offset of the balance between Ca2+-dependent and -independent pathways. One possible explanation is that LTH may enhance Ca2+-independent contraction in the fetus; however, resolving these pathways was not a focus of the present investigation. Nevertheless, in preliminary studies, we have shown that Rho kinase, which is important to Ca2+-independent PA contraction in rodents,62–66 also is important to 5-HT-induced stimulation of PAs from fetal sheep.67
Of further note, the finding that Ca2 + signals were reduced following antenatal LTH in PA myocytes from fetal sheep is similar to the effect of LTH in PA myocytes from adult rats, in which ET-1, angiotensin II, and ATP-dependent cytosolic Ca2+ signaling were depressed.22,68 However, the roles for voltage-dependent and -independent Ca2 + entry pathways appear distinct in PAs from LTH fetal sheep. The maintenance of CaL-dependent pathways in PAs from LTH fetal sheep is dissimilar from the reduced role for CaL channels during ET-1 stimulation of rat PA myocytes following LTH 22 and the enhancement in CaL function by LTH in PA myocytes from newborn pigs.23 The effective reduction in 5-HT-elicited contraction by ten millimoles per liter Ni2+, extracellular Ca2+ removal, KB-R7943, the combination of SKF-96365 and nifedipine, or verapamil, compared to the modest reduction in contraction due to nifedipine and diltiazem, suggest that voltage-independent Ca2 + entry is predominantly responsible for 5-HT contraction before birth.19,20,69,70 The pharmacological profiling we performed indicates that TRP channels, but not reverse-mode NCX activity, are critical to Ca2 +-dependent 5-HT-elicited PA contraction in fetal sheep.49,53 A prominent role for TRP channels was not surprising, since they contribute significantly to contraction and Ca2 + signaling in vascular myocytes of other species.24,71–73 The inhibition in 5-HT-dependent contraction by inorganic and organic blockers provides additional details regarding the importance of various TRP channels and changes due to antenatal LTH. The considerable inhibition of contraction by verapamil, as compared to nifedipine and diltiazem, is notable as verapamil is also a CaL blocker. However, unlike the other CaL inhibitors, verapamil also inhibits TRPC3 channels,74 suggesting that this channel is important to 5-HT-elicited contraction. Based on the reduction in contraction by the various inhibitors tested and the expression of TRP gene products in PA myocytes of mice, rats, dogs, and fetal sheep, the most likely candidates include, TRPC3, 5, and 6.24,73,75–77 These findings are important as there is evidence that chronic hypoxia increases TRPC1 and 6 expression in rats 24 and because TRPC6 expression is coupled to proliferation of rat PA myocytes 27 and myocytes from idiopathic pulmonary hypertension patients.30 Even though TRP gene expression in PA myocytes from fetal sheep and the specific changes due to LTH remain to be determined, the pharmacological comparisons we performed support the proposal that TRP channels are significant to hypoxia-related pulmonary hypertension in newborn sheep.3
5-Hydroxytryptamine is linked etiologically to hypoxia-induced pulmonary hypertension and PPHN.10,15,78–81 The present study establishes that antenatal maternal hypoxic stress alters 5-HT-mediated PA contraction in the fetus, with selective changes in receptor- and Ca2+-dependent signaling processes. These changes are unique when compared to adult rats, mice,13 ,21,58 and newborn pigs 23 that are thought to be more responsive to long-term hypoxic stress than sheep.82 We speculate that the adaptive responses in fetal sheep may restrict the development of pulmonary hypertension following birth.3 However, the present study also raises a number of important issues that require additional studies, such as which TRP channel isoforms are involved in the acclimatization response to LTH and whether the changes persist in the newborn. Nonetheless, we believe that the original insights provided by comparing the effects of antenatal LTH acclimatization responses in fetal sheep PAs to other species, such as rats, mice, and pigs, will help us better understand pathogenesis of pulmonary disorders.
Footnotes
The work reported in this study was performed at Loma Linda University, Loma Linda, California and University of Mississippi, Oxford, Mississippi.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Science Foundation Grants MRI 0619774 and MRI 0923559 (SMW) and National Institutes of Health (NIH) Grants R01HL95973 (ABB), P01 HD-031226 (LDL), and R01 HL-064867 (WJP), as well as a University of Mississippi graduate student fellowship and a Sigma Xi research fellowship RG and research experience opportunity programs funded by NIH NCRR Grant P20 RR-016476 ML and US Department of Education Ronald E. McNair Post-Baccalaureate Achievement Summer Program (AD).
References
- 1. Gabbay E, Reed A, Williams TJ. Assessment and treatment of pulmonary arterial hypertension: an Australian perspective in 2006. Intern Med J. 2007;37(1):38–48 [DOI] [PubMed] [Google Scholar]
- 2. Danhaive O, Margossian R, Geva T, Kourembanas S. Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol. 2005;25(7):495–499 [DOI] [PubMed] [Google Scholar]
- 3. Herrera EA, Riquelme RA, Ebensperger G, et al. Long-term exposure to high-altitude chronic hypoxia during gestation induces neonatal pulmonary hypertension at sea level. Am J of Physiol Reg Int Comp Physiol. 2010;299(6):R1676–R1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao Y, Portugal AD, Liu J, et al. Preservation of cGMP-induced relaxation of pulmonary veins of fetal lambs exposed to chronic high altitude hypoxia: role of PKG and Rho kinase. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L889–L896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao Y, Portugal AD, Negash S, Zhou W, Longo LD, Usha Raj J. Role of Rho kinases in PKG-mediated relaxation of pulmonary arteries of fetal lambs exposed to chronic high altitude hypoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L678–L684 [DOI] [PubMed] [Google Scholar]
- 6. Sheng L, Zhou W, Hislop AA, Ibe BO, Longo LD, Raj JU. Role of epidermal growth factor receptor in ovine fetal pulmonary vascular remodeling following exposure to high altitude long-term hypoxia. High Alt Med Biol. 2009;10(4):365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90(4):1291–1335 [DOI] [PubMed] [Google Scholar]
- 8. Toga H, Hibler S, Ibe BO, Raj JU. Vascular effects of platelet-activating factor in lambs: role of cyclo- and lipoxygenase. J Appl Physiol. 1992;73(6):2559–2566 [DOI] [PubMed] [Google Scholar]
- 9. Bixby CE, Ibe BO, Abdallah MF, et al. Role of platelet-activating factor in pulmonary vascular remodeling associated with chronic high altitude hypoxia in ovine fetal lambs. Am J Physiol Lung Cell Mol Physiol. 2007;293(6):L1475–L1482 [DOI] [PubMed] [Google Scholar]
- 10. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587 [DOI] [PubMed] [Google Scholar]
- 11. MacLean MR, Herve P, Eddahibi S, Adnot S. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br J Pharmacol. 2000;131(2):161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morecroft I, MacLean MR. 5-hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br J Pharmacol. 1998;125(1):69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT1B-receptor knockout mice and the 5-HT1B/1D-receptor antagonist GR127935. Circ Res. 2001;89(12):1231–1239 [DOI] [PubMed] [Google Scholar]
- 14. Heffner JE, Sahn SA, Repine JE. The role of platelets in the adult respiratory distress syndrome. Culprits or bystanders? Am Rev Respir Dis. 1987;135(2):482–492 [DOI] [PubMed] [Google Scholar]
- 15. Herve P, Launay JM, Scrobohaci ML, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995;99(3):249–254 [DOI] [PubMed] [Google Scholar]
- 16. Wilson SM, Mason HS, Ng LC, et al. Role of basal extracellular Ca2+ entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol. 2005;144(2):252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slodzinski MK, Blaustein MP. Physiological effects of Na+/Ca2+ exchanger knockdown by antisense oligodeoxynucleotides in arterial myocytes. Am J Physiol Cell Physiol. 1998;275(1):C251–C259 [DOI] [PubMed] [Google Scholar]
- 18. Slodzinski MK, Juhaszova M, Blaustein MP. Antisense inhibition of Na+/Ca2+ exchange in primary cultured arterial myocytes. Am J Physiol Cell Physiol. 1995;269(5):C1340–C1345 [DOI] [PubMed] [Google Scholar]
- 19. Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2005;288(2):C245–C252 [DOI] [PubMed] [Google Scholar]
- 20. Zhang S, Dong H, Rubin LJ, Yuan JX. Upregulation of Na+/Ca2+ exchanger contributes to the enhanced Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2007;292(6):C2297–C2305 [DOI] [PubMed] [Google Scholar]
- 21. Rodat L, Savineau JP, Marthan R, Guibert C. Effect of chronic hypoxia on voltage-independent calcium influx activated by 5-HT in rat intrapulmonary arteries. Pflugers Arch. 2007;454(1):41–51 [DOI] [PubMed] [Google Scholar]
- 22. Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca(2+) channels, resting [Ca(2+)](i), and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L884–L894 [DOI] [PubMed] [Google Scholar]
- 23. Hirenallur SD, Haworth ST, Leming JT, et al. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L915–L924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin MJ, Leung GP, Zhang WM, et al. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95(5):496–505 [DOI] [PubMed] [Google Scholar]
- 25. Jernigan NL, Broughton BR, Walker BR, Resta TC. Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle after chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290(3):L517–L525 [DOI] [PubMed] [Google Scholar]
- 26. Zhang S, Patel HH, Murray F, et al. Pulmonary artery smooth muscle cells from normal subjects and IPAH patients show divergent cAMP-mediated effects on TRPC expression and capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1202–L1210 [DOI] [PubMed] [Google Scholar]
- 27. Yu Y, Sweeney M, Zhang S, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284(2):C316–C330 [DOI] [PubMed] [Google Scholar]
- 28. Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L962–L969 [DOI] [PubMed] [Google Scholar]
- 29. Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L144–L155 [DOI] [PubMed] [Google Scholar]
- 30. Yu Y, Fantozzi I, Remillard CV, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101(38):13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landsberg JW, Yuan JX. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci. 2004;19(2):44–50 [DOI] [PubMed] [Google Scholar]
- 32. Ibe BO, Portugal AM, Chaturvedi S, Raj JU. Oxygen-dependent PAF receptor binding and intracellular signaling in ovine fetal pulmonary vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L879–L886 [DOI] [PubMed] [Google Scholar]
- 33. Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, alpha 1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Heart Circ Physiol. 1996;270(3):H915–H923 [DOI] [PubMed] [Google Scholar]
- 34. Kamitomo M, Longo LD, Gilbert RD. Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol Renal Physiol. 1994;266(6):R1778–R1785 [DOI] [PubMed] [Google Scholar]
- 35. Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol. 2009;297(6):H2242–H2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xue Q, Ducsay CA, Longo LD, Zhang L. Effect of long-term high-altitude hypoxia on fetal pulmonary vascular contractility. J Appl Physiol. 2008;104(6):1786–1792 [DOI] [PubMed] [Google Scholar]
- 37. Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563(pt 2):409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward JP, Snetkov VA. Determination of signaling pathways responsible for hypoxic pulmonary vasoconstriction: use of the small vessel myograph. Methods Enzymol. 2004;381:71–87 [DOI] [PubMed] [Google Scholar]
- 39. Goyal R, Mittal A, Chu N, Zhang L, Longo LD. Alpha1-adrenergic receptor subtype function in fetal and adult cerebral arteries. Am J Physiol Heart Circ Physiol. 2010;298(6):H1797–H1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holford NH, Sheiner LB. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981;6(6):429–453 [DOI] [PubMed] [Google Scholar]
- 41. Morecroft I, Heeley RP, Prentice HM, Kirk A, MacLean MR. 5-Hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol. 1999;128(3):730–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jahnichen S, Glusa E, Pertz HH. Evidence for 5-HT2B and 5-HT7 receptor-mediated relaxation in pulmonary arteries of weaned pigs. Naunyn Schmiedebergs Arch of Pharmacol. 2005;371(1):89–98 [DOI] [PubMed] [Google Scholar]
- 43. Jaw SP, Hussong MJ, Matsumoto RR, Truong DD. Involvement of 5-HT2 receptors in posthypoxic stimulus-sensitive myoclonus in rats. Pharmacol Biochem Behav. 1994;49(1):129–131 [DOI] [PubMed] [Google Scholar]
- 44. Wilson SM, Mason HS, Smith GD, et al. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol. 2002;543(3):917–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Millard RW, Lathrop DA, Grupp G, Ashraf M, Grupp IL, Schwartz A. Differential cardiovascular effects of calcium channel blocking agents: potential mechanisms. Am J Cardiol. 1982;49(3):499–506 [DOI] [PubMed] [Google Scholar]
- 46. Merritt JE, Armstrong WP, Benham CD, et al. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271(2):515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol. 2007;152(1):101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th Ed Br J Pharmacol. 2009;158(suppl 1):S1–S254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Ann Rev Physiol. 2006;68:619–647 [DOI] [PubMed] [Google Scholar]
- 50. Krautwurst D, Degtiar VE, Schultz G, Hescheler J. The isoquinoline derivative LOE 908 selectively blocks vasopressin-activated nonselective cation currents in A7r5 aortic smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 1994;349(3):301–307 [DOI] [PubMed] [Google Scholar]
- 51. Guibert C, Marthan R, Savineau JP. 5-HT induces an arachidonic acid-sensitive calcium influx in rat small intrapulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1228–L1236 [DOI] [PubMed] [Google Scholar]
- 52. Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79(3):763–854 [DOI] [PubMed] [Google Scholar]
- 53. Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361(1):230–236 [DOI] [PubMed] [Google Scholar]
- 54. Niu CF, Watanabe Y, Iwamoto T, et al. Electrophysiological effects of SN-6, a novel Na+/Ca2+ exchange inhibitor on membrane currents in guinea pig ventricular myocytes. Ann N Y Acad Sci. 2007;1099:534–539 [DOI] [PubMed] [Google Scholar]
- 55. Goyal R, Creel KD, Chavis E, Smith GD, Longo LD, Wilson SM. Maturation of intracellular calcium homeostasis in sheep pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L905–L914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dempsie Y, MacLean MR. Pulmonary hypertension: therapeutic targets within the serotonin system. Br J Pharmacol. 2008;155(4):455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dempsie Y, Morecroft I, Welsh DJ, et al. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation. 2008;117(22):2928–2937 [DOI] [PubMed] [Google Scholar]
- 58. MacLean MR, Morecroft I. Increased contractile response to 5-hydroxytryptamine1-receptor stimulation in pulmonary arteries from chronic hypoxic rats: role of pharmacological synergy. Br J Pharmacol. 2001;134(3):614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. MacLean MR, Sweeney G, Baird M, McCulloch KM, Houslay M, Morecroft I. 5-hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br J Pharmacol. 1996;119(5):917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morecroft I, Loughlin L, Nilsen M, et al. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Therap. 2005;313(2):539–548 [DOI] [PubMed] [Google Scholar]
- 61. Morecroft I, Pang L, Baranowska M, et al. In vivo effects of a combined 5-HT1B receptor/SERT antagonist in experimental pulmonary hypertension. Cardiovasc Res. 2010;85(3):593–603 [DOI] [PubMed] [Google Scholar]
- 62. Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L674–L685 [DOI] [PubMed] [Google Scholar]
- 63. Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L797–L806 [DOI] [PubMed] [Google Scholar]
- 64. Oka M, Homma N, McMurtry IF. Rho kinase-mediated vasoconstriction in rat models of pulmonary hypertension. Methods Enzymol. 2008;439:191–204 [DOI] [PubMed] [Google Scholar]
- 65. Nagaoka T, Gebb SA, Karoor V, et al. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol. 2006;100(3):996–1002 [DOI] [PubMed] [Google Scholar]
- 66. Guilluy C, Eddahibi S, Agard C, et al. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179(12):1151–1158 [DOI] [PubMed] [Google Scholar]
- 67. Goyal R, Nguyen D, Loftin M, et al. Contributions of PKC, RhoA and ERK signaling to serotonergic contractility of pulmonary arteries from chronic hypoxic fetal and adult sheep. FASEB J. 2008;22:1150–1153 [Google Scholar]
- 68. Bonnet S, Belus A, Hyvelin JM, Roux E, Marthan R, Savineau JP. Effect of chronic hypoxia on agonist-induced tone and calcium signaling in rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L193–L201 [DOI] [PubMed] [Google Scholar]
- 69. Kita S, Iwamoto T. Inhibitory mechanism of SN-6, a novel benzyloxyphenyl Na+/Ca2+ exchange inhibitor. Ann N Y Acad Sci. 2007;1099(1):529–533 [DOI] [PubMed] [Google Scholar]
- 70. Niu CF, Watanabe Y, Ono K, et al. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur J Pharmacol. 2007;573(1-3):161–169 [DOI] [PubMed] [Google Scholar]
- 71. Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280(48):39786–39794 [DOI] [PubMed] [Google Scholar]
- 72. Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L848–L858 [DOI] [PubMed] [Google Scholar]
- 73. Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290(6):L1267–L1276 [DOI] [PubMed] [Google Scholar]
- 74. Zhu X, Jiang M, Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J Biol Chem. 1998;273(1):133–142 [DOI] [PubMed] [Google Scholar]
- 75. Resnik ER, Keck M, Sukovich DJ, Herron JM, Cornfield DN. Chronic intrauterine pulmonary hypertension increases capacitative calcium entry in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L953–L959 [DOI] [PubMed] [Google Scholar]
- 76. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L104–L113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol. 2001;280(5):C1184–C1192 [DOI] [PubMed] [Google Scholar]
- 78. Eddahibi S, Raffestin B, Pham I, et al. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol Heart Circ Physiol. 1997;272(3):H1173–H1181 [DOI] [PubMed] [Google Scholar]
- 79. Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax. 1999;54(2):161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marcos E, Fadel E, Sanchez O, et al. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94(9):1263–1270 [DOI] [PubMed] [Google Scholar]
- 81. Nagaoka T, Muramatsu M, Sato K, McMurtry I, Oka M, Fukuchi Y. Mild hypoxia causes severe pulmonary hypertension in fawn-hooded but not in tester moriyama rats. Resp Physiol. 2001;127(1):53–60 [DOI] [PubMed] [Google Scholar]
- 82. Tucker A, McMurtry IF, Reeves JT, Alexander AF, Will DH, Grover RF. Lung vascular smooth muscle as a determinant of pulmonary hypertension at high altitude. Am J Physiol. 1975;228(3):762–767 [DOI] [PubMed] [Google Scholar]