Abstract
The aim of this study was to determine the influence of maternal undernutrition (MUN) on maternal and offspring adrenal steoridogenic enzymes. Pregnant Sprague-Dawley rats were 50% food-restricted from day 10 of gestation until delivery. Control animals received ad libitum food. Offspring were killed on day 1 of life (P1) and at 9 months. We determined the messenger RNA (mRNA) expression of steroidogneic enzymes by real-time reverse transcriptase polymerized chain reaction (RT-PCR). Maternal undernutrition inhibited maternal adrenal expression of P450 cholesterol side-chain cleavage enzyme (CYP11A1), 11 beta-hydroxylase (CYP11B1), aldosterone synthase (CYP11B2), and adrenocorticotropic hormone (ACTH) receptor (ACTH-R; MC2 gene) compared with control offspring. There was a marked downregulation in the expression of CYP11B1, CYP11B2, 11 β-hydroxysteroid dehydrogenase type 1 and 2 (HSD1 and HSD2), CYP11A1, ACTH receptor, steroidogenic acute regulatory protein (STAR), and mineralocorticoid receptor (MCR; NR3C2 gene) mRNA in P1 MUN offspring (both genders), with no changes in glucocorticoid receptor (GCR). Quantitative immunohistochemical analysis confirmed the PCR data for GCR and MCR in P1 offspring and demonstrated lower expression of leptin receptor protein (Ob-Ra/Ob-Rb) and mRNA in P1 MUN offspring. In 9-month adult male MUN offspring, the expression of HSD1, CYP11A1, CYP11B2, Ob-Ra/Ob-Rb, and GCR mRNA were significantly upregulated with a trend toward an increase in ACTH-R and a decrease in 17 alpha-hydroxylase (CYP17A1) expression. In adult female MUN offspring, similar to males, the expression of CYP11A1, ACTH-R, and Ob-Rb mRNA were increased, whereas GCR and CYP17A1 mRNA were decreased. These results indicate that the adrenal gland is a target of nutritional programming. In utero undernutrition has a global suppressive effect on maternal and P1 offspring adrenal steroidogenic enzymes in association with reduced circulating corticosterone levels in P1 offspring, which may be secondary to a negative feedback from elevated maternal GC levels and or leptin levels in MUN dams. Gender-specific differences in steroidogenic enzyme expression were found in adult MUN offspring. The common finding of increased ACTH receptor expression in MUN adults of both genders suggests an increased sensitivity of these offspring to stress.
Keywords: adrenal, steroidogenesis, STAR protein, leptin receptor, ACTH receptor, maternal undernutrition, glucocorticoids, fetal programming
An adverse intrauterine environment induces fetal growth restriction and is associated with development of metabolic syndrome in adult life.1 These adverse intrauterine conditions are associated with maternal stress leading to excess glucocorticoid production which has significant impact on the fetus in terms of growth and later development of adult diseases.2,3 Several studies have shown hyperactivity of offspring hypothalamic-pituitary-adrenal axis caused by maternal undernutrition.2,3 Most of these studies have focused primarily on characterization of the adult offspring hypothalamic-pituitary axis and changes in expression of GC/MC receptors in the central nervous system.4,6–8 The effect of maternal undernutrition on expression of adrenal GC/MC receptors remains elusive. Glucocorticoids play an essential role in the stress response and have been proposed to alter signal transduction in the adrenal cortex thereby regulating adrenocorticotropic hormone (ACTH) sensitivity.9–11 In addition to negative feedback regulation at the hypothalamic-pituitary level, glucocorticoid feedback may occur at the adrenal level,2,3 mediated by adrenal glucocorticoid receptor (GCR)/mineralocorticoid receptor (MCR).2,3 Adrenal ACTH receptor (ACTH-R)2,3 mediates a long-loop feedback axis, by which the hypothalamus/pituitary regulate the expression of steroidogenic enzymes.
The effect of maternal undernutrition on adrenal steroidogenic enzyme expression has been examined in sheep where timing of nutritional stress, that is preconception versus gestational differentially influences the expression of steroidogenic enzymes. Periconceptional nutrient restriction in sheep was reported not to alter the expression of adrenal ACTH-R (MC2), steroidogenic acute regulatory protein (STAR), side-chain cleavage enzyme (CYP11A1), 17 alpha-hydroxylase (CYP17A1), and 3βHSD messenger RNA (mRNA),16 whereas gestational undernutrition increased the expression of ACTH-R and STAR mRNA.17 In a rat model of fetal programming in which dexamethasone was administered to dams from day 13 of gestation to term, adult offspring adrenals showed no changes in weight or morphology and had a significant increase ACTH-R expression in both genders with no changes in STAR or CYP11A1 mRNA.18 The investigators suggested that the increase in ACTH-R expression in this species may account for exaggerated GC/MC response of these offspring to stress.18 Maternal undernutrition also alters rat adrenal medullary structure and function, with defective adrenaline secretion in food-restricted offspring.19
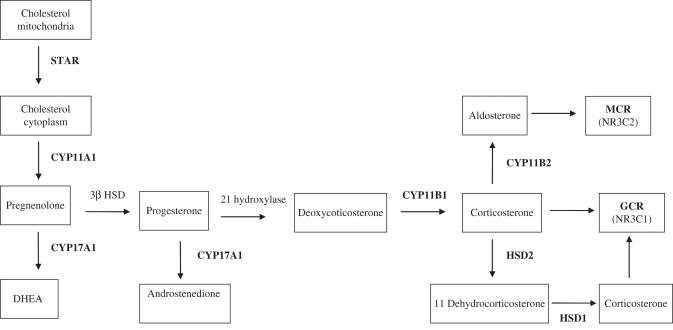
Since maternal undernutrition is associated with excess maternal GC production which may play a vital role in programming offspring hypertension in the offspring, we sought to characterize maternal as well as offspring adrenal expression of major steroidogenic enzymes involved in glucocorticoid/mineralocorticoid synthesis along with their respective receptors (see Figure 1 ) and ACTH-Rs. In addition, we determined the adrenal expression of leptin receptor as leptin has a direct effect on adrenal steroidogenic enzyme expression20,21 and was previously reported to show a decline in circulating levels at birth followed by an increase in adult life in MUN offspring.22 We hypothesized that elevated maternal leptin and corticosterone in response to undernutrition programs offspring adrenal steroidogenic enzymes, GC/MC, and leptin receptor expression in a way that would increase their vulnerability for development of hypertension.
Figure 1.
Schematic demonstration of the steroidogenic pathway in rat adrenal gland. The expression level of the bolded enzymes were determined.
Materials and Methods
Animals/Specimen Handling
The study was approved by the Animal Use and Care Committee at Los Angeles Biomedical Research Institute at the Harbor-UCLA Medical Center. We used a well-characterized animal model of fetal programming developed by our group.22 In this model, first-time-pregnant Sprague Dawley rats (Charles River Laboratories, Inc, Hollister, California) were housed in a facility with constant temperature and humidity and a controlled 12-hour light-12-hour dark cycle. At 10 days of gestation, rats were provided either an ad libitum diet of standard laboratory chow (Lab Diet 5001, Brentwood, Missouri: protein 23%, fat 4.5%, metabolizable energy 3030 kcal/kg) or 50% food-restricted diet determined by quantification of normal intake in the ad libitum-fed rats. The respective diets were given from day 10 of pregnancy to term. Maternal body weights and the food intake were recorded daily. At day 1 after birth (P1), all offspring from food-restricted and control rat dams were cross-fostered to rat dams fed ad libitum, and litter size was culled to 4 males and 4 females per dam. Offspring were weaned at 3 weeks of age to ad libitum standardized laboratory chow. Animals were anesthetized under isofluroane gas. Adrenal glands were dissected and cleaned of adherent fat, snap frozen in liquid nitrogen, and stored at −80°C for later RNA extraction. For immunohistochemical (IHC) analysis, tissues were fixed for 24 hours in 4% paraformaldehyde and subsequently stored in 70% ethanol.
Real Time RT-PCR Analysis
RNA was extracted using RNAqueous-4PCR kit (Ambion, Austin, Texas). The RNA was then treated to remove trace amounts of DNA using Turbos DNAse (Ambion), and its integrity determined by gel electrophoresis. Synthesis of complementary DNA (cDNA) was accomplished using Superscript III 1st Strand Synthesis Supermix for quantitative reverse transcriptase polymerized chain reaction (qRT-PCR kit; Invitrogen, Carlsbad, California). Specific rat primers used to quantify mRNA levels were designed using Probe and Primer Design software (Table 1 ). The DNA sequence for each enzyme was obtained from the National Center for Biotechnology Information (NCBI). Real time RT-PCR analysis was conducted using cDNA diluted at a 1:10 concentration. 18S was used as an internal control. Polymerized chain reaction was performed with the ABI-Prism 7000 Sequence System (Applied Biosystems, Foster City, CA) at the following conditions: 10 minutes at 95°C for 1 cycle, 15 seconds at 95°C, and 1 minute at 60°C for 40 cycles. All samples were run in triplicate and each experiment was replicated at least twice. Control PCR samples replaced cDNA with water. ABI Sequence Detection System 1.6 software (Applied Biosystems) was used to select a threshold level of fluorescence that was in the linear phase of the PCR product accumulation. Results from the reverse transcription-PCR assay was determined as the difference between the C T for a specific mRNA gene and the C T for a reference mRNA, normalized to 18S threshold expression, and was expressed as fold change with the formula 2-ΔΔCT.23
Table 1.
Primer Sequences Used for Real Time RT-PCR Analysis
| Primer | Sequence (5′-3′) Forward | Sequence (5′-3′) Reverse |
|---|---|---|
| CYP11B1 | GGCACATACGAGCTGGTGAGT | GTCCTCCTGCCTGCATCTCT |
| CYP11B2 | TGCTGCTTGGGCAAAGGT | CTTTTCGCCCTACCGACTTG |
| ACTH-R | TATCTCAAGCCTCGTGGCAGTT | GCTCCCATGCTCGGAAGAT |
| CYP11A1 | TCAAGCAGCAAAACTCTGGA | CGCTCCCCAAATACAACACT |
| HSD1 | CAGTTCTGCGCAAAGATGAG | TGGGTAGGGCTCACAGAAAT |
| HSD2 | TCTTTGGTGCACTTGAGCTG | CTGGATGATGCTGACCTTGA |
| GCR | ATAAAAGCCTGAGGGGAGGA | TCCTCTGCTGCTTGGAATCT |
| MCR | ACGCTGTGAGACTGGATTTC | AGTTACCCGGAGACACATGA |
| STAR | AGGAAAGCCAGCAGGAGAATG | GTCCATGGGCTGGTCTAGCA |
| CYP17A1 | TGAATGGGACCAGCCAGATC | CAGCTCCGAAGGGCAAGTAA |
Abbreviations: CYP11B1, 11-beta hydroxylase; CYP11B2, aldosterone synthase; ACTH-R, ACTH receptor (MC2); CYP11A1, side chain cleavage enzymes; HSD, hydroxysteroid dehydrogenase; GCR (NR3C1), glucocorticoid receptor; MCR, mineralocorticoid receptor (NR3C2); CYP17A1, 17 alpha-hydroxylase.
Immunohistochemistry
Using specific antibodies to GC receptor alpha, MC receptor, and leptin receptor (Ob-Ra and Ob-Rb; Santa Cruz) IHC analysis was performed as previously described.24,25 Four separate fields were digitally photographed and analyzed in a blinded fashion at a magnification of ×40 by Image Pro Plus (MediaCybernetics, Version 4) software. The mean Integrated Optical Density (IOD) obtained using this software was statistically analyzed.
Radioimmunoassay (RIA)
Plasma corticosterone (CORT) was determined by RIA using a commercial kit (Diagnostic Products Corp, Los Angeles, California). Sensitivity at 90% intercept was 8 ng/mL. Intra and interassay coefficients of variation were 4% and 6%, respectively.
Statistical Analysis
All data was initially analyzed by 2-way ANOVA, with gender and diet as covariates. Since the P1 data showed no gender differences with the exception of leptin-receptor, the results from males and females for all endpoints were combined and analyzed by Student t-test. In case of adult groups, gender differences were found with a number of endpoints, and therefore male and female data were analyzed separately by Student t-test or Mann-Whitney U test when indicated. The SigmaStat and Prism software was used for all analysis. Significance was established at P < .05.
Results
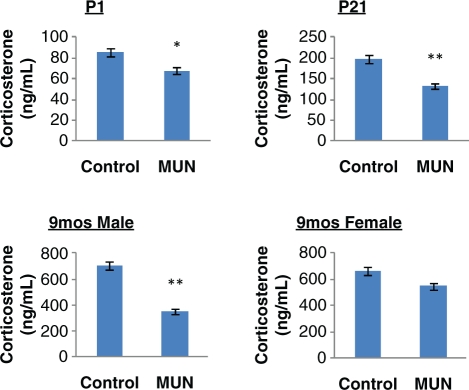
Offspring body weights and adrenal weights are shown in Table 2 . As demonstrated in this table, adult body weights in MUN offspring were higher in both males and females, confirming our prior studies. Although the absolute adrenal weights in adult MUN offspring were not different than the controls, when expressed in terms of body weight, relative adrenal weights in both genders were significantly lower in the MUN offspring. Blood pressure of the adult MUN offspring of both genders were previously reported to be higher.25 Maternal plasma levels of CORT were higher in MUN dams compared to controls (Control: 332 ± 43 ng/mL; MUN: 623 ± 84 ng/mL, P < .05, N = 6). Plasma levels (pooled from 4 to 6 animals in case of P1 offspring only, mixed gender) of CORT determined by RIA was significantly lower in P1 (P < .05), P21 (P < .01), and adult 9-month-old male (P < .01) MUN offspring compared with respective age-matched controls. There was a trend (P = 0.1) toward lower CORT levels in 9-month-old adult MUN females (Figure 2 ). Leptin levels in the offspring were previously reported to be lower at birth and higher in the adult MUN offspring compared with control offspring.22
Table 2.
The Effect of Maternal Undernutrition (MUN) on Adult Offspring Body and Adrenal Weightsa
| Control | MUN | P Value | |
|---|---|---|---|
| Males | |||
| Body weight (g) | 647 ± 17 | 742 ± 20 | <.01 |
| Adrenal weight (mg) | 71.2 ± 1.9 | 69.6 ± 2.2 | NS |
| Relative adrenal weight | 0.0117 ± 0.0001 | 0.0093 ± 0.0003 | <.05 |
| Females | |||
| Body weight (g) | 350 ± 18 | 420 ± 15 | <.01 |
| Adrenal weight (mg) | 100.4 ± 6.4 | 90.8 ± 4.3 | <.07 |
| Relative adrenal weight | 0.0295 ± 0.0013 | 0.0233 ± 0.0022 | <.05 |
a Values are expressed as mean ± SEM relative adrenal weight = adrenal weight (g)/body weight (g) × 100.
Figure 2.
Plasma corticosterone levels determined by RIA in P1, P21 (mixed gender), adult 9-month male and female offspring (fasting) in control and MUN offspring. N = 6 per group. * P < .05, ** P < .01.
Real time RT-PCR was performed using the primers listed in Table 1. The mRNA expression of bolded enzymes in the steroidogenic pathway shown in Figure 1 were determined by real time RT-PCR. As shown in Table 3 , maternal adrenal mRNA expression of CYP11B1 and CYP11B2, enzymes involved in CORT and aldosterone synthesis, respectively, were significantly reduced in MUN dams, along with an inhibition of ACTH-R (MC2) expression. Similar to maternal mRNA profiles, adrenal expression of steroidogenic enzymes in P1 MUN offspring were mostly lower as compared with controls (Table 4 ). In these offspring, the expression of 11 beta-hydroxylase (CYP11B1), CYP11B2, HSD1, HSD2, CYP 11A1, ACTH-R (MC2), STAR, and the MCR (NR3C2) mRNA were significantly downregulated in the P1 MUN offspring, with no significant changes in GCR (NR3C1) and CYP17A1 mRNA. In adult offspring (Table 5 ), gender differences were found. In male MUN offspring, adrenal expression of HSD1, CYP11B2 (aldosterone synthase), CYP11A1, and GCR were significantly upregulated with a trend toward an increase in ACTH-Receptor (P = .07) and reduced CYP17A1 expression. In contrast to males, in female MUN offspring, the expression of GCR was significantly decreased, with no changes in HSD1. In similarity to males, the expression of CYP11A1 was increased and there was a significant decrease in CYP17A1 mRNA expression in female MUN adrenals. When data from adult males and females were combined for statistical analysis, the expression of ACTH-R was significantly increased (1.8-fold, P = .02) and that of CYP17A1 decreased 1.9-fold (P = .04).
Table 3.
Expression of Adrenal Steroidogenic Enzymes in Maternal Adrenals on Day 21 of Gestationa
| Gene | Fold Change | P Value |
|---|---|---|
| CYP11B1 | −9.0 ± 0.03 | .001 |
| CYP11B2 | −5.9 ± 0.07 | .001 |
| ACTH-R (MC2) | −2.0 ± 0.11 | .05 |
| CYP11A1 | −2.3 ± 0.03 | .001 |
| HSD1 | 1.1 ± 0.15 | NS |
| HSD2 | −1.7 ± 0.29 | NS |
| GCR (NR3C1) | −1.2 ± 0.13 | NS |
| MCR (NR3C2) | 1.3 ± 0.45 | NS |
| STAR | −2.0 ± 0.10 | NS |
| CYP17A1 | −3.2 ± 0.13 | NS |
Abbreviations: CYP11B1, 11-beta hydroxylase; CYP11B2, aldosterone synthase; ACTH-R, ACTH receptor (MC2); CYP11A1, side chain cleavage enzymes; HSD, hydroxysteroid dehydrogenase; GCR (NR3C1), glucocorticoid receptor; MCR, mineralocorticoid receptor (NR3C2); CYP17A1, 17 alpha-hydroxylase.
a Negative numbers signify lower expression in MUN vs control dams. Values are expressed as mean fold change compared to control ± SEM. N = 4 control and 6 MUN dams.
Table 4.
Expression of Adrenal Steroidogenic Enzymes mRNA in P1 Offspringa
| Gene | Fold Change | P Value |
|---|---|---|
| CYP11B1 | −5.9 ± 0.04 | .01 |
| CYP11B2 | −5.9 ± 0.03 | .04 |
| ACTH-R (MC2) | −4.2 ± 0.06 | .05 |
| CYP11A1 | −4.0 ± 0.06 | .05 |
| HSD1 | −2.9 ± 0.06 | .01 |
| HSD2 | −5.3 ± 0.06 | .03 |
| STAR | −3.8 ± 0.05 | .01 |
| CYP17A1 | −3.3 ± 0.14 | NS |
| GCR (NR3C1) | −2.2 ± 0.18 | NS |
| MCR (NR3C2) | −2.7 ± 0.08 | .02 |
| Ob-Ra (female) | 1.2 ± 0.007 | .02 |
| Ob-Rb (female) | −1.0 ± 0.009 | NS |
| Ob-Ra (male) | −3.0 ± 0.003 | .001 |
| Ob-Rb (male) | −4.8 ± 0.001 | .001 |
Abbreviations: CYP11B1, 11-beta hydroxylase; CYP11B2, aldosterone synthase; ACTH-R, ACTH receptor (MC2); CYP11A1, side chain cleavage enzymes; HSD, hydroxysteroid dehydrogenase; GCR (NR3C1), glucocorticoid receptor; MCR, mineralocorticoid receptor (NR3C2); CYP17A1, 17 alpha-hydroxylase.
a Values are expressed as mean fold change ± SEM compared to control. Negative numbers signify decreased expression in MUN as compared with controls. N = 11 per group (5 males/6 females).
Table 5.
Adrenal Steroidogenic Enzymes mRNA in 9-Month Adult Male and Female Offspringa
| Gene | Fold Change | P Value Male | Fold Change | P Value Female |
|---|---|---|---|---|
| CYP11B1 | −1.1 ± 0.07 | NS | −1.3 ± 0.06 | NS |
| CYP11B2 | 1.5 ± 0.13 | .05 | 1.1 ± 0.10 | NS |
| ACTH-R | 1.9 ± 0.57 | NS | 1.7 ± 0.22 | .02 |
| CYP11A1 | 1.9 ± 0.5 | .001 | 1.3 ± 0.28 | .03 |
| HSD1 | 2.9 ± 0.16 | .001 | −1.1 ± 0.09 | NS |
| HSD2 | 1.1 ± 0.03 | NS | 1.0 ± 0.18 | NS |
| GCR | 2.2 ± 0.008 | .001 | −1.3 ± 0.02 | .04 |
| MCR | −1.1 ± 0.45 | NS | 1.1 ± 0.09 | NS |
| STAR | 1.0 ± 0.19 | NS | −1.1 ± 0.06 | NS |
| CYP17A1 | −1.5 ± 0.04 | NS | −2.3 ± 0.03 | .04 |
| Ob-Ra | 2.9 ± 0.1 | .002 | 1.2 ± 0.03 | NS |
| Ob-Rb | 2.5 ± 0.03 | .001 | 1.6 ± 0.05 | .001 |
Abbreviations: CYP11B1, 11-beta hydroxylase; CYP11B2, aldosterone synthase; ACTH-R, ACTH receptor (MC2); CYP11A1, side chain cleavage enzymes; HSD, hydroxysteroid dehydrogenase; GCR (NR3C1), glucocorticoid receptor; MCR, mineralocorticoid receptor (NR3C2); CYP17A1, 17 alpha-hydroxylase. a Values are expressed as mean fold change ± SEM compared to control. N = 4 males and 4 females.
a Values are expressed as mean fold change ± SEM compared to control. N = 4 males and 4 females.
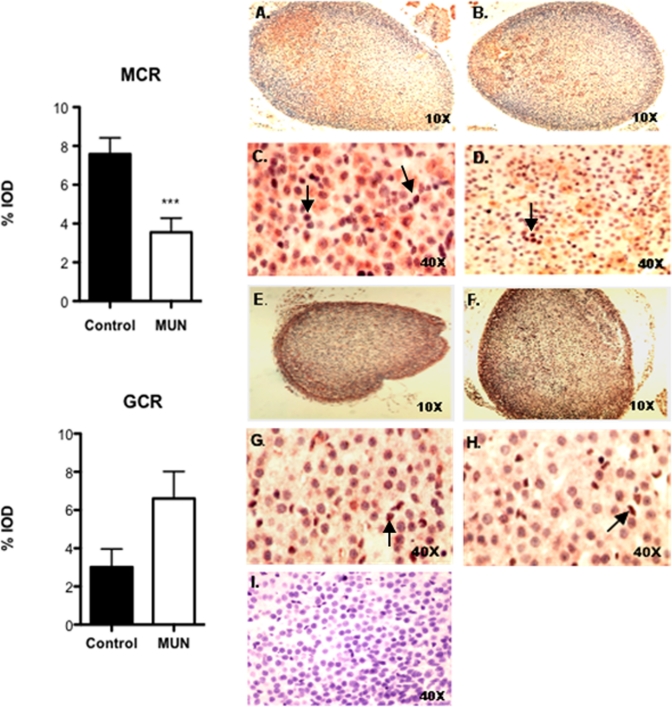
We also determined the expression of GCR (NR3C1) and MCR (NR3C2) proteins in P1 offspring adrenals by quantitative IHC. As demonstrated in Figure 3 , both MC receptor (panels C and D) and GC receptor (panels G and H) staining was primarily nuclear. In case of MC receptor, staining pattern was patchy and involved the 3 layers of adrenal cortex equally, with scant expression in adrenal medullary cells (panels A and B). In contrast, GC receptor staining was uniformly distributed throughout the adrenal cortex mostly concentrated in the zona glomerulosa layer (panels E and F). There was a significant decrease in percentage integrated optical density (IOD) in MC receptor expression in MUN offspring, whereas an opposite pattern of expression was obtained for GC receptors although this change did not achieve statistical significance (P = .06).
Figure 3.
Demonstrates immunohistochemical localization of mineralocorticoid receptor (MCR) and glucocorticoid receptor (GCR) in P1 control and MUN adrenals. Brown stain indicated by arrows represents positive staining. Panels A-D show MCR staining, with panels A and E representing control adrenals and panels B and D, MUN adrenals. Panels E-H show GCR staining, with panels E and G representing controls and panels F and H, MUN adrenals. Panel I is the negative control. Results of quantitative analysis of staining intensity expressed as Integrated Optical Density (IOD) based on evaluation of 4 high-power fields by Image Pro Plus software is shown in the bar plots. N = 3 males and 3 females from different litters. *** P < .001 by Mann Whitney U test.
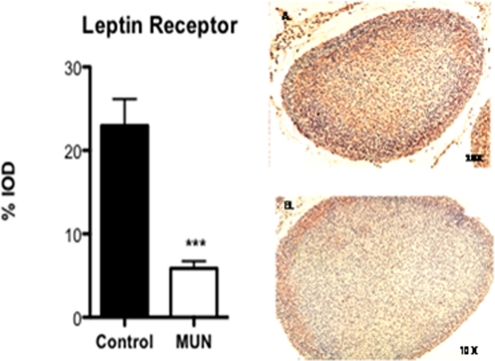
The expression of leptin receptor in MUN adrenals was also determined by quantitative IHC in P1 adrenals (Figure 4 ), and by real time PCR in P1 (Table 4) and adult adrenals (Table 5). As shown in this figure, the pattern of expression of leptin receptor was similar to GC receptor, concentrated primarily in the adrenal cortex with scant staining in the medullary cells. There was a marked decrease in % IOD in leptin receptor expression (P < .001) in the P1 MUN adrenals as compared with controls. Real time RT-PCR showed a marked decrease in expression of both Ob-Ra and Ob-Rb in P1 male MUN adrenals, whereas in females there was a slight increase in Ob-Ra and no change in Ob-Rb mRNA expression. In adult MUN male and female offspring, the expression of Ob-Rb mRNA was significantly increased. The expression of Ob-Ra mRNA was significantly higher in adult male MUN offspring with a trend toward an increase in females. The magnitude of changes in adult Ob-Ra /Ob-Rb mRNA expression were more pronounced for male MUN offspring as compared with females.
Figure 4.
Immunohistochemical localization (×10) of leptin receptor in P1 adrenal of control and MUN offspring (N = 3 males and 3 females). Bar plot is a summary of the staining intensity expressed as % IOD in the 2 dietary groups.
Discussion
In this study, the effect of maternal undernutrition on maternal and offspring adrenal steroidogenic enzyme mRNA expression in the newborn and adult periods was determined. We hypothesized that elevated maternal leptin and corticosterone in response to undernutrition programs offspring adrenal steroidogenic enzymes, GC/MC, and leptin receptors. In support of this hypothesis, we found a generalized inhibition of steroidogenic enzymes, GC/MC, and leptin receptors most likely secondary to a negative feedback from elevated maternal corticosterone and leptin levels. By 9 months, adrenal profiles of steroidogenic enzymes in MUN offspring markedly changed in a gender-specific manner, reflecting not only the effects of early exposure to elevated maternal corticosterone and leptin but also gender-specific differences in adaptive responses. In adult MUN male adrenals, a significant increase in CYP11A1, CYP11B2, HSD1, and GCR mRNA expression, a trend toward an increase in ACTH-R, and a decrease in CYP17A1 mRNA expression was found, whereas in adult female MUN adrenals, there was a similar mRNA expression profile as males with respect to CYP 11A1, HSD1, ACTH-R, but an opposite pattern of expression with respect to GCR and no changes in HSD1 expression. In contrast to the neonatal MUN offspring, adult adrenal leptin receptor expression was higher in both genders.
There is considerable evidence linking fetal exposure to excess glucocorticoids and development of metabolic syndrome later in life.2,3 Maternal undernutrition stress induces excess maternal production of corticosterone as demonstrated by our data, and by others.26 Furthermore, overexpression of placental 11 HSD-2 in MUN dams, which inactivates active GC such as corticosterone to inactive 11–keto forms, is inhibited,2,3 resulting in overexposure of the fetus to GC which in turn would cause growth restriction and other adult chronic disease.2,3 The importance of excess maternal GC to the pathogenesis of metabolic syndrome in the offspring was previously demonstrated in the protein restriction model of programming in which offspring hypertension was prevented by treatment of pregnant dams with GC synthesis inhibitors and recreated by administration of corticosterone to the dams.2,3
Our data indicate that GC and MC receptors are primarily localized in the adrenal cortex. The expression of GC/MC receptors as shown here and by others9 suggests that the adrenal gland is a target for feedback regulation by circulating GC and MC.33 Elevated levels of maternal GC in response to undernutrition would be expected to inhibit maternal and fetal adrenal steroidogenic enzymes as demonstrated by our data. Our data in the neonatal offspring, showing inhibition of enzymes involved in corticosterone (CYP11B1) and aldosterone (CYP11B2), supports this negative feedback regulation of offspring adrenal steroidogenic enzymes by maternal GC and is in agreement with the findings of Lesage et al.28 In addition, in neonatal MUN offspring, other important components of steroidogenesis such as STAR protein, which drives the movement of cholesterol from the outer to the inner mitochondrial membrane,13 and CYP11A1, which is the side-chain cleavage enzyme that is involved in the early steps of steroidogenesis, were both inhibited. This generalized inhibition of steroidogenic enzyme mRNA expression in the P1 offspring explains their lower circulating levels of CORT and could signify reduced stress reactivity and increased inflammation.34 In the MUN dams, although both CYP11B1 and CYP11B2 were suppressed, CORT levels were elevated. This discrepancy could potentially be secondary to a nutritional stress-driven central CRF drive in these dams, which would stimulate ACTH secretion.35 In contrast to MUN dams, in the P1 offspring the central expression of MCR/GCR and responsiveness to stress is not fully developed.36,37 The maturation of negative feedback loop axis for both GCR and MCR appears to be tissue-dependant in rats. In case of GCR, GC-induced inhibition of GCR mRNA is evident between 2 and 7 days of life in liver and between 7 and 14 days in the brain,38 whereas for MCR, negative feedback was operative in the kidney but not brain in neonates.36 Currently, there are no studies that have addressed the maturation of the negative feedback loop between circulating GC/MC and adrenal steroidogenic enzymes. Our finding of reduced circulating CORT levels in P1 offspring is in agreement with Lesage et al who also used a 50% food restriction model and reported an increase in CORT levels at birth in the food-restricted offspring, which then decreased 2 hours later,28 and with human data showing lower cortisol levels in individuals with the metabolic syndrome.39 Similarly, in another model of programming employing low-sodium diet during pregnancy, fetal levels of CORT were reduced in the exposed fetuses and this was associated with decreased adrenal expression of 11 beta hydroxylase.40 Fetal exposure to long-term hypoxia also inhibited the expression of key enzymes regulating cortisol biosynthesis in the ovine fetus,41 whereas in a protein-restriction model of fetal programming plasma CORT levels were unaffected,42 illustrating the importance of the nature of stress in fetal programming.
The adrenal response to nutritional programming is highly species-dependant. Gestational undernutrition resulted in an increased mRNA expression of ACTH-R and STAR in the adrenals during late gestation in sheep17 and in ACTH levels and response to corticotropin-releasing hormone (CRH).16 Coulter et al also found a decreased level of expression of STAR in the growth-restricted fetus; however, this did not affect adrenal steroidogenesis.43 Based on these findings, these investigators suggested that in the ovine growth-restricted fetus, steroidogenesis may not be ACTH-dependent.43 In another study using the sheep model, brief undernutrition in utero did not alter adult adrenal protein expression of P450C17 and P45011B1.44
The global inhibition of adrenal steroidogenic enzymes in the P1 MUN adrenals could also be secondary to elevated maternal leptin levels in MUN dams, which could directly inhibit offspring steroidogenic enzymes, and inhibit offspring leptin receptor expression, as demonstrated by our data. Our group previously reported that maternal food restriction as other types of systemic stress45 results in elevated leptin levels most likely from placental and adipose tissue origin.46 Elevated maternal leptin could also directly inhibit fetal adrenal steroidogenic enzymes as leptin was reported to have a direct adrenal suppressive effect on steroidogenic enzymes,20,21,47 and on STAR protein expression.48 Exposure to high levels of leptin would also enhance GC negative feedback in the brain through increased expression of GCR in the hippocampus and paraventricular nucleus as previously reported.49
The profile of adrenal steroidogenic enzymes in the adult MUN offspring changed during the course of development in a gender-specific manner, demonstrating different adaptational strategies in male versus female MUN offspring. Others have also reported on gender differences in basal cortisol levels or responsiveness to stress in adult offspring of other species such as sheep50 and guinea pigs.6 Our data showed that the generalized inhibition of steroidogenic enzymes was no longer evident in the adult MUN offspring. In adult male MUN offspring, the adrenal expression of HSD1, CYP11A1, CYP11B2, and GCR were markedly increased, a pattern opposite to that of the neonates. In contrast to males, adult MUN female profile of steroidogenic enzymes showed fewer changes compared with controls, with only an inhibition of CYP17A1 and an increase in CYP11A1 expression. In adult male MUN offspring, a trend toward an inhibition of CYP17A1 was found. This inhibitory trend in CYP17A1 expression was also noted in the P1 offspring. CYP17A1 is a key enzyme in the steroidogenic pathway, catalyzing the addition of a hydroxyl group to pregnenolone and progesterone, thereby controlling the amount of substrates for downstream steps for synthesis of mineralocorticoids, glucocorticoids, androgens, and estrogens. Inhibition of CYP17A1 in the MUN offspring in the different age group offspring could account for the lower basal circulating CORT levels in them and could potentially lead to reduced synthesis of sex hormone levels in both genders.
In adult MUN offspring of both genders, there was an increase in adrenal CYP11A1 mRNA (cholesterol side-chain cleavage enzyme) expression. This enzyme converts cholesterol into pregneolone, and its expression is principally regulated by angiotensin II, luteinizing hormone (LH), and ACTH.51 Previous studies have demonstrated an activation of the renin-angiotensin system in MUN offspring.52 Furthermore, the adrenal expression of angiotensin receptor 1b is increased in these offspring,53 suggesting increased responsiveness of the adrenal to circulating angiotensin II. Elevated angiotensin II levels and increased responsiveness to its effects in MUN offspring would be expected to stimulate the expression of CYP11A1 mRNA as demonstrated by our data. The more pronounced inhibition of basal CORT levels in adult male compared with female MUN offspring could also be secondary to negative feedback inhibition of CORT at the adrenal level due to (1) increased HSD1 mRNA expression which would lead to increased active GC levels within the adrenal thereby inhibiting other steroidogenic enzymes locally and (2) increased GCR expression in male adrenals would make these tissues more sensitive to circulating CORT which stimulates HSD1 expression.54 Gender-related differences in GCR/MCR imbalance and steroidogenic enzyme expression in adult MUN offspring could also account for a relative protection of female MUN offspring from development of programmed hypertension as compared with males55,56 since in males but not females the expression of aldosterone synthesizing enzyme, CYP11B2, was increased. Our data also showed an increase in leptin receptor expression in MUN adrenals particularly in males. Although both male and female MUN have hyperleptinemia, leptin levels in male MUN are higher compared with females,22 yet leptin receptor expression is also higher in males compared with females, suggesting a probable leptin resistance at the adrenal level that develops during the course of development and is more exaggerated in MUN males.
The common finding of increased adrenal ACTH-R expression in adult males and females would suggest an increased sensitivity of these animals to the effects of stress. Our data are in agreement with Waddell et al who reported increased adrenal expression of the ACTH-R in 6-month-old offspring and increased stress-induced levels of plasma corticosterone in a rat model of programming in which dams received dexamthasone during gestation.18 In humans, low birth weight is associated with increased urinary glucocorticoid excretion in children57 and with elevated basal plasma cortisol concentration58 and greater adrenocortical responsiveness to ACTH in adults.59 This increased responsiveness to stress has been proposed to contribute to the pathogenesis of chronic diseases.58
In summary, our data demonstrate that the adrenal gland is an important target for nutritional programming effects. The changing pattern of GC/MC and ACTH-R in the adrenal would imply differing sensitivities of this gland to circulating GC/MC and ACTH during the course of development. The programmed adrenal expression of steroidogenic enzymes, GC, and leptin receptors in a gender-specific and developmentally regulated manner supports an important contribution of the adrenal gland to the development of metabolic syndrome in MUN offspring and the gender differences in response to maternal undernutrition.
Acknowledgment
We wish to thank Ms Jeannie Park and Ms Diane Park for their help in preparation of this manuscript.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: NIH RO3 HD054920-01 (O.Khorram) and University of California San Diego Chancellor’s Research Scholarship (N. Khorram).
Reference
- 1. Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301(6746):259–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59(3):279–289 [DOI] [PubMed] [Google Scholar]
- 3. Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dutriez-Casteloot I, Breton C, Coupe B, et al. Tissue-specific programming expression of glucocorticoid receptors and 11 beta-HSDs by maternal perinatal undernutrition in the HPA axis of adult male rats. Horm Metab Res. 2008;40(4):257–261 [DOI] [PubMed] [Google Scholar]
- 5. Vieau D, Sebaai N, Leonhardt M, et al. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2007;32(suppl 1):S16–S20 [DOI] [PubMed] [Google Scholar]
- 6. Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280(5):E729–E739 [DOI] [PubMed] [Google Scholar]
- 7. Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Dupouy JP. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinol. 2002;75(1):45–54 [DOI] [PubMed] [Google Scholar]
- 8. Lesage J, Dufourny L, Laborie C, et al. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J Neuroendocrinol. 2002;14(2):135–143 [DOI] [PubMed] [Google Scholar]
- 9. Yang K, Challis JR. Fetal and adult sheep adrenal cortical cells contain glucocorticoid receptors. Biochem Biophys Res Commun. 1989;162(2):604–611 [DOI] [PubMed] [Google Scholar]
- 10. Darbeida H, Naaman E, Durand P. Glucocorticoid induction of the maturation of ovine fetal adrenocortical cells. Biochem Biophys Res Commun. 1987;145(3):999–1005 [DOI] [PubMed] [Google Scholar]
- 11. Picard-Hagen N, Darbeida H, Durand P. Glucocorticoids enhance the cholesterol side-chain cleavage activity of ovine adrenocortical mitochondria. J Steroid Biochem Mol Biol. 1995;55(1):57–65 [DOI] [PubMed] [Google Scholar]
- 12. Root B, Abrassart J, Myers DA, Monau T, Ducsay CA. Expression and distribution of glucocorticoid receptors in the ovine fetal adrenal cortex: effect of long-term hypoxia. Reprod Sci. 2008;15(5):517–528 [DOI] [PubMed] [Google Scholar]
- 13. Manna PR, Dyson MT, Stocco DM. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod. 2009;15(6):321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xing Y, Parker CR, Edwards M, Rainey WE. ACTH is a potent regulator of gene expression in human adrenal cells. J Mol Endocrinol. 2010;45(1):59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mountjoy KG, Bird IM, Rainey WE, Cone RD. ACTH induces up-regulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol. 1994;99(1):R17–R20 [DOI] [PubMed] [Google Scholar]
- 16. Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biol Reprod. 2002;66(5):1562–1569 [DOI] [PubMed] [Google Scholar]
- 17. Edwards LJ, Bryce AE, Coulter CL, McMillen IC. Maternal undernutrition throughout pregnancy increases adrenocorticotrophin receptor and steroidogenic acute regulatory protein gene expression in the adrenal gland of twin fetal sheep during late gestation. Mol Cell Endocrinol. 2002;196(1-2):1–10 [DOI] [PubMed] [Google Scholar]
- 18. Waddell BJ, Bollen M, Wyrwoll CS, Mori TA, Mark PJ. Developmental programming of adult adrenal structure and steroidogenesis: effects of fetal glucocorticoid excess and postnatal dietary omega-3 fatty acids. J Endocrinol. 2010;205(2):171–178 [DOI] [PubMed] [Google Scholar]
- 19. Molendi-Coste O, Grumolato L, Laborie C, et al. Maternal perinatal undernutrition alters neuronal and neuroendocrine differentiation in the rat adrenal medulla at weaning. Endocrinology. 2006;147(6):3050–3059 [DOI] [PubMed] [Google Scholar]
- 20. Walker CD, Salzmann C, Long H, Otis M, Roberge C, Gallo-Payet N. Direct inhibitory effects of leptin on the neonatal adrenal and potential consequences for brain glucocorticoid feedback. Endocr Res. 2004;30(4):837–844 [DOI] [PubMed] [Google Scholar]
- 21. Hsu HT, Chang YC, Chiu YN, Liu CL, Chang KJ, Guo IC. Leptin interferes with adrenocorticotropin/3’,5'-cyclic adenosine monophosphate (cAMP) signaling, possibly through a Janus kinase 2-phosphatidylinositol 3-kinase/Akt-phosphodiesterase 3-cAMP pathway, to down-regulate cholesterol side-chain cleavage cytochrome P450 enzyme in human adrenocortical NCI-H295 cell line. J Clin Endocrinol Metab. 2006;91(7):2761–2769 [DOI] [PubMed] [Google Scholar]
- 22. Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R91–R96 [DOI] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 24. Khorram O, Momeni M, Desai M, Ross MG. Nutrient restriction in utero induces remodeling of the vascular extracellular matrix in rat offspring. Reprod Sci. 2007;14(1):73–80 [DOI] [PubMed] [Google Scholar]
- 25. Khorram O, Khorram N, Momeni M, et al. Maternal undernutrition inhibits angiogenesis in the offspring: a potential mechanism of programmed hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R745–R753 [DOI] [PubMed] [Google Scholar]
- 26. Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R955–R962 [DOI] [PubMed] [Google Scholar]
- 27. Langley-Evans SC, Phillips GJ, Benediktsson R, et al. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17(2-3):169–172 [DOI] [PubMed] [Google Scholar]
- 28. Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142(5):1692–1702 [DOI] [PubMed] [Google Scholar]
- 29. Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(suppl 3):U49–U62 [DOI] [PubMed] [Google Scholar]
- 30. Seckl JR. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr Opin Pharmacol. 2004;4(6):597–602 [DOI] [PubMed] [Google Scholar]
- 31. Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30(6):1525–1530 [DOI] [PubMed] [Google Scholar]
- 32. Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15(5):537–544 [DOI] [PubMed] [Google Scholar]
- 33. Loose DS, Do YS, Chen TL, Feldman D. Demonstration of glucocorticoid receptors in the adrenal cortex: evidence for a direct dexamethasone suppressive effect on the rat adrenal gland. Endocrinology. 1980;107(1):137–146 [DOI] [PubMed] [Google Scholar]
- 34. Gessi S, Merighi S, Borea PA. Glucocorticoid’s pharmacology: past, present and future. Curr Pharm Des. 2010;16(32):3540–3553 [DOI] [PubMed] [Google Scholar]
- 35. Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–292, vii [DOI] [PubMed] [Google Scholar]
- 36. Kalinyak JE, Bradshaw JG, Perlman AJ. The role of development and adrenal steroids in the regulation of the mineralocorticoid receptor messenger RNA. Horm Metab Res. 1992;24(3):106–109 [DOI] [PubMed] [Google Scholar]
- 37. Patchev VK, Hayashi S, Orikasa C, Almeida OF. Ontogeny of gender-specific responsiveness to stress and glucocorticoids in the rat and its determination by the neonatal gonadal steroid environment. Stress. 1999;3(1):41–54 [DOI] [PubMed] [Google Scholar]
- 38. Kalinyak JE, Griffin CA, Hamilton RW, Bradshaw JG, Perlman AJ, Hoffman AR. Developmental and hormonal regulation of glucocorticoid receptor messenger RNA in the rat. J Clin Invest. 1989;84(6):1843–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bahr V, Pfeiffer AF, Diederich S. The metabolic syndrome X and peripheral cortisol synthesis. Exp Clin Endocrinol Diabetes. 2002;110(7):313–318 [DOI] [PubMed] [Google Scholar]
- 40. Bibeau K, Battista MC, Houde V, Brochu M. Fetal adrenal gland alterations in a rat model of adverse intrauterine environment. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R899–R911 [DOI] [PubMed] [Google Scholar]
- 41. Myers DA, Hyatt K, Mlynarczyk M, Bird IM, Ducsay CA. Long-term hypoxia represses the expression of key genes regulating cortisol biosynthesis in the near-term ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1707–R1714 [DOI] [PubMed] [Google Scholar]
- 42. Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996;126(6):1578–1585 [DOI] [PubMed] [Google Scholar]
- 43. Coulter CL, McMillen IC, Bird IM, Salkeld MD. Steroidogenic acute regulatory protein expression is decreased in the adrenal gland of the growth-restricted sheep fetus during late gestation. Biol Reprod. 2002;67(2):584–590 [DOI] [PubMed] [Google Scholar]
- 44. Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JR. Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology. 2003;144(7):2933–2940 [DOI] [PubMed] [Google Scholar]
- 45. Konishi N, Otaka M, Odashima M, et al. Systemic stress increases serum leptin level. J Gastroenterol Hepatol. 2006;21(7):1099–1102 [DOI] [PubMed] [Google Scholar]
- 46. Jelks A, Belkacemi L, Han G, Chong WL, Ross MG, Desai M. Paradoxical increase in maternal plasma leptin levels in food-restricted gestation: contribution by placental and adipose tissue. Reprod Sci. 2009;16(7):665–675 [DOI] [PubMed] [Google Scholar]
- 47. Kruse M, Bornstein SR, Uhlmann K, Paeth G, Scherbaum WA. Leptin down-regulates the steroid producing system in the adrenal. Endocr Res. 1998;24(3-4):587–590 [DOI] [PubMed] [Google Scholar]
- 48. Cherradi N, Capponi AM, Gaillard RC, Pralong FP. Decreased expression of steroidogenic acute regulatory protein: a novel mechanism participating in the leptin-induced inhibition of glucocorticoid biosynthesis. Endocrinology. 2001;142(8):3302–3308 [DOI] [PubMed] [Google Scholar]
- 49. Proulx K, Clavel S, Nault G, Richard D, Walker CD. High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology. 2001;142(11):4607–4616 [DOI] [PubMed] [Google Scholar]
- 50. Gardner DS, Van Bon BW, Dandrea J, et al. Effect of periconceptional undernutrition and gender on hypothalamic-pituitary-adrenal axis function in young adult sheep. J Endocrinol. 2006;190(2):203–212 [DOI] [PubMed] [Google Scholar]
- 51. Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood). 2009;234(8):880–907 [DOI] [PubMed] [Google Scholar]
- 52. Riviere G, Michaud A, Breton C, et al. Angiotensin-converting enzyme 2 (ACE2) and ACE activities display tissue-specific sensitivity to undernutrition-programmed hypertension in the adult rat. Hypertension. 2005;46(5):1169–1174 [DOI] [PubMed] [Google Scholar]
- 53. Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS ONE. 2010;5(2):e9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shimojo M, Whorwood CB, Stewart PM. 11 beta-Hydroxysteroid dehydrogenase in the rat adrenal. J Mol Endocrinol. 1996;17(2):121–130 [DOI] [PubMed] [Google Scholar]
- 55. Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50(4):679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roghair RD, Segar JL, Volk KA, et al. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R651–R662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clin Endocrinol (Oxf). 1996;45(6):721–726 [DOI] [PubMed] [Google Scholar]
- 58. Phillips DI, Barker DJ, Fall CH, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome?. J Clin Endocrinol Metab. 1998;83(3):757–760 [DOI] [PubMed] [Google Scholar]
- 59. Reynolds RM, Walker BR, Syddall HE, et al. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86(1):245–250 [DOI] [PubMed] [Google Scholar]