Abstract
Maternal fever and/or chorioamnionitis at term are associated with an increased prevalence of adverse neurobehavioral outcomes in exposed offspring. Since the mechanisms of such injury are currently unknown, the objectives of this study were to elucidate whether intrauterine inflammation at term results in fetal brain injury. Specifically, we assessed brain injury by investigating the cytokine response, white matter damage, and neuronal injury and viability. A mouse model of intrauterine inflammation at term was utilized by injecting lipopolysaccharide (LPS), or normal saline into uterine horn. Compared to saline-exposed, LPS-exposed fetal brains had significantly increased IL-1β and IL-6 messenger RNA (mRNA) expression (P < .05 for both) and IL-6 protein levels by enzyme-linked immunosorbent assay (ELISA; P < 0.05). Fetal neurons were affected by the intrauterine and fetal brain inflammation, as demonstrated by significantly decreased microtubule-associated protein 2 (MAP2) mRNA expression and a decrease in immunocytochemical staining (a marker of neuronal cytoskeleton development; P < .05), altered neuronal morphology (P < 0.05), and delayed neurotoxicity (P < .05). These fetal neuronal changes occurred without overt changes in white matter damage markers. Marker of astrocyte development and astrogliosis (glial fibrillary acidic protein [GFAP]) did not show an increase; pro-oligodendrocyte marker (PLP1/DM20) was not significantly changed (P > .05). These studies may provide a critical mechanism for the observed long-term adverse neurobehavioral outcomes after exposure to chorioamnionitis at term.
Keywords: chorioamnionitis, mouse model of intrauterine inflammation, neuroinflammation, neuronal injury, delayed neurotoxicity
Introduction
Chorioamnionitis, an inflammation of the fetal membranes, complicates up to 10% of all pregnancies and, specifically, up to 2% of labors at term.1,2 The presence of maternal fever and/or chorioamnionitis at term has been shown to be associated with adverse neurodevelopmental outcomes.3–5 Specifically, chorioamnionitis increases the risk for neonatal encephalopathy to up to 12.5% (independent of neonatal sepsis)5 and the risk for cerebral palsy (CP) by 2- to 12-fold in term infants.3,6–12 Chorioamnionitis is thought to play a role in the development of fetal brain injury due to elevated circulating cytokines and coexistence of inflammatory and thrombotic lesions.12,13
To date, most of the basic science research regarding the effect of intrauterine inflammation has focused on investigating mechanisms of adverse neurological outcomes in the context of preterm birth.14–19 Specifically, in animal models, it has been demonstrated that exposure to intrauterine inflammation in the preterm period is associated with (1) an increase in proinflammatory cytokines in the fetal brain; (2) white matter damage (WMD), and (3) neuronal injury.14–19 However, in term gestations, the mechanisms of the associated neurobehavioral findings remain unknown. Despite the assumption that the term brain might be more resistant to injury,20 epidemiologic studies indicate that chorioamnionitis at term has a higher rate of CP than in preterm infants.9 Hence, our hypothesis is that chorioamnionitis at term adversely affects fetal brain development. Therefore, for these studies, we used a mouse model of intrauterine inflammation at term to determine whether an exposure to prenatal inflammation at the end of gestation induces fetal brain injury. Specifically, the objectives of this study were to investigate whether exposure to intrauterine inflammation at term caused an inflammatory response in the fetal brain, WMD, and/or neuronal injury.
Materials and Methods
Mouse Model of Intrauterine Inflammation at Term
CD-1 out-bred, timed pregnant mice (Charles River Laboratories, Wilmington, Massachusetts) were utilized. Guidelines for the care and use of animals were approved by the University of Pennsylvania. Mice were mated and day of plug was considered embryonic day 1 (E1). As delivery day in CD-1 mice routinely occurs on E19, survival surgery and intrauterine injections of LPS or normal saline (NS) were performed on day 18.5 of gestation as previously reported.21 Briefly, a continuous isofluorane/oxygen anesthesia was supplied by a mask that fits over the mouse’s face. After deep anesthesia was reached, a mini-laparotomy was performed in the lower abdomen. The right uterine horn was isolated and either lipopolysaccharide (LPS, 250 μg/dam in 100 μL NS; from Escherichia coli, 055:B5, Sigma Chemical Co, St Louis, Missouri) or an equal volume of NS (control) was infused into the uterus between 1st and 2nd gestational sac closest to the cervix. Routine closure was performed and the dams were recovered in individual cages. Dams (both control and LPS exposed) were humanely euthanized 6 hours after surgery by utilizing carbon dioxide (CO2). At this time, 4 fetuses per dam were immediately removed from the lower uterine horns (the same proximity to LPS infusion site) for either primary cortical neuronal cultures or for the assessment of messenger ribonucleic acid (mRNA) expression as described below.
Quantitative Polymerase Chain Reaction for Expression of Cytokines and Markers of Fetal Brain Development in Whole Fetal Brains
Fetal brains were isolated as per our prior reports.15,16 Total RNA was extracted from whole fetal brains with Trizol (Invitrogen). Complimentary deoxyribonucleic acid (cDNA) was generated with high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, California). Primer sets, conjugated to Taqman MGB probes, were used for quantitative polymerase chain reaction (QPCR; Applied Biosystems). The expression of the following gene targets was investigated: (1) proinflammatory cytokines (IL-1β, IL-6, TNF-α and IL-10); (2) markers of fetal brain development (microtubule-associated protein 2 [MAP2], a neuronal marker; glial fibrillary acidic protein (GFAP), an astrocyte marker and marker of astrogliosis; and proteolipid protein 1 (PLP1/DM20), a marker of pro-oligodendrocytes. Quantitative polymerase chain reactions were carried out with equivalent dilutions of each cDNA sample on the Applied Biosystems Model 7900 sequence detector PCR machine, as previously reported from our laboratory.15,17,22,23 The relative abundance of the target of interest was divided by the relative abundance of 18S in each sample to generate a normalized abundance for the target of interest. In all, 4 fetal brains were extracted per dam and constituted 1 sample. A total of 6 dams (n = 6) were utilized for the LPS group and 9 dams (n = 9) were utilized for the control group. All samples were analyzed in duplicate. The duplicate results for each dam were averaged prior to the statistical analysis.
Enzyme-Linked Immunosorbent Assay for Cytokine Expression
Commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, Minnesota) were used to investigate the expression of IL-1β and IL-6. The total amount of protein in fetal brain specimens was measured by colorimetric bicinchoninic acid (BCA) Protein Assay Reagent kit (Fisher Scientific, Pittsburgh, Pennsylvania) per the manufacturer’s instructions. Six dams were utilized per treatment group. In all, 3 fetal brains from right lower uterine horn represented each dam (one n). All tests were performed in duplicate.
Primary Cortical Neuronal Cultures
Using sterile technique, E18.5 fetal brains were harvested and placed into Petri dishes containing cold Ca++/Mg++-free Hank’s Balanced Salt Solution (HBSS; Invitrogen, Carlsbad, California), pH 7.4. The fetal cortex was separated from meninges, olfactory bulbs, brain stem, and cerebellum. Each cortex was minced, placed in 4-mL neurobasal medium (NBM) containing 0.03% Trypsin (Invitrogen) and incubated for 15 minutes at 37°C and 5% CO2. Brain tissue was removed and placed in 4.5 mL NBM containing 10% fetal bovine serum (FBS) and allowed to settle to inactivate the trypsin. The medium was decanted and replaced with NBM supplemented with B-27 vitamin (Invitrogen) and 0.5 mmol/L l-glutamine and cells were dissociated by trituration. This media combination, NBM in the absence of FBS, allows for the selective growth of neurons and not glia (astrocytes or microglia).15,24–26 Cells were plated at 4 × 104 cells/mL density on poly-l-lysine (1 mg/mL; Sigma-Aldrich, St Louis, Missouri)-coated glass coverslips, using 6- and 12-well culture plates. Groups were plated to equal density for each experiment. For each experiment, 4 fetal brains from 1 dam constituted 1 culture. A total of 6 dams (n = 6) from the LPS group and 9 dams (n = 9) from the control group were utilized for the analysis and the comparison of the dendritic counts. All experiments were performed in triplicate to assure the consistency of the results.
Immunocytochemistry
Cortical cell cultures were fixed and stained at days in vitro (DIV) 3, 10, and 14 to assess morphologic changes between the treatment groups, using double immunofluorescence as previously reported.15,16 A mouse monoclonal antibody to MAP2 (Sigma-Aldrich) was used to identify dendrites and cell bodies at a dilution of 1:100. A rabbit polyclonal antibody to 200 kDa Neurofilament protein (NF-200, Sigma-Aldrich) was used to label the entire cell at a dilution of 1:400. Alexa Fluor goat anti-mouse 488 (Invitrogen) and Alexa Fluor goat anti-rabbit 568 (Invitrogen) were used for immunofluorescence at 1:500 dilution. Confocal microscopy (Leica SP2 Confocal) was utilized for the morphological evaluation of the neurons.
Quantitative Analysis of Dendritic Processes From Cortical Culture Experiments
Dendrite processes were analyzed at DIV 3, using previously described techniques.15,24–26 Briefly, the cells were selected at random using at least 3 coverslips for each condition. In all, 1 coverslip represented 4 fetal brains from 1 dam and 3 different dams were used for each condition in order to quantify the processes emanating from each cell body. Neurons from each treatment group were evaluated at a final image magnification of ×400. Individual neurons were selected at random if they were clearly defined and not overlapping with other neurons. Approximately, 10 neurons were evaluated from each coverslip. Each coverslip represented 1 dam (with pooled cultures from 4 fetal brains from that same dam). This experiment was repeated 3 times. Fluorescent images were recorded and analyzed using a Dell Latitude D620, using an image processing program (Image J 1.37v).
Quantification of Neuronal Viability
The comparison of cell viability between the treatment groups was done at DIV 3 and 14 of fetal cortical neurons, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Mitochondrial Activity Assay (Sigma-Aldrich) to measure the mitochondrial activity. Cell viability on DIV 3 reflects neurotoxicity associated with an acute insult, while DIV 14 viability indicates the degree of delayed neurotoxicity from an in vivo exposure to LPS administration. Briefly, reconstituted MTT solution was added in the amount of 10% of the total volume per well. Plates were incubated at 37°C for 2 hours. Following the incubation period, 1 mL MTT solubilization solution was added to each well and the tissue culture plates were placed on shaker for 10 minutes; the remaining precipitate was solubilized by trituration. The absorbance of the samples was assessed at 2 different wavelengths (570 and 690 nm). The difference between the measurements was calculated to obtain normalized absorbance (viability of cells). Each well represented a primary neuronal culture. For each dam, 12 replicates of primary cortical culture were used. A total of 6 dams were utilized per each treatment group and time point.
Statistical Analysis
The data were tested for normality. For normally distributed data, a t test was used to compare the values between the treatment groups. For data which were not normally distributed, Wilcoxon-Mann-Whitney test was utilized. Specifically, for the QPCR, ELISA analysis, and the MTT assay a t test was used. For the comparison of dendritic counts, the analysis was performed with Wilcoxon-Mann-Whitney test.
Results
Term Intrauterine Inflammation Increases Proinflammatory Cytokine mRNA Expression in Whole Fetal Brain
We sought to investigate whether the intrauterine inflammation at term elicited a proinflammatory cytokine response. In whole fetal brains, at E18.5, mRNA expression of IL-1β and IL-6 were significantly increased between the treatment groups (12.4-fold increase IL-1β in LPS exposed; P = .017, t test; 3.2-fold increase in IL-6; P = .04, t test; Figure 1 ). The cytokines, IL-10, and TNF-α, were not differentially expressed between groups (P > .05, t test for both; Figure 1).
Figure 1.
Quantitative polymerase chain reaction evaluation of cytokine release in whole fetal brains in response to intrauterine inflammation at E18.5. Bar graphs represent means and standard errors (P < .05, t test; n = 6-9 animals per treatment group). In whole fetal brains, at E18.5, mRNA expression of IL-1β and IL-6 were significantly increased between the treatment groups (12.4-fold increase IL-1β in LPS-exposed; P = .017, t test; 3.2-fold increase in IL-6; P = .04, t test). IL-10 and TNF-α were not differentially expressed between groups (P > .05, t test for both). *P < .05.
IL-6 Protein Levels are Increased in Whole Fetal Brains 6 Hours After Exposure to Intrauterine Inflammation
Message RNA levels were found to be increased for the IL-1β and IL-6. Therefore, protein levels of these cytokines at the same time point were assessed. While there was an increase in IL-1β in the LPS-exposed fetal brains (Figure 2A), this did not reach statistical significance (1.9-fold increase; P > .05; t test). IL-6 protein levels in the LPS-exposed fetal brains were statistically significantly increased as compared to NS-exposed fetal brains (2.6-fold increase; P < .05, t test; Figure 2B).
Figure 2.
Enzyme-linked immunosorbent assay evaluation for the IL-1β and IL-6 expression in whole fetal brains at E18.5 of gestation. (A) The results of ELISA for IL-1β and (B) the results for IL-6 proinflammatory cytokines. Bar graphs represent means and standard errors (P > .05, t test for both groups; n = 6 animals per treatment group). There was a 1.9-fold increase in IL-1β protein level in the LPS-exposed fetal brains over the NS-exposed fetal brains; this did not reach statistical significance (P > .05; t test; [A]). IL-6 protein level in the LPS-exposed fetal brains was statistically significantly increased as compared to NS-exposed fetal brains (2.6-fold increase; P < .05, t test; [B]). *P < .05.
Term Intrauterine Inflammation Decreases Astrocyte and Neuronal Marker mRNA Expression
Glial fibrillary acidic protein, an astrocytic marker and a marker for astrogliosis, and MAP-2, a neuronal marker, were both decreased in the brains of LPS-exposed fetuses (P = .02, and P = .04, t test, respectively, Figure 2). Proteolipid protein/DM20, pro-oligodendrocyte marker, was not significantly different between the groups (P > .05, t test, Figure 3 ).
Figure 3.
Quantitative polymerase chain reaction evaluation of markers of fetal brain development in whole fetal brains in response to intrauterine inflammation at E18.5. Bar graphs represent means and standard errors (*P < .05, t test; n = 6-9 animals per treatment group). Glial fibrillary acidic protein, an astrocytic marker and a marker for astrogliosis, and MAP-2, a neuronal marker, were both decreased in the brains of LPS-exposed fetuses (P = .02, and P = .04, t test, respectively). PLP/DM20, pro-oligodendrocyte marker was not significantly different between groups (P > .05, t test). *P < .05.
Neuronal Morphology Change in Neuronal Culture of LPS-Exposed Cells
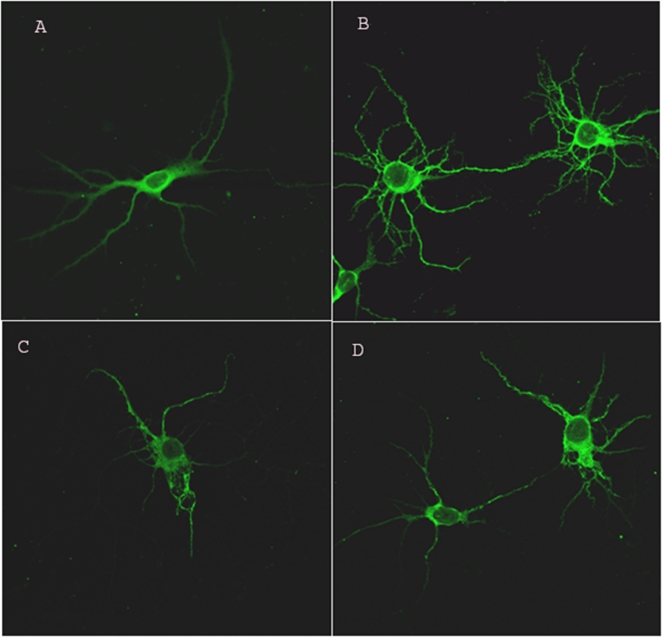
Since the mRNA levels of MAP-2, a cytoskeletal neuronal maker, were found to be decreased, we set to investigate the neuronal injury in vitro and to assess the protein levels of MAP-2 by immunohistochemistry. Saline-exposed neurons (control) had numerous dendritic processes and a discrete cytoskeletal morphology at DIV 3 (Figure 4A and B). In contrast, the LPS-exposed neurons demonstrated decreased growth and abnormal cytoskeletal morphology (Figure 4C and D).
Figure 4.
Immunocytochemistry for microtubule-associated protein 2 (MAP2; a neuronal cytoskeletal marker) in primary neuronal cortical cultures at DIV 3. (A and B) The neuronal morphology in control (normal saline [NS]) group. The control cells demonstrated numerous processes and discrete cellular morphology. The LPS-exposed neurons (C and D) demonstrate a decreased growth of dendrites, abnormal morphology, including overall decreased MAP2 staining in single cells as well as with cell-cell interaction. Magnification ×400, Zoom 2.
Quantitative Analysis of Dendritic Processes
As a means to quantify the neuronal injury, the number of dendritic processes was recorded from cultured neurons at DIV 3. The use of the number of processes on cultured neurons as an objective method to document neuronal injury is well established in the literature.25–27 The fetal neurons exposed to LPS in vivo demonstrated a decreased number of dendritic processes compared to saline-exposed neurons (P < .05, Wilcoxon-Mann-Whitney test; Figure 5 ). The experiment had consistent results across 3 trials.
Figure 5.
Number of processes of cultured neuronal cells at DIV 3. Bar graphs represent medians and standard deviations for each group; n = 6-9 animals per treatment group. There were significantly less processes present on LPS-exposed cells at DIV 3 (P < .001, Wilcoxon-Mann-Whitney test). *P < .05.
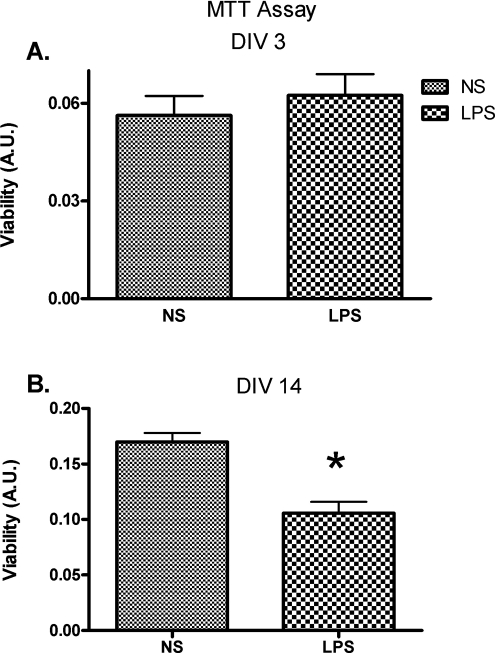
Exposure to Term Intrauterine Inflammation Decreases Neuronal Viability at DIV 14
Neuronal cell viability in the cortical cultures was assessed at DIV 3 and 14. Statistical analysis demonstrated no significant difference in DIV 3 viability between the treatment groups (Figure 6A; P < 0.05, t test). However, there was a difference in cell viability between the treatment groups at DIV 14 (Figure 6B; P > .05, t test).
Figure 6.
Neuronal cell viability at DIV 3 and DIV 14. Cell viability was assessed with a mitochondrial activity assay. Bar graphs represent means and standard errors for each group. There was no statistically significant difference between cell viability of the 2 treatment groups at DIV 3 ([A]; P > .05, t test). On the contrary, at DIV 14, there was a statistically significant difference between the treatment groups, with less neuronal viability in the LPS-exposed group ([B]; P < .05, t test). A.U. indicates absorbance units. *P < .05.
Discussion
The principal finding of our study was that intrauterine inflammation at term results in fetal brain injury as demonstrated by increase in proinflammatory cytokines in fetal brain and neuronal damage with delayed neurotoxicity. In these studies, intrauterine inflammation did not result in changes associated with WMD such as astrogliosis (increase in GFAP marker) or decrease in pro-oligodendrocyte marker.
Our study underlines the importance of neuronal injury in response to intrauterine inflammation as a unifying mechanism, leading to neurodegenerative and neurobehavioral disorders from prenatal exposure to inflammation. Neuronal injury, in this study, is characterized by an abnormal cytoskeletal formation, based on the decreased MAP2 mRNA levels and protein levels, and a decrease in dendritic arbor. Abnormalities in cytoskeletal structure and neuronal arborization have been shown to impact synaptic connectivity and result in neurodegenerative and neurobehavioral disorders.28–32 Specifically, dendritic arborization has been found to play a role in neurobehavioral disorders such as the Rett syndrome and autism.33–35 The cumulative evidence suggests that neurodegenerative and neurobehavioral disorders are associated with cytoskeletal alterations in neurons that, in turn, have an altered synaptic connectivity. The response of the fetal brain to intrauterine inflammation may have a similar implication.
In addition to the observed neuronal injury, our results suggest a delayed neurotoxicity as LPS-exposed fetal neurons exhibited delayed changes in neuronal viability. The presence of delayed neurotoxicity indicates that neurons are continuously affected by the in vivo insult and the manifestations of such injury may not be acute. We hypothesize that the neuronal changes and delayed neurotoxicity observed in this model may be mechanistically responsible for neurobehavioral deficits, such as CP, present in offspring exposed to chorioamnionitis at term. As there are no proposed mechanisms of neuronal injury in the setting of chorioamnionitis at term, these data are crucial for directing new research and therapeutic directions for neuroprophylaxis in a setting of chorioamnionitis at term.
While animal models of fetal and neonatal brain injury following an inflammation have inherent limitations, such studies are not possible in humans. Animal models have been designed to elucidate the effect of inflammation on fetal and neonatal brain development. 17–19,36–38 However, these are either systemic models of inflammation with intraperitoneal infusion of LPS36,38 or these models concentrate on preterm brain development.18,19,37 Chorioamnionitis represents a local, intrauterine infection and is unique from the clinical scenarios observed with systemic infections, such as pyelonephritis and pneumonia. Therefore, the use of an appropriate model which mimics intrauterine inflammation is of paramount importance in order to investigate the effects of in utero inflammation on the fetal brain. Hence, the utilization of such animal model represents a strength of our study. While neuronal development of rodents is similar to humans, 1 of the possible limitations is that at this gestational age, we could only evaluate pro-oligodendrocyte markers and not the direct changes in oligodendrocytes since the maturation of the oligodendrocytes in mice is not complete until postnatal day 21.
It is noted that this model is not meant to be a specific model of CP. The model is used to accurately mimic fetal exposure to inflammation as occurs in most human cases of term chorioamnionitis. As such, we can explore the effect of intrauterine inflammation on brain development. Future work will need to determine whether the exposed offspring will have motor deficits that are similar to CP. Recent reports using a rabbit model of intrauterine inflammation provide biological plausibility that inflammatory exposure to a rodent fetus will elicit motor deficits.39 In this rabbit model, the prenatal administration of endotoxin results in behavioral abnormalities that are similar to movement disorders observed in CP.
White matter damage, including astrogliosis, and the changes in the pro-oligodendrocyte precursors have been studied extensively in other models.17–19,36,38,40 However, while WMD may be sufficient for adverse neurobehavioral outcomes, it may not be necessary as CP and other adverse neurobehavioral outcomes are known to occur in the absence of WMD.41–45 Our study is the first, to date, demonstrating an effect of intrauterine inflammation at term on neuronal morphology and viability without an overt changes in glial markers (GFAP and PLP/DM20) indicating WMD. It is possible that changes in white mater occur later in the pathogenesis of term brain injury and neurons are the primary site of initial injury.
These studies may provide a possible explanation for the observed long-term adverse neurobehavioral outcomes after exposure to inflammation at term. Further investigation of mechanisms involved in neuronal injury and viability is critical in order to recognize the possibilities for novel therapeutic strategies.
Conclusion
Intrauterine inflammation at term results in altered neuronal morphology and delayed neurotoxicity. As there are no proposed mechanisms of neuronal injury in the setting of chorioamnionitis at term, these data are crucial for directing new research directions for the prevention of neuronal injury and adverse neurobehavioral outcomes in full-term gestations.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: supported by the NIH: 5-RO1-HD046544-0 (MAE) and supported part by the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania. The project described was supported by Grand Number UL1RR024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1. Soper DE, Mayhall CG, Froggatt JW. Characterization and control of intraamniotic infection in an urban teaching hospital. Am J Obstet Gynecol. 1996;175(2):304–309 discussion 309–310 [DOI] [PubMed] [Google Scholar]
- 2. Soper DE, Mayhall CG, Dalton HP. Risk factors for intraamniotic infection: a prospective epidemiologic study. Am J Obstet Gynecol. 1989;161(3):562–566 discussion 566–568 [DOI] [PubMed] [Google Scholar]
- 3. Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):387–392 [DOI] [PubMed] [Google Scholar]
- 4. Becroft DM, Thompson JM, Mitchell EA. Placental chorioamnionitis at term: epidemiology and follow-up in childhood. Pediatr Dev Pathol. 2010; 13(4): 282-90. 2009; [DOI] [PubMed] [Google Scholar]
- 5. Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol. 2008;198(1):49.e41–e46 [DOI] [PubMed] [Google Scholar]
- 6. Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. I. Univariate analysis of risks. Am J Dis Child. 1985;139(10):1031–1038 [DOI] [PubMed] [Google Scholar]
- 7. Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. Multivariate analysis of risk. N Engl J Med. 1986;315(2):81–86 [DOI] [PubMed] [Google Scholar]
- 8. Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–211 [PubMed] [Google Scholar]
- 9. Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–1424 [DOI] [PubMed] [Google Scholar]
- 10. Wu YW, Croen LA, Shah SJ, Newman TB, Najjar DV. Cerebral palsy in a term population: risk factors and neuroimaging findings. Pediatrics. 2006;118(2):690–697 [DOI] [PubMed] [Google Scholar]
- 11. Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–2684 [DOI] [PubMed] [Google Scholar]
- 12. Neufeld MD, Frigon C, Graham AS, Mueller BA. Maternal infection and risk of cerebral palsy in term and preterm infants. J Perinatol. 2005;25(2):108–113 [DOI] [PubMed] [Google Scholar]
- 13. Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44(4):665–675 [DOI] [PubMed] [Google Scholar]
- 14. Nitsos I, Rees SM, Duncan J, et al. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig. 2006;13(4):239–247 [DOI] [PubMed] [Google Scholar]
- 15. Burd I, Bentz AI, Chai J, et al. Inflammation-induced preterm brith alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88(9):1872–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burd I, Bentz A, Gonzalez J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59(1):50–55 [DOI] [PubMed] [Google Scholar]
- 18. Debillon T, Gras-Leguen C, Leroy S, Caillon J, Roze JC, Gressens P. Patterns of cerebral inflammatory response in a rabbit model of intrauterine infection-mediated brain lesion. Brain Res Dev Brain Res. 2003;145(1):39–48 [DOI] [PubMed] [Google Scholar]
- 19. Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70(4):570–579 [DOI] [PubMed] [Google Scholar]
- 20. Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30(9):473–478 [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez JM, Ofori E, Burd I, Chai J, Scholler N, Elovitz MA. Maternal mortality from systemic illness: unraveling the contribution of the immune response. Am J Obstet Gynecol. 2009;200(4):430.e431–e438 [DOI] [PubMed] [Google Scholar]
- 22. Elovitz MA, Mrinalini C. Can medroxyprogesterone acetate alter Toll-like receptor expression in a mouse model of intrauterine inflammation?. Am J Obstet Gynecol. 2005;193(3 Pt 2):1149–1155 [DOI] [PubMed] [Google Scholar]
- 23. Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163(5):2103–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monnerie H, Le Roux PD. Glutamate receptor agonist kainate enhances primary dendrite number and length from immature mouse cortical neurons in vitro. J Neurosci Res. 2006;83(6):944–956 [DOI] [PubMed] [Google Scholar]
- 25. Monnerie H, Le Roux PD. Reduced dendrite growth and altered glutamic acid decarboxylase (GAD) 65- and 67-kDa isoform protein expression from mouse cortical GABAergic neurons following excitotoxic injury in vitro. Exp Neurol. 2007;205(2):367–382 [DOI] [PubMed] [Google Scholar]
- 26. Monnerie H, Shashidhara S, Le Roux PD. Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: involvement of NMDA receptors. J Neurosci Res. 2003;74(5):688–700 [DOI] [PubMed] [Google Scholar]
- 27. Coronas V, Arnault P, Roger M. Cortical diffusible factors increase MAP-2 immunoreactive neuronal population in thalamic cultures. Neurosci Res. 2002;43(1):57–67 [DOI] [PubMed] [Google Scholar]
- 28. Benitez-King G, Ramirez-Rodriguez G, Ortiz L, Meza I. The neuronal cytoskeleton as a potential therapeutic target in neurodegenerative diseases and schizophrenia. Curr Drug Targets CNS Neurol Disord. 2004;3(6):515–533 [DOI] [PubMed] [Google Scholar]
- 29. Whitford KL, Dijkhuizen P, Polleux F, Ghosh A. Molecular control of cortical dendrite development. Annu Rev Neurosci. 2002;25:127–149 [DOI] [PubMed] [Google Scholar]
- 30. Whitford KL, Marillat V, Stein E, et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33(1):47–61 [DOI] [PubMed] [Google Scholar]
- 31. Holgado A, Ferreira A. Synapse formation proceeds independently of dendritic elongation in cultured hippocampal neurons. J Neurobiol. 2000;43(2):121–131 [DOI] [PubMed] [Google Scholar]
- 32. Martone ME, Hu BR, Ellisman MH. Alterations of hippocampal postsynaptic densities following transient ischemia. Hippocampus. 2000;10(5):610–616 [DOI] [PubMed] [Google Scholar]
- 33. Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222(1):51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fatemi SH. The role of Reelin in pathology of autism. Mol Psychiatry. 2002;7(9):919–920 [DOI] [PubMed] [Google Scholar]
- 35. Abuhatzira L, Shemer R, Razin A. MeCP2 involvement in the regulation of neuronal alpha-tubulin production. Hum Mol Genet. 2009;18(8):1415–1423 [DOI] [PubMed] [Google Scholar]
- 36. Rousset CI, Chalon S, Cantagrel S, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59(3):428–433 [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Hagberg H, Zhu C, Jacobsson B, Mallard C. Effects of intrauterine inflammation on the developing mouse brain. Brain Res. 2007;1144:180–185 [DOI] [PubMed] [Google Scholar]
- 38. Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47(1):64–72 [DOI] [PubMed] [Google Scholar]
- 39. Saadani-Makki F, Kannan S, Lu X, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199(6):651.e651–e657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Rousset CI, Hagberg H, Mallard C. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11(5):343–353 [DOI] [PubMed] [Google Scholar]
- 41. Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19 [DOI] [PubMed] [Google Scholar]
- 42. Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264–3272 [DOI] [PubMed] [Google Scholar]
- 43. Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51–58 [DOI] [PubMed] [Google Scholar]
- 44. Rademaker KJ, Uiterwaal CS, Beek FJ, et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed. 2005;90(6):F489–F493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuban KC, Allred EN, O’Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]