Abstract
Objective: To investigate if a diagnosis of diminished ovarian reserve (DOR) is associated with a differential gene profile of ovarian granulosa cells (GCs) in infertile women undergoing in vitro fertilization (IVF). Design: Prospective Cohort Study. Setting: Academic IVF Program. Patients: Infertile women <38 years were prospectively enrolled into 2 groups: normal ovarian reserve (NOR, follicle-stimulating hormone [FSH] < 10 mIU/mL, n = 4) and DOR (FSH ≥ 10.0 mIU/mL, n = 4). Interventions: Cumulus (C) and mural (M) GCs were isolated at egg retrieval; messenger RNA was extracted and transcribed. Main Outcome Measure(s): Differential gene expression in cerebellar granule cells (CGCs) in the 2 groups was assessed by cDNA microarray. Microarray findings were validated by quantitative real-time polymerase chain reaction (qRTPCR) in CGCs and explored in multinucleated giant cells (MGCs). Results: Of the 1256 differentially regulated genes identified in CGCs of women with DOR, the insulin-like growth factor (IGF) family was a biologically relevant gene family of a priori interest. Downregulation of IGF1 and IGF2 ligands (−3.28- and −2.54–fold, respectively), and their receptors, (−3.53- and −1.32-fold downregulation of IGF1R and IGF2R, respectively) was identified in luteinized CGCs in women with DOR compared to those with NOR. Downregulation of both IGF1 and IGF 2 ligands (−4.35- and 3.89-fold, respectively) was furthermore observed in MGCs in women with DOR compared to those with NOR; no differences in the expression of respective receptors were however observed in MGCs in the 2 groups. Conclusions: Components of the IGF gene family are downregulated in GCs of women with DOR. These findings maybe contributory to the reproductive compromise observed in women with DOR, and merit further exploration.
Keywords: granulosa cell, cumulus, mural, diminished ovarian reserve, microarray, insulin-like growth factor, gene expression, ovary
Introduction
The Practice Committee of the American Society of Reproductive Medicine defines ovarian reserve as reflecting a woman’s reproductive potential with respect to quantity and quality of residual ovarian follicles and oocytes.1 Abnormalities in folliculogenesis and in oocyte competence are suggested to partly underlie the poor reproductive performance in the context of aging and declining ovarian reserve.2 Of the available spectrum of biochemical and morphometric markers reflecting ovarian reserve status, early follicular phase serum levels of follicle-stimulating hormone (FSH) remain one of the most commonly utilized of the available parameters in clinical practice.3,4 Suboptimal ovarian responses to the attempts at controlled ovarian hyperstimulation (COH) and subpar reproductive success are well recognized in women demonstrating evidence of diminished ovarian reserve (DOR).3,5
Anatomical proximity and functional interdependence describe the relationships between ovarian germ cells and the surrounding somatic cells (ie granulosa cells [GCs]).6 A bidirectional communication between oocytes and GCs is essential for oocyte and follicular growth and is crucial for fertility.7 Transfer of critical molecules through gap junction exchange, as well as via paracrine signaling, affords a means of communication between the developing oocyte and the surrounding GCs.8 Oocytes secrete potent mitogenic factors (members of the transforming growth factor-β superfamily: growth differentiation factor [GDF]-9, GDF-9B also known as bone morphogenetic protein [BMP]-15, BMP-6, and the activins) that influence GC’s growth, development, differentiation, and function, including mucification, expansion, and ovulation.8,9 These oocyte contributors in turn are regulated by GC factors (ie kit ligand, FSH, insulin-like growth factor [IGF], and androgens) that play critical roles in oocyte biology.10,11 Optimal progression of folliculogenesis is thus driven by both oocyte and GC-derived contributions.
During folliculogenesis, 2 distinct subtypes of GCs, mural (M) and cumulus (C), are identified. Cerebellar granule cells are proximate to the developing oocyte, demonstrate a high rate of proliferation, exhibit lower steroidogenic capacity, and express very low levels of luteinizing hormone (LH) receptor expression compared to the mural GCs.8 Cerebellar granule cells produce an extracellular matrix and undergo expansion, phenomena that are imperative for normal oocyte development, ovulation, and fertility.8,12Multinucleated giant cells, in contrast, occupy a peripheral location within the developing follicle, are mitotically active, express high levels of LH receptors, exhibit robust steroidogenic activity, and are the integral constituents of the postovulatory corpus luteum.
Meiotic arrest of oocytes encased within the antral follicles is attributed to suppressive influences of the surrounding GCs7; indeed, in 1935, Pincus and Enzmann described the spontaneous resumption of meiosis in oocytes if removed from the antral follicle and cultured in a supportive medium.13 Reciprocally, the oocyte plays key roles in the differentiation of the 2 GC subtypes. This interdependence is evident in the experiments utilizing removal of the oocyte from the oocyte-cumulus complex; in the oocyte-denuded complexes, CGCs lose their distinct characteristics and adopt a phenotype characteristic of mural GCs (ie reduced DNA synthesis capacity and increased progesterone secretion), whereas the cells maintain the CGC phenotype if cocultured with oocytes stripped of surrounding GCs.11,14
Given the critical roles of GCs in the biology of folliculogenesis and for oocyte development, these cells have the potential to provide meaningful information regarding their respective oocytes. In addition, GCs are identified as a valuable area of study of reproductive biology, given the ease of access during assisted reproductive technique procedures.
Limited data are suggestive of GC compromise in the setting of declining ovarian reserve. Decreased GC proliferation and increased apoptosis are described in GCs obtained from infertile women with DOR15–17; more recently, our group reported an inverse relationship between increasing FSH levels (suggestive of declining ovarian reserve) and declining GC viability.18
Hypothesizing that a differential pattern of gene expression in luteinized GCs accompanies a diagnosis of DOR in young women (age <38), this study pursues a global differential gene expression analysis of luteinized cumulus GCs utilizing a microarray platform with a focus on genes with the previously recognized roles in ovarian folliculogenesis. Differentially expressed genes of interest were subsequently validated within the 2 GC compartments using quantitative real-time polymerase chain reaction (qRTPCR).
Materials and Methods
Patients
This study was reviewed and approved by the Institutional Review Board and patients provided written consent. Granulosa cells were collected from infertile women undergoing in vitro fertilization (IVF). Inclusion criteria were age <38 (to minimize confounding influences of advancing age19). As per the clinical practice guidelines, early follicular FSH levels (days 1-3) ≥10 mIU/mL identified those with DOR, whereas levels <10 mIU/mL were taken to reflect normal ovarian reserve (NOR). Follicle-stimulating hormone was assayed using Roche Elecsys 1010 Immunoanalyzer, Indianapolis, lower detection limit (sensitivity): <0.1 mIU/mL, cross-reactivity (specificity): <0.1%, intra-assay variability: CV 1.7%, interassay variability: CV 4.2%. In those in whom FSH levels were available from a prior menstrual cycle, the highest value was taken to reflect ovarian reserve.20,21 Women with ovulatory dysfunction, endometriosis, and tubal disease with hydrosalpinx were excluded to minimize etiological influences that could potentially alter the GC gene expression.22–24 Patients underwent suppression of ovulation and controlled ovarian hyperstimulation (COH) according to the established protocols. Ovarian downregulation was achieved by use of a gonadotropin-releasing hormone (GnRH) agonist in a luteal protocol for 6 out of 8 patients (3 in each group), whereas the remaining 2 received GnRH antagonist (1 in each group). Standard formulations of gonadotropins (75 units per ampule) were used for COH, and the ovarian response was monitored by serum estradiol levels and serial ultrasound evaluation of follicular development. Oocyte maturation was triggered with 10 000 IU of human chorionic gonadotropin when a minimal of 3 dominant follicles had attained a size of 17mm or more. Ultrasound guided transvaginal retrieval of oocytes was performed approximately 34 hours after HCG administration.
Granulosa Cell Isolation
Mural GCs
During transvaginal oocyte retrieval, follicular fluid aspirates from follicles averaging ≥14 mm in diameter were collected. The first follicular aspirate from each ovary was excluded to eliminate contamination with vaginal epithelial cells (personal observation). Oocytes were identified and transferred into modified human tubal fluid (mHTF; Conception Technology, San Diego, California) + 5% plasmanate (Bayer Corporation, Elkhart, Indianapolis). Follicular fluid aspirates from each patient were pooled and GCs were harvested utilizing established methods25; briefly, follicular fluid aspirate was treated with 40% Upper Phase Density Gradient (Pureception, Sage BioPharma, Trumbull, Connecticut) and centrifugation (10 minutes × 2000 rpm at room temperature [RT]). The interphase layer of GCs was extracted and washed in phosphate-buffered saline (PBS, Fisher Scientific, Pittsburgh, Pennsylvania) and centrifuged (10 minutes × 2000 rpm at RT). The supernatant was discarded and the cells were resuspended in PBS. Enzymatic digestion with Collagenase IA (Sigma Chemical Co, St Louis, Missouri) was performed to disperse GCs and DNase (Sigma Chemical Co) to digest any stray genomic DNA. Multinucleated giant cells were then washed in PBS and cell suspensions were nutated for 20 minutes with anti-CD45 immunomagnetic beads (Dynal Biotech ASA, Oslo, Norway) followed by application to a magnet to achieve leukocyte depletion.26 The GCs were washed in PBS, centrifuged, pelleted, and frozen at −80°C in RLT buffer (RNeasy kit, Qiagen, Valencia, California) activated with 2-mercaptoethanol (Fisher Scientific, Fairlawn, New Jersy).
Cumulus GCs
Following isolation of oocytes, the CGCs were isolated by mechanical stripping. Enzymatic digestion with Cumulase (Halozyme Therapeutics, San Diego, California) and Collagenase IA to disperse GCs, and DNase (Sigma Chemical Co, St Louis, Missouri) to digest any stray genomic DNA was performed, in combination with further mechanical stripping. Cells were then washed in mHTF + 5% plasmanate and centrifuged at 2000 rpm for 10 minutes and the pellets were frozen at −80°C in RLT buffer (RNeasy kit, Qiagen, Valencia, California) activated with 2-mercaptoethanol (Fisher Scientific).
RNA Extraction
RNeasy Mini-kit (Qiagen, Valencia, California) was utilized using the established protocols.27 The RNA quantity was measured using the NanoDrop ND-1000 spectrophotomoter (NanoDrop Technologies, Wilmington, Delaware). The RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California).
Cumulus GC Microarray Analysis
Microarray targets were generated from the CGCs using the NuGEN Ovation v2.0 Biotin RNA Amplification and Labeling System (NuGEN Inc, San Carlos, California).28 Labeled cDNA was hybridized to Affymetrix Human Genome U133 + 2.0 GeneChips (Affymetrix, Santa Clara, California). Microarray data analysis was performed using Stratagene Array Assist Enterprise software package (La Jolla, California). All microarray data were normalized at the probe-level utilizing Robust Multichip Analysis (RMA).29
Quantitative Real-Time Polymerase Chain Reaction
Amplifications of the genes of interest and β-actin were performed in triplicate wells. Polymerase chain reaction targets were created from the template RNA using the manufacturer’s protocols (WT-Ovation RNA Amplification System, NuGEN Inc). Gene-specific primers and probes were designed using ProbeFinder software according to the manufacturer’s protocols (Exiqon ProbeLibrary, Roche-Applied Science, Indianapolis). Reactions were performed in a total volume of 10 μL using Taqman Master Mix reagent (Applied Biosystems, Foster City, California); 2 μL of cDNA/sample was used as a template for the reaction, with 900 nM forward and reverse primers, and 10 nM Exiqon probe. Thermal cycling conditions included 2 minutes at 50°C and 10 minutes at 95°C, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Negative controls did not express the genes of interest (data not shown). Threshold cycle (C T) values greater than 40 cycles indicated the absence of the gene of interest. Results are expressed as relative fold changes in genes, using the 2−ΔΔCT method.30
Statistical Analysis
Data distribution was assessed. Student t test compared patient and IVF cycle characteristics between the 2 groups (DOR and NOR) for data demonstrating Gaussian distribution; skewed data (no. of eggs retrieved and duration of COH) were compared between the 2 groups using nonparametric Mann-Whitney 2-sample rank-sum test. Student t test was used to assess the differences in microarray data between the 2 groups. Stata 10 (Statacorp, College Station, Texas) and P < .05 was considered of statistical significance. A difference in gene expression of greater than 2.0-fold was considered as biologically meaningful, and of potential clinical significance.31
Results
In total, 8 women <38 years were prospectively enrolled (4 each diagnosed with DOR and NOR met study inclusion criteria). Patient characteristics for the 2 groups (NOR vs DOR) are summarized in Table 1 . Continuous data are reported as mean ± standard deviation (SD) (for normally distributed data) or median (interquartile range—IQR) for skewed data.
Table 1.
Characteristics of the study sample, categorized by normal vs diminished ovarian reservea
| Variable | NOR (n = 4) | DOR (n = 4) | P Value |
|---|---|---|---|
| Age (years)b | 28.75 ± 6.18 | 34 ± 2.16 | .160 |
| Body mass index (kg/m2)b | 26.62 ± 3.50 | 23.55 ± 4.14 | .300 |
| FSH (mIU/mL)b | 5.73 ± 2.81 | 12.58 ± 3.81 | <.028* |
| # Ampules of Gonadotropinb | 27.37 ± 6.70 | 55.75 ± 7.5 | .001* |
| Duration of COH (days)c | 12.0 (IQR 1-12) | 12.5 (IQR 12-13) | .342 |
| Peak estradiol (pg/mL)bd | 3043.25 ± 858.0 | 1984.25 ± 605.97 | .090 |
| # Eggs retrievedc | 12 (IQR 9-15) | 9.5 (IQR 7.5-12.5) | .564 |
a Peak estradiol on the hyperstimulation standard deviation (SD; for normally distributed data) or median (interquartile range—DOR: diminished ovarian reserve (FSH ≥ 10.0 mIU/mL), NOR, normal ovarian reserve (FSH < 10 mIU/mL), COH, controlled ovarian hyperstimulation.
b Data expressed as mean ± SD.
c Data expressed as median (IQR).
d Serum estradiol on the day of hCG administration.
p < 0.05.
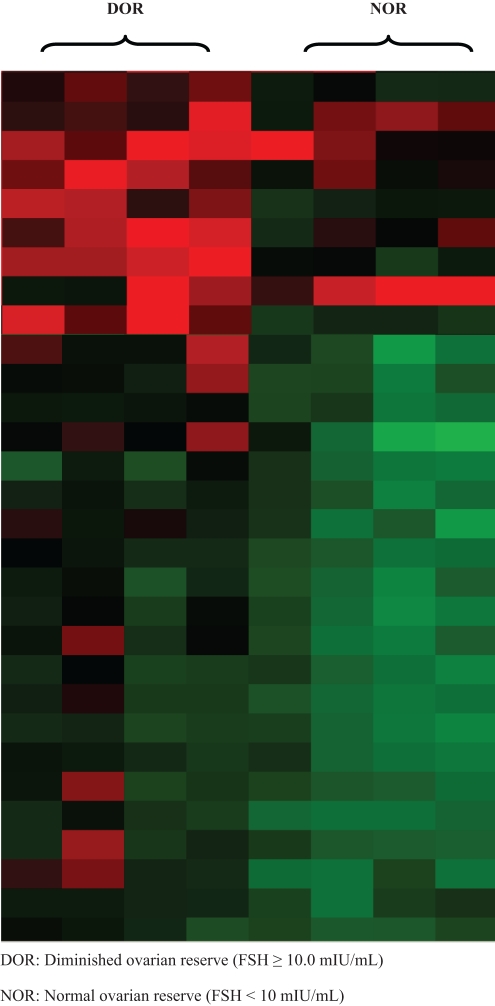
In a relatively young population of infertile women, a diagnosis of DOR was associated with a differential gene expression profile of CGC. Using RMA, a robust normalization schema,29 1256 statistically significant (P < .05) differentially expressed genes with a 2.0-fold or greater difference in expression was identified between the DOR and the NOR groups; 843 genes were observed to be downregulated and 413 were upregulated in the DOR, as compared with the NOR group. Figure 1 is a heat map, showing the result of a hierarchical clustering algorithm of the most differentially expressed genes.32
Figure 1.
Cluster analysis highlighting a subset of genes that were differentially expressed between the DOR and the NOR women. Each column represents the expression of a patient and each row represents a gene. Red denotes upregulation of gene expression and green denotes downregulation of gene expression.
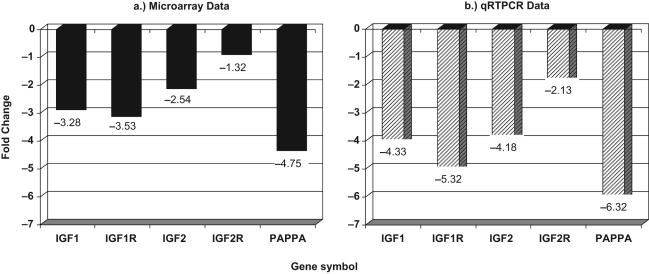
As per our a priori focus on the genes of functional relevance, the IGF family was identified as a biologically relevant gene cluster of interest from the differentially regulated genes.33 A differential downregulation in the expressions of members of the IGF family was observed in luteinized CGCs in the DOR compared to the NOR group (Figure 2 ). Another biologically relevant gene observed to be differentially expressed in the DOR population was pregnancy-associated plasma protein-A (PAPP-A). Pregnancy-associated plasma protein-A is a well-recognized protease of IGFBP4, and a mediator of IGF action,34 and was significantly downregulated in CGCs of the DOR compared to the NOR group (Figure 2). These microarray findings were validated using qRTPCR (Figure 2).
Figure 2.
Differential expression of IGF family of genes in cumulus GC in DOR compared to NOR (referent expression normalized as 0). (A) Microarray data and (B) validation of microarray findings by qRTPCR.
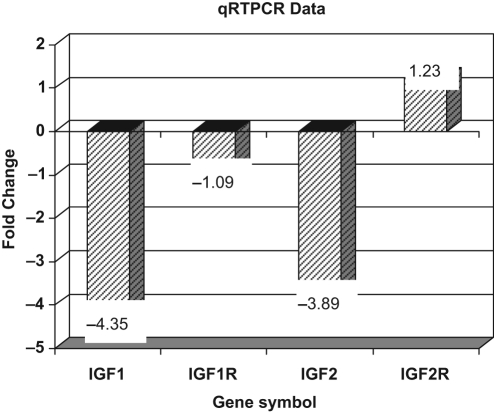
Expression of the IGF ligands and receptors noted to be differentially expressed in the CGCs was then explored in MGCs in the 2 groups by qRTPCR. Consistent with the findings in the CGCs, a downregulation of both the IGF1 and the IGF2 ligands was observed in MGCs of women with DOR compared to those with NOR (Figure 3 ). In contrast to the findings noted in CGCs, the expression of genes for IGF-1 and IGF-2 receptors was comparable in the MGCs in the 2 groups (Figure 3).
Figure 3.
Differential expression of IGF family of genes in mural GC in DOR compared to NOR (referent expression normalized as 0).
Discussion
During the process of folliculogenesis, complex interactions between the oocyte and the surrounding GCs are recognized, as are enduring effects of this interplay on the potential for postfertilization embryonic development.35 Oocyte-derived factors are recognized to influence multiple processes during folliculogenesis including formation of the oocyte-cumulus complex, synthesis of progesterone, modulation of signaling pathways, and suppression of luteinization in the preovulatory cumulus GCs.35 The study of gene expression in CGCs thus holds potential for meaningful insights into the processes that reflect on the health of the developing oocyte.
Although utilization of the high-throughput gene microarray technology for the study of GCs is increasingly being reported, a single published study by Chin et al has thus far explored the pattern of gene expression in MGCs in women with DOR compared to those with NOR.36 These authors report a distinct expression profile in the MGCs from the DOR group; our overall approach in utilizing a microarray platform is thus similar, as our findings are on differentially expressed gene profile in the setting of DOR. Our approach of systematically targeting the individual GC compartments for the previously substantiated genes of functional relevance in the context of folliculogenesis, as well as subsequent confirmation of the microarray data by qRTPCR provide meaningful and novel additions to the existing literature.
An intraovarian IGF system, including ligands, receptors, binding proteins, and binding protein proteases has been described.33 Both IGF1 and IGF2 have been shown to stimulate GC proliferation and differentiation; a synergism with gonadotropins in augmenting estradiol and progesterone production by GCs via upregulation of aromatization of androgen precursors37–41 is described. Prior studies show that IGF2 is abundantly expressed in human GCs of preovulatory follicles and is the dominant IGF ligand (as opposed to IGF1) within human GCs.42,43 Our findings of significant downregulation of IGF2 in both cumulus and mural GCs in women with DOR are novel and not previously reported. Given the recognized role of the IGF family in ovarian steroidogenesis,44 the observed downregulation in the expressions of the IGF ligands in the mural GCs may explain the suboptimal serum estradiol levels during COH that are commonly noted in the context of DOR.3 Our observations thus may be of relevance in advancing our understanding of the altered ovarian biology in the setting of DOR.
Cellular effects of IGFs are modulated by a series of specific binding proteins and binding protein proteases, in addition to the expression levels of the respective receptors. Six IGF-binding proteins (IGFBP-1-6) have been identified to regulate IGF bioavailability.45 All IGFBPs exhibit inhibitory effects on IGF expression although some also have stimulatory influences. Insulin-like growth factor-binding protein-4 has exclusively inhibitory effects and has been found in increased abundance in small antral follicles.46 Increased expression of IGFBP-4 protease, also identified as PAPP-A,34 is described in the dominant follicles; excess PAPP-A by decreasing IGFBP4 activity allows availability of the free IGF ligand within the dominant follicle, and hence facilitating the mitogenic and gonadotropin-augmenting activities of the IGF ligand.47 The marked decrease in expression of PAPP-A within GCs of women with DOR, as shown, may further contribute to an IGF-deficient intraovarian milieu in the setting of DOR.
Accruing data suggest that compromised ovarian reserve may be salvageable with exogenous supplementation with DHEA (dehydroepiandrosterone), an adrenal steroid and an essential precursor for ovarian steroid hormones.48–51 While mechanisms that may underlie the purported therapeutic efficacy of DHEA for ovarian physiology are far from understood, Casson et al were the first to suggest an upregulation of IGF-1 axis with DHEA use as a mechanism for improved folliculogenesis with DHEA.48,52 Our observations of a downregulated ovarian IGF axis within GCs of women with DOR strengthen the argument proposed by Casson et al, and offer a potential for guiding future research on this subject.
Limitations to our study include a small sample size and utilization of FSH as the biomarker reflecting ovarian reserve. The sample size constraints are secondary to the stringent inclusion and exclusion criteria; we specifically attempted to minimize the potential for microarray data from being confounded by inclusion of conditions for which prior gene expression signature profiles have been suggested, such as endometriosis, polycystic ovary syndrome (PCOS), and the presence of hydrosalpinges.22–24
Our study design allocated patients based on early follicular phase FSH levels, a strategy that mimics our clinical practice. Although newer, possibly more sensitive and specific markers are identified for evaluating ovarian reserve status (eg Anti-Mullerian Inhibiting Substance and Inhibin B),53,54 elevated FSH is well-recognized and a fairly accurate marker for identifying women with a poor prognosis for success following IVF55; the choice reflected our practice standard screening methodology during the recruitment period. The need for significantly more ampules of gonadotropin during COH, the lower estradiol levels on the day of HCG administration, and lesser number of eggs retrieved, all identify that the patients categorized under DOR based on the early follicular-phase FSH indeed exhibited poorer ovarian reserve parameters compared to those with NOR, defined as per specified FSH-based criteria.
Controversy exists with regard to the presence of IGF1 messenger RNA within GCs of the human ovary. Prior reports by el-Roeiy et al,42 and Zhou and Bondy43 using in situ hybridization and immunohistochemistry techniques, showed an absence of IGF1 mRNA transcripts within the human GC. In contrast, Voutilainen et al, utilizing RT-PCR revealed IGF1 in the theca and stromal compartments, as well as in GCs of smaller size follicles when 32 amplification cycles were used.56 IGF1 is a 70 amino acid polypeptide that shares significant (approximately, 70%) sequence homology with IGF2.33 While a possibility of overlap in the detection of these transcripts is plausible, current techniques for qRTPCR are more precise, and small copy numbers of IGF1 transcript can be detected in luteinized cumulus and mural GCs of preovulatory follicles and may explain our ability to demonstrate the expression of IGF1 in both the mural and the cumulus luteinized human GCs. Utilization of super-physiologic conditions, that is COH with exogenous gonadotropins and the absence of information regarding expression levels of the respective growth factors are additional limitations. It has been suggested previously that gonadotropins regulate IGF2 mRNA expression and secretion in human luteal GCs in vitro57; while exogenous gonadotropins may activate transcription of IGF genes and hence confound results, the differential in expression of genes of interest was identified in a comparable setting in the 2 groups (DOR and NOR), and hence our findings may indeed be construed as valid.
In summary, we herein provide evidence that young women with DOR exhibit a distinct GC genotype. Downregulation of the IGF1 and IGF2 ligands and respective receptors in the cumulus GCs and downregulation of the IGF1 and 2 ligands in the mural GCs are identified in the setting of DOR. Our data allow us to propose that inefficiencies in the intraovarian IGF system may contribute to the steroidogenic incompetence and decreased reproductive capacity that is previously described in women with DOR.
Acknowledgments
The authors gratefully acknowledge the invaluable contributions of the following colleagues: Athena Zapantis, BS and Michael Nihsen, MS during cell collection; and Andrew Brooks, PhD, of the Environmental and Occupational Health Science Institute, Robert Wood Johnson Medical School, Piscataway, NJ during microarray analysis.
All research described in this manuscript was performed in the Division of Reproductive Endocrinology & Infertility, Department of Obstetrics, Gynecology & Women’s Health, Albert Einstein College of Medicine, Bronx, NY, USA and Montefiore’s Institute for Reproductive Medicine and Health, Hartsdale, NY, USA. A portion of this work has been presented as an oral presentation at the Annual Meeting of the American Society for Reproductive Medicine, October 2007.
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: supported by Ferring Pharmaceuticals, Inc (to SJ); NIH (grant number 5K12 RR17672 to LP); and NIH (grant number K24 CD41978 to NS) .
References
- 1. Medicine PCotASoR Aging and infertility in women. Fertil Steril. 2006;86(4):S248–S252 [DOI] [PubMed] [Google Scholar]
- 2. Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11(10):2217–2222 [DOI] [PubMed] [Google Scholar]
- 3. Scott RT, Toner JP, Muasher SJ, Oehninger S, Robinson S, Rosenwaks Z. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;51(4):651–654 [DOI] [PubMed] [Google Scholar]
- 4. Macklon NS, Fauser BC. Ovarian reserve. Semin Reprod Med. 2005;23(3):248–256 [DOI] [PubMed] [Google Scholar]
- 5. Muasher SJ, Oehninger S, Simonetti S, et al. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50(2):298–307 [DOI] [PubMed] [Google Scholar]
- 6. Canipari R. Oocyte-granulosa cell interactions. Hum Reprod Update. 2000;6(3):279–289 [DOI] [PubMed] [Google Scholar]
- 7. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122(6):829–838 [DOI] [PubMed] [Google Scholar]
- 8. Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82-83:431–446 [DOI] [PubMed] [Google Scholar]
- 9. Strauss JF, Williams CJ. The ovarian life cycle. In: Strauss JF, Barbieri RL. (Eds.), Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. Philadelphia, PA: Elsevier Saunders Press; 2004:213–254 [Google Scholar]
- 10. Armstrong DT, Xia P, de Gannes G, Tekpetey FR, Khamsi F. Differential effects of insulin-like growth factor-I and follicle-stimulating hormone on proliferation and differentiation of bovine cumulus cells and granulosa cells. Biol Reprod. 1996;54(2):331–338 [DOI] [PubMed] [Google Scholar]
- 11. Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod. 2000;63(3):839–845 [DOI] [PubMed] [Google Scholar]
- 12. McKenzie LJ, Pangas SA, Carson SA, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19(12):2869–2874 [DOI] [PubMed] [Google Scholar]
- 13. Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med. 1935;62(5):665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138(1):16–25 [DOI] [PubMed] [Google Scholar]
- 15. Seifer DB, Charland C, Berlinsky D, et al. Proliferative index of human luteinized granulosa cells varies as a function of ovarian reserve. Am J Obstet Gynecol. 1993;169(6):1531–1535 [DOI] [PubMed] [Google Scholar]
- 16. Nakahara K, Saito H, Saito T, et al. Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil Steril. 1997;67(2):302–308 [DOI] [PubMed] [Google Scholar]
- 17. Nakahara K, Saito H, Saito T, et al. The incidence of apoptotic bodies in membrana granulosa can predict prognosis of ova from patients participating in in vitro fertilization programs. Fertil Steril. 1997;68(2):312–317 [DOI] [PubMed] [Google Scholar]
- 18. Greenseid K, Jindal S, Zapantis A, Nihsen M, Hurwitz J, Pal L. Declining ovarian reserve adversely influences granulosa cell viability. Fertil Steril. 2009;91(6):2611–2615 [DOI] [PubMed] [Google Scholar]
- 19. Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25(4):957–968 [DOI] [PubMed] [Google Scholar]
- 20. Scott RT, Jr, Hofmann GE, Oehninger S, Muasher SJ. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;54(2):297–302 [DOI] [PubMed] [Google Scholar]
- 21. Abdalla H, Thum MY. Repeated testing of basal FSH levels has no predictive value for IVF outcome in women with elevated basal FSH. Hum Reprod. 2006;21(1):171–174 [DOI] [PubMed] [Google Scholar]
- 22. Hughes C, Elgasim M, Layfield R, Atiomo W. Genomic and post-genomic approaches to polycystic ovary syndrome—progress so far: Mini Review. Hum Reprod. 2006;21(11):2766–2775 [DOI] [PubMed] [Google Scholar]
- 23. Matsuzaki S, Canis M, Vaurs-Barrière C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertil Steril. 2005;84(suppl 2):1180–1190 [DOI] [PubMed] [Google Scholar]
- 24. Daftary GS, Taylor HS. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril. 2002;78(3):577–580 [DOI] [PubMed] [Google Scholar]
- 25. Hurwitz JM, Jindal S, Greenseid K, et al. Reproductive aging is associated with altered gene expression in human luteinized granulosa cells. Reprod Sci. 2010;17(1):56–67 [DOI] [PubMed] [Google Scholar]
- 26. Neurauter AA, Bonyhadi M, Lien E, et al. Cell isolation and expansion using Dynabeads. Adv Biochem Eng Biotechnol. 2007;106:41–73 [DOI] [PubMed] [Google Scholar]
- 27. Vogelstein B, Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci USA. 1979;76(2):615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dafforn A, Chen P, Deng G, et al. Linear mRNA amplification from as little as 5 ng total RNA for global gene expression analysis. Biotechniques. 2004;37(5):854–857 [DOI] [PubMed] [Google Scholar]
- 29. Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193 [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 31. Moll AG, Lindenmeyer MT, Kretzler M, Nelson PJ, Zimmer R, Cohen CD. Transcript-specific expression profiles derived from sequence-based analysis of standard microarrays. PLoS One. 2009;4(3):e4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brazma A, Vilo J. Gene expression data analysis. FEBS Lett. 2000;480(1):17–24 [DOI] [PubMed] [Google Scholar]
- 33. Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–582 [DOI] [PubMed] [Google Scholar]
- 34. Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC. Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab. 1999;84(12):4742–4745 [DOI] [PubMed] [Google Scholar]
- 35. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–2180 [DOI] [PubMed] [Google Scholar]
- 36. Chin KV, Seifer DB, Feng B, Lin Y, Shih WC. DNA microarray analysis of the expression profiles of luteinized granulosa cells as a function of ovarian reserve. Fertil Steril. 2002;77(6):1214–1218 [DOI] [PubMed] [Google Scholar]
- 37. Adashi EY, Resnick CE, D’Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6(3):400–420 [DOI] [PubMed] [Google Scholar]
- 38. Christman GM, Randolph JF, Jr, Peegel H, Menon KM. Differential responsiveness of luteinized human granulosa cells to gonadotropins and insulin-like growth factor I for induction of aromatase activity. Fertil Steril. 1991;55(6):1099–1105 [DOI] [PubMed] [Google Scholar]
- 39. Erickson GF, Garzo VG, Magoffin DA. Insulin-like growth factor-I regulates aromatase activity in human granulosa and granulosa luteal cells. J Clin Endocrinol Metab. 1989;69(4):716–724 [DOI] [PubMed] [Google Scholar]
- 40. Erickson GF, Garzo VG, Magoffin DA. Progesterone production by human granulosa cells cultured in serum free medium: effects of gonadotrophins and insulin-like growth factor I (IGF-I). Hum Reprod. 1991;6(8):1074–1081 [DOI] [PubMed] [Google Scholar]
- 41. Kamada S, Kubota T, Taguchi M, Ho WR, Sakamoto S, Aso T. Effects of insulin-like growth factor-II on proliferation and differentiation of ovarian granulosa cells. Horm Res. 1992;37(4-5):141–149 [DOI] [PubMed] [Google Scholar]
- 42. el-Roeiy A, Chen X, Roberts VJ, LeRoith D, Roberts CT, Jr, Yen SS. Expression of insulin-like growth factor-I (IGF-I) and IGF-II and the IGF-I, IGF-II, and insulin receptor genes and localization of the gene products in the human ovary. J Clin Endocrinol Metab. 1993;77(5):1411–1418 [DOI] [PubMed] [Google Scholar]
- 43. Zhou J, Bondy C. Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod. 1993;48(3):467–482 [DOI] [PubMed] [Google Scholar]
- 44. Monget P, Bondy C. Importance of the IGF system in early folliculogenesis. Mol Cell Endocrinol. 25 2000;163(1-2):89–93 [DOI] [PubMed] [Google Scholar]
- 45. Rechler MM, Clemmons DR. Regulatory actions of insulin-like growth factor-binding proteins. Trends Endocrinol Metab. 1998;9(5):176–183 [DOI] [PubMed] [Google Scholar]
- 46. el-Roeiy A, Chen X, Roberts VJ, et al. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1-6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab. 1994;78(6):1488–1496 [DOI] [PubMed] [Google Scholar]
- 47. Conover CA, Faessen GF, Ilg KE, et al. Pregnancy-associated plasma protein-a is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology. 2001;142(5):2155. [DOI] [PubMed] [Google Scholar]
- 48. Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–2132 [DOI] [PubMed] [Google Scholar]
- 49. Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. Fertil Steril. 2005;84(3):756. [DOI] [PubMed] [Google Scholar]
- 50. Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91(2):644–646 [DOI] [PubMed] [Google Scholar]
- 51. Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor-responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010;25(10):2496–2500 [DOI] [PubMed] [Google Scholar]
- 52. Casson PR, Santoro N, Elkind-Hirsch K, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–110 [DOI] [PubMed] [Google Scholar]
- 53. Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–546 [DOI] [PubMed] [Google Scholar]
- 54. Seifer DB, Lambert-Messerlian G, Hogan JW, Gardiner AC, Blazar AS, Berk CA. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67(1):110–114 [DOI] [PubMed] [Google Scholar]
- 55. Barnhart K, Osheroff J. Follicle stimulating hormone as a predictor of fertility. Curr Opin Obstet Gynecol. 1998;10(3):227–232 [DOI] [PubMed] [Google Scholar]
- 56. Voutilainen R, Franks S, Mason HD, Martikainen H. Expression of insulin-like growth factor (IGF), IGF-binding protein, and IGF receptor messenger ribonucleic acids in normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81(3):1003–1008 [DOI] [PubMed] [Google Scholar]
- 57. Ramasharma K, Li CH. Human pituitary and placental hormones control human insulin-like growth factor II secretion in human granulosa cells. Proc Natl Acad Sci USA. 1987;84(9):2643–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]