Abstract
Human endometrium, a steroid hormone-dependent tissue, displays complex cellular regulation mediated by nuclear receptors (NRs). The NRs interact with histone-modifying and DNA-methylating/-demethylating enzymes in the transcriptional complex. We investigated NRs, their coregulators, and associated signaling pathways in endometrium across the normal menstrual cycle and in endometriosis, an estrogen-dependent, progesterone-resistant disorder. Endometrial tissue was processed for analysis of 84 genes using NR and coregulator polymerase chain reaction (PCR) arrays. Select genes were validated by immunohistochemistry. Ingenuity pathway analysis identified DNA methylation and transcriptional repression signaling as the most affected pathway in endometrium in women with versus without endometriosis, regardless of cycle phase. Thyroid hormone receptor (THR) and vitamin D receptor (VDR) pathways were also regulated in normal and disease endometrium by activation of TH or vitamin D regulated genes. These data support the involvement of the epigenome in steroid hormone response of normal endometrium throughout the cycle and abnormalities in endometrium in women with endometriosis.
Keywords: endometriosis, nuclear receptors, coregulators, DNA methylation, histone deacetylases
Introduction
Steroid hormone-responsive tissues display complex regulation of tissue-specific cellular functions mediated by nuclear receptors (NRs). The involvement of NRs with coregulators and other recruited proteins in the transcriptional complex is essential for target gene regulation.1 The NRs interact with histone-modifying enzymes and DNA-methylating/-demethylating enzymes in the transcriptional complex, and histone modification and DNA methylation are interrelated in regulating gene expression and chromatin remodeling.2,3 Thus, steroid hormone-responsive tissues have epigenetic constituents regulating normal physiologic functions, which may be vulnerable to modifications that alter these intended functions, resulting in human disease.
Human endometrium is a steroid hormone-responsive tissue whose cellular components, in response to cyclic changes in circulating ovarian-derived estradiol (E2) and progesterone (P4), undergo proliferation, angiogenesis, differentiation, and apoptosis, in preparation of embryonic implantation. Abnormalities in endometrial steroid hormone responsiveness have profound effects on pregnancy establishment, maintenance, and outcomes and result in infertility, miscarriage, and pregnancy complications, as well as cancer.4 In endometriosis, which is present in 6% to 10% of reproductive age women5 and up to 50% of women with infertility,4,6 there is increasing evidence that epigenetic modifications of steroid hormone receptors (estrogen receptor 2 [ESR2], progesterone receptor B [PGRB]) and transcription factors (HOXA10, SF1) involved in steroid hormone gene regulation and biosynthesis result in resistance to P4 action within the endometrium and subsequently abnormal receptivity to embryonic implantation.7 The progesterone receptor is a member of the superfamily of NRs whose transcriptional activity is regulated by various cofactors.8 Decreased expression of PGRs,9 hypermethylation of the PGRB promoter,10 abnormal regulation and expression of the PGR coactivator, Hydrogen peroxide-inducible clone-5 (HIC5),11 and local E2 biosynthesis6 contribute to resistance to P4 action in this tissue.7 Abrogated P4 signaling results in enhanced estrogen responsiveness, with a characteristic persistent proliferative phenotype of eutopic endometrium, as well as ectopic lesions in the pelvis and is accompanied by increased cell survival and invasive capacity.4,6,12
In the current study, we found cycle-dependent expression of specific NRs, coregulators, and genes engaged in a complex orchestra of signaling pathways and chromatin remodeling across the menstrual cycle and dysregulation of key players in steroid hormone, thyroid hormone, and vitamin D signaling, as well as chromatin remodeling, in the presence of endometriosis. These novel findings are significant to the biology of steroid hormone action within the endometrium and likely other tissues and in the pathogenesis and pathophysiology of endometriosis.
Materials and Methods
Tissue Collection
Tissue samples of proliferative (PE), early secretory (ESE), and midsecretory endometrium (MSE) were collected from hysterectomy or biopsy specimens. Control samples were obtained from women undergoing surgery for benign disease and who did not have a history of endometriosis. Endometrial tissue was immediately frozen in liquid nitrogen and then stored at −80°C. Disease samples were obtained from women who had moderate/severe endometriosis (stage III/IV). The study participants in both the disease and no disease categories were 22 to 45 years old and had regular menstrual cycles, had not been on hormonal therapy within 3 months of tissue sampling, and were documented not to be pregnant. The profile of the participant annotation is provided in Table 1. All tissue samples were collected after written, informed consent, through The University of California, San Francisco (UCSF)/National Institutes of Health (NIH) Human Endometrial Tissue and DNA Bank, under full review and with approval of the Committee on Human Research of the UCSF.
Table 1.
Participant Characteristics: Disease and Control Specimensa
| Patient ID | Type of | ||||||
|---|---|---|---|---|---|---|---|
| Sample | Cycle Phase | Age (yr) | Diagnoses | Ethnicity | Mode of Collection | Medications | |
| ST-84 | Disease | Pro | 37 | PP, SE, leiomyoma | Caucasian | Pipelle biopsy | Hydroxyzine, flax seed oil, fish oil |
| 508 | Disease | Pro | 25 | PP, SE, bleeding | Caucasian | Pipelle biopsy | Atenolol, sorrel, burdock root |
| 587 | Disease | Pro | 37 | PI, SE, liver endo | Caucasian | Pipelle biopsy | |
| 651 | Disease | Pro | 37 | PI, SE, endometrioma, leiomyoma | Caucasian | Pipelle biopsy | Advair, rhinocort |
| 575b | Disease | Pro | 26 | SE, left endometrioma, pelvic adhesions | Unknown | Pipelle biopsy | |
| 589b | Disease | Pro | 48 | Bilateral endometrioma, leiomyomata | Asian | Hysterectomy | Cytomel |
| ST90b | Disease | Pro | 42 | PP, SE, ovary with follicular cyst | Caucasian | Pipelle Biopsy | |
| 455 | Control | Pro | 39 | Menorrhagia, leiomyoma | Caucasian | Hysterectomy | Levothyroxine, cytomel |
| 469 | Control | Pro | 42 | Leiomyoma | Caucasian | Hysterectomy | Synthroid, motrin |
| 604 | Control | Pro | 44 | Uterine prolapse, cystocele, rectocele | Caucasian | Pipelle Biopsy | Imitrex |
| 693 | Control | Pro | 46 | Bladder laceration | Caucasian | Hysterectomy | |
| 607 | Disease | ES | 24 | SE, bilateral ovaries, right ovarian cyst | Asian | Pipelle biopsy | |
| 684 | Disease | ES | 36 | SE, leiomyoma | Caucasian | Hysterectomy | |
| ST-112 | Disease | ES | 38 | PP, SE, bleeding | Caucasian | Pipelle biopsy | |
| ST-130 | Disease | ES | 35 | SE, adenomyosis, right ovarian cyst | Caucasian | Pipelle biopsy | |
| UC-24 | Control | ES | 45 | Menorrhagia, leiomyomata | Black | Hysterectomy | Hydrochlorothiazide, topamax |
| UC-26 | Control | ES | 34 | Menometrorrhagia, leiomyoma | Caucasian | Hysterectomy | Prednisone, zyrtec, folic acid |
| 629 | Control | ES | 46 | Leiomyoma | Caucasian | Hysterectomy | Iron |
| 680 | Control | ES | 34 | Uterine prolapse | Caucasian | Hysterectomy | Zoloft, phentermine |
| 544 | Disease | MS | 46 | SE, endocervical polyp, leiomyoma | Caucasian | Pipelle biopsy | |
| ST-37 | Disease | MS | 32 | SE, bleeding, abnormal ovarian pathology | Unknown | Pipelle biopsy | |
| ST-91 | Disease | MS | 20 | PP, SE, adenomyosis, pelvic adhesions | Caucasian | Pipelle biopsy | Advil, percocet, metoclopraminde |
| ST-96 | Disease | MS | 31 | PP, SE, PCOS, right ovarian cyst | Caucasian | Pipelle biopsy | Tylenol, claritin |
| 463 | Control | MS | 48 | Leiomyoma, prolapse | Caucasian | Hysterectomy | Zyrtec, iron, elavil |
| 501c | Control | MS | 49 | Leiomyoma, menorrhagia | Caucasian | Hysterectomy | Prozac, calcium, iron |
| 610 | Control | MS | 50 | PP, hormonal imbalance | Caucasian | Hysterectomy | |
| 626 | Control | MS | 42 | Prolapse, cystocele, rectocele | Caucasian | Hysterectomy | Allegra, advil |
| 648c | Control | MS | 45 | Leiomyoma | Caucasian | Hysterectomy | Lexapro, ambien |
| 705b | Control | MS | 46 | Left ovarian cyst, dysmenorrhea | Caucasian | Hysterectomy | |
Abbreviations: Pro, proliferative; ES, early secretory; MS, mid-secretory; PP, pelvic pain; SE, severe endometriosis; PI, peritoneal endometriosis; PCOS, polycystic ovarian disease; endo, endometriosis; PCR, polymerase chain reaction.
a All disease specimens were taken from participants surgically staged with moderate/severe endometriosis.
b Sample used for Immunohistochemistry (IHC) validation but not PCR array analysis.
c Sample used only for PCR array analysis.
RNA Isolation and Complementary DNA Synthesis
Total RNA was isolated from PE (n = 4 with disease; n = 4 controls), ESE (n = 4 with disease; n = 4 controls), and MSE (n = 4 with disease; n = 5 controls). Menstrual cycle phase was determined by at least 2 pathologists. The endometrial tissue was processed using Trizol reagent (Invitrogen, Carlsbad, California) and purified using the NucleoSpin RNA II Kit (Marcherey-Nagel, Bethlehem, Pennsylvania), which incorporates DNase treatment in the purification process. Ribonucleic acid concentration and quality (260/280 ratio) were assessed by the Nanodrop instrument (Thermo Scientific, Wilmington, Delaware). Reverse transcription was performed on 1 μg of template to generate double-stranded complementary DNA (cDNA) using RT2 Fist Strand kit (SA Biosciences, Frederick, Maryland). The cDNA was stored at −20°C.
Polymerase Chain Reaction Array and Ingenuity Pathway Analysis
Real-time quantitative polymerase chain reaction (RT2 qPCR) Master Mixes (SA Biosciences) were used for the human nuclear receptors and coregulators RT2 profiler PCR array (SA Biosciences). The 384-well PCR arrays were chosen with precoated primers for 84 genes of interest and 5 housekeeping genes. There were also genomic DNA controls, 3 replicate reverse transcription controls and 3 replicate positive PCR controls (SA Biosciences). The 384-well PCR array plates were run on ABI 7900HT (Applied Biosystems, Carlsbad, California) in the Genomics Core at the UCSF. The Cycle threshold (Ct) values were provided for the genes, and an online Web-based PCR array data analysis Web site provided by SA Biosciences was used to generate fold changes. ΔΔCt was generated using PCR Array Data Analysis Web Portal (SA Biosicences) and the significance for ≥1.5-fold differences was assessed by a 2-tailed t test at P ≤ .05. Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, California) evaluated the participating networks and canonical pathways.
Validation by Immunohistochemistry
Indirect immunostaining was performed with paraffin sections of disease and no disease tissue specimens (PE, n = 3 and n = 4; ESE, n=4; and n = 4; MSE, n = 4 and n = 4, respectively) using antibodies against histone deacetylase 1 (HDAC1), thyroid hormone receptorα (THRA), and DEAD box polypeptide 5 ([DDX5] all from Abcam, Cambridge, Massachusetts). After deparaffinization and rehydration, heat-mediated antigen retrieval13 was performed in a 1× citrate buffer pH 6.0 for 15 minutes at 95°C. The sections were incubated with the primary antibodies (at concentrations: 1.25 μg/mL HDAC1; 2.5 μg/mL THRA; 2 μg/mL DDX5) in blocking buffer overnight at 4°C. Human placenta was used as a positive control, and incubation with the corresponding nonimmune immunoglobulin (Ig) isotypes used as a negative control.14–16 After washing with phosphate-buffered saline (PBS)-Tween 0.01%, the secondary antibody (prediluted as supplied in ImmPRESS Reagent Kit, Vector Laboratories, Burlingame, California) was applied for 30 minutes at room temperature. Diaminobenzidine–hydrogen peroxide solution (DAB Kit; Vector Laboratories) was subsequently added for color development. Sections were then counterstained with hematoxylin (Vector Laboratories) and mounted with Clarion mounting medium (Sigma-Aldrich, St. Louis, Missouri). A Leica microscope (Leica Microsystems, Ltd, Wetzlar, Germany) was used to visualize the staining and to photograph the results.
Qualitative Evaluation of Immunohistochemistry
The staining intensity of the tissues was scored on a scale of 0 = no staining, 1 = faint staining, 2 = moderate staining, and 3 = strong staining. Each disease and no disease sample (PE, n = 3 and n = 4; ESE n = 4 and n = 4; MSE n = 4 and n = 4, respectively) was analyzed 3 times by 1 investigator, blinded to the identities of the samples, and the results were verified by 2 other observers. The average values from the biological replicates are presented.
Results
Gene Expression: Endometrium in the Absence of Endometriosis
In the transition from the proliferative (E2 dominant) to early secretory phase (beginning of P4 action), analysis of endometrial NR/coregulator expression in controls (Table 2) revealed increased expression of peroxisome proliferator-activated receptor gamma (PPARG), NOTCH2, and nuclear coactivator 1 ([NCOA1], also known as steroid receptor coactivator 1 [SRC1], a histone acetylase [HAT] member), along with decreased expression of retinoic acid receptor alpha (RARA) and nuclear receptor subfamily 4, group A (NR4A1). In MSE compared to ESE, RARA was the only upregulated gene, while downregulation of HDAC3, vitamin D receptor (VDR), ESR1, cAMP responsive element binding protein (CREB)-binding protein (CREBBP), and other genes were observed (Table 2). In MSE (peak P4 action on the tissue), compared to PE (E2 dominant) increased expression of retinoic acid receptor-related orphan receptor A (RORA), the aryl hydrocarbon receptor nuclear translocator (ARNT), and nuclear corepressor 1 (NCOR1), among others, were observed (Table 2). Decreased expression of HDAC3, metastasis-associated protein 1 (MTA1, a component of the Dermatomyositis-specific autoantigen (Mi-2)/nucleosome remodeling and deacetylating complex that acts as a potent corepressor of ESR), as well as ESR1 was observed (Table 2), Interestingly, THRA, retinoid X receptor gamma (RXRG), and the androgen receptor (AR) were also downregulated in MSE versus PE (Table 2).
Table 2.
Nuclear Receptor and Coregulator Expression in the Endometrium of Women Without Endometriosis
| Gene | Fold Change |
|---|---|
| Control endometrium: early secretory vs proliferative phase | |
| PPARG | 4.4 |
| NOTCH2 | 3.0 |
| NCOA1 | 2.8 |
| PPARA | 1.8 |
| RARA | −3.7 |
| NR4A1 | −6.1 |
| Control endometrium: mid-secretory vs early secretory phase | |
| RARA | 2.64 |
| HDAC3 | −1.5 |
| NONO | −1.6 |
| CREBBP | −1.7 |
| MED12 | −1.9 |
| VDR | −3.4 |
| ESR1 | −3.6 |
| RXRG | −6.7 |
| Control endometrium: mid-secretory vs proliferative phase | |
| RORA | 3.3 |
| NR1H3 | 2.2 |
| ARNT | 2.0 |
| MED13 | 1.9 |
| NCOR1 | 1.9 |
| MED24 | −1.6 |
| MED12 | −1.6 |
| HDAC3 | −1.7 |
| MTA1 | −1.8 |
| THRA | −2.3 |
| NR0B2 | −2.8 |
| RXRG | −2.8 |
| ESR1 | −4.0 |
| AR | −4.2 |
Abbreviations: AR, androgen receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; CREBBP, CREB-binding protein; ESR1, estrogen receptor 1; HDAC3, histone deacetylase 3; MED12, mediator complex subunit 12; MED13, mediator complex subunit 13; MED24, mediator complex subunit 24; MTA1, metastasis-associated 1; NCOA1, nuclear coactivator 1; NCOR1, nuclear receptor corepressor 1; NONO, nou-POU domain containing, octamer-binding; NR0B2, nuclear receptor subfamily 0, group B, member 2; NR1H3, nuclear receptor subfamily 1, group H, member 3; NR4A1, nuclear receptor subfamily 4, group A, member 1; PPARA, peroxisome proliferator-activated receptor alpha; PPARG, peroxisome proliferator-activated receptor gamma; RARA, retinoic acid receptor alpha; RORA, retinoic acid receptor-related orphan receptor A; RXRG, retinoid X receptor gamma; THRA, thyroid hormone receptor alpha.
Gene Expression: Endometrium in the Presence of Endometriosis
In the setting of endometriosis, HDAC1 and HDAC2, which interact directly with proteins recruited to steroid hormone receptors and are part of the transcriptional complex, were upregulated in the proliferative (E2-dominant) phase, compared to controls without disease, and MTA2, a potent ESR corepressor was downregulated (Table 3). In the early secretory phase (beginning of P4 action and where P4 resistance is first observed in endometrium of women with severe endometriosis12), even higher expression of HDAC1 and HDAC2 in endometrium from women with versus without endometriosis was observed, compared to the proliferative phase (Table 3).
Table 3.
Nuclear Receptor and Coregulator Expression in the Endometrium of Women With Versus Without Endometriosis
| Gene | Fold Change |
|---|---|
| Proliferative phase | |
| DDX5 | 7.6 |
| HDAC2 | 4.2 |
| NCOA1 | 3.9 |
| ITGB3PB | 3.6 |
| COPS2 | 3.3 |
| RBPJ | 3.2 |
| NCOR1 | 2.9 |
| HDAC1 | 2.8 |
| MED17 | 2.8 |
| MED4 | 2.7 |
| BRD8 | 2.5 |
| MED14 | 2.3 |
| ANRT | 2.2 |
| NCOA6 | 1.5 |
| THRA | −2.7 |
| MTA1 | −2.8 |
| MED16 | −3.8 |
| NR4A1 | −4.7 |
| NR2F6 | −5.7 |
| Mid-secretory phase | |
| RARB | 1.7 |
| Early secretory phase | |
| DDX5 | 11.3 |
| HDAC2 | 6.3 |
| NCOA1 | 5.9 |
| ITGB3PB | 5.4 |
| NR2C2 | 5.3 |
| COPS2 | 4.9 |
| RBPJ | 4.8 |
| MED1 | 4.6 |
| NR2C1 | 4.4 |
| NCOR1 | 4.3 |
| HDAC1 | 4.2 |
| MED17 | 4.2 |
| MED4 | 4.1 |
| BRD8 | 3.8 |
| NCOA3 | 3.4 |
| MED14 | 3.4 |
| ARNT | 3.4 |
| NR3C2 | 3.1 |
| NR3C1 | 3.1 |
| NR1D2 | 2.9 |
| NCOA6 | 2.2 |
| NR2F2 | 1.7 |
| MED16 | −2.6 |
Abbreviations: BRD8, bromodomain containing 8; COPS2, COP9 constitutive photomorphogenic homolog subunit 2 (arabidopsis); DDX5, DEAD (Asp-Glu Ala-Asp) box polypeptide 5; HDAC1, histone deacetylase 1; HDAC2, histone deacetylase 2; ITGB3BP, integrin beta 3 binding protein (beta3-endonexin) MED1, mediator complex subunit 1; MED14, mediator complex subunit 14; MED16, mediator complex subunit 16; MED17, mediator complex subunit 17 MED17, mediator complex subunit 17; MED4, mediator complex subunit 4; NCOA3, nuclear receptor coactivator 3; NCOA6, nuclear receptor coactivator 6 NR1D2, nuclear receptor subfamily 1, group D, member 2; NR2C1, nuclear receptor subfamily 2, group C, member 1; NR2C2, nuclear receptor subfamily 2 group C member 2; NR2F2, nuclear receptor subfamily 2, group F, member 2; NR2F6, nuclear receptor subfamily 2, group F, member 1; NR3C1, nuclear receptor subfamily 3, group C, member 1; NR3C2, nuclear receptor subfamily 3, group C, member 2; NR4A1, nuclear receptor subfamily 4, group A, member 1; RBPJ, recombination signal binding protein for immunoglobulin kappa J region.
Upregulation of a number of other transcription factors was observed, including NCOA1, NCOR1, and NCOA3 (which recruits p300/CBP (C-terminal Src kinase (Csk)-binding protein) -associated factor and CREB-binding protein), and recombination signal-binding protein for Ig kappa J region (RBPJ, a DNA-binding protein that interacts with the Notch intracellular domain to activate transcription of genes that inhibit cell differentiation).17,18
Overall, of the 3 phases of the menstrual cycle investigated herein, the early secretory phase had the highest number of statistically significantly upregulated genes in endometrium from women with versus without disease (Table 3). The most highly upregulated gene in the proliferative and early secretory phases was DDX5, a transcriptional repressor that associates with HDAC1.19 In the mid-secretory phase, only 1 gene, RARB, was upregulated in disease versus controls (Table 3).
Ingenuity Pathway Analysis
Endometrium in the absence of endometriosis
In the transition from the proliferative to the early secretory phase, the cellular development/embryonic development/gene expression network was upregulated, based on PPARA, PPARG, and NCOA1 expression involved in the PPAR-signaling pathway (Table 2). In the early to mid-secretory transition, Ingenuity Pathway Analysis (IPA) analysis revealed the drug metabolism/embryonic development/lipid metabolism network affected, with upregulation of retinoic acid-mediated apoptosis signaling, based on the upregulation of RARA and downregulation of RXRG (Table 2). In the comparison of the mid-secretory phase to the proliferative phase, the gene expression/cell signaling/nutritional disease network was downregulated, based on the downregulation of THRA and ESR1 in the estrogen receptor-signaling pathway and the downregulation of HDAC3 and upregulation of NCOR1 in the thyroid hormone (TR)/RXR pathway (Table 2). For a more comprehensive list of networks and pathways revealed by IPA, see supplemental data (Supplement Tables 1-3).
Endometrium in the setting of endometriosis
IPA identified DNA methylation and transcriptional repression signaling as the most affected pathway in endometrium in women with versus without disease, regardless of the cycle phase.
In the proliferative phase, gene expression/cell signaling was the first ranked affected network, and DNA methylation and transcriptional repression signaling were the first ranked canonical pathway to be upregulated in endometrium from women with versus without disease. The genes involved in these processes were HDAC1, HDAC2, and MTA1 (Table 3). The THR/RXR pathway was affected by the downregulation of THRA expression in the proliferative phase in endometriosis, compared to control samples (Table 3). In disease versus no disease in the early secretory phase, IPA analysis revealed THRA and VDR pathway activation, by the upregulation of NCOA1, NCOA3, NCOR1, and mediator complex subunit 1 (MED1) gene expression (Table 3). A more comprehensive list of networks and pathways revealed by IPA is provided in the supplemental data (Supplement Tables 4-6).
Table 4.
Nuclear Immunostaining Intensities of DDX5, HDAC1, and THRA in Cycling Endometrium of Women With and Without Diseasea
| Disease PE | No Disease PE | Disease ESE | No Disease ESE | Disease MSE | No Disease MSE | |
|---|---|---|---|---|---|---|
| DDX5 | ||||||
| Luminal epitheliumb | 2.75 ± 0.25 | 2.25 ± 0.25 | NA | 3.00 ± 0.00 | 2.00 ± 0.00 | NA |
| Glandular epithelium | 2.50 ± 0.00 | 2.00 ± 0.29 | 2.38 ± 0.31 | 1.63 ± 0.38 | 2.75 ± 0.25 | 1.75 ± 0.14 |
| Stromal cells | 1.83 ± 0.17 | 2.00 ± 0.29 | 2.00 ± 0.29 | 2.13 ± 0.24 | 2.25 ± 0.32 | 1.75 ± 0.14 |
| HDAC1 | ||||||
| Luminal epitheliumb | NA | 2.00 ± 1.00 | 1.17 ± 0.93 | 0.83 ± 0.60 | NA | NA |
| Glandular epithelium | 2.67 ± 0.17 | 2.67 ± 0.33 | 2.25 ± 0.43 | 2.13 ± 0.38 | 2.13 ± 0.13 | 2.13 ± 0.24 |
| Stromal cells | 2.50 ± 0.00 | 2.83 ± 0.17 | 2.63 ± 0.38 | 2.25 ± 0.60 | 1.63 ± 0.43 | 1.38 ± 0.52 |
| THRA | ||||||
| Luminal epitheliumb | 2.50 ± 0.29 | NA | 2.00 ± 0.50 | 0.25 ± 0.14 | 1.17 ± 0.17 | NA |
| Glandular epithelium | 2.67 ± 0.33 | 3.00 ± 0.00 | 2.13 ± 0.24 | 1.50 ± 0.58 | 1.63 ± 0.24 | 2.17 ± 0.44 |
| Stromal cells | 1.67 ± 0.60 | 2.17 ± 0.83 | 1.88 ± 0.24 | 1.75 ± 0.48 | 1.00 ± 0.29 | 1.67 ± 0.73 |
Abbreviations: NA, not available; DDX5, DEAD (Asp-Glu-Ala-Asp) box polypeptide 5; HDAC1, histone deacetylase 1; THRA, thyroid hormone receptor alpha; RXRG, retinoid X receptor gamma; SEM standard error of the mean.
a Values represent mean immunostaining intensities ± SEM.
b Some paraffin sections did not contain luminal epithelium.
Protein Validation by Immunohistochemistry
To validate the PCR array results and determine cellular localization of corresponding proteins, immunohistochemistry of the most highly upregulated genes (DDX5 and HDAC1), as well as THRA proteins was performed. Average staining intensities are presented in Table 4.
DDX5 protein
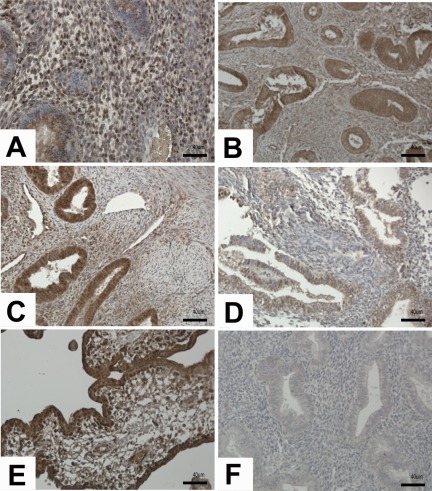
DDX5 immunostaining was observed in the nuclei of luminal and glandular epithelium and stromal cells. In PE from women with endometriosis variable immunoreactivity was observed in glandular epithelium (Figure 1A). Controls had more consistent expression of DDX5 (Figure 1B) but with similar intensities as the disease samples. In the early secretory phase, stronger immunoreactivity was observed in tissue from women with endometriosis, especially in the glandular and luminal epithelia (Figure 1C and D). Stromal cells had variable expression in samples from women with and without disease. Equal intensities of immunoreactivity were observed in the mid-secretory phase of women with and without the disease (data not shown). The increase of DDX5 protein observed in the disease samples in the early secretory phase is consistent with the upregulation of DDX5 gene expression in this phase of the cycle.
Figure 1.
Results of immunostaining for DEAD box polypeptide 5 (DDX5) in disease and no disease endometrium. Immunoreactivity of DDX5 was observed equally in the proliferative phase of both disease (A) and no disease (B) endometrium. Number of positively stained glandular epithelial cells increased in early secretory phase of disease (C), and decreased in no disease (D). Human placenta was used as positive control (E) and nonimmune isotype as negative control (F). Magnification, ×20. Scale bar, 40 µm.
HDAC1 protein
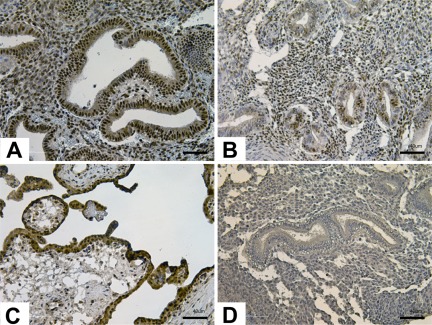
Nuclear immunostaining of HDAC1 was observed in luminal and glandular epithelium and stromal cells. In the proliferative phase, equal intensities were observed in glandular epithelium and stromal cells (data not shown). Stronger HDAC1 immunoreactivity was observed in the early secretory phase, especially in the glandular epithelium of disease samples (Figure 2A and B). In the mid-secretory phase, similar HDAC1 immunoreactivity was observed in stromal cells; whereas glandular epithelium had sporadic immunoreactivity regardless of the presence of disease (data not shown). While similar intensities in the proliferative phase are in contrast with the gene expression results of HDAC1, stronger immunoreactivity observed in glandular epithelium of disease samples in the early secretory phase is consistent with the PCR array analysis.
Figure 2.
Results of immunostaining for histone deacetylase 1 (HDAC1) in disease and no disease endometrium. In the early secretory phase, stronger immunoreactivity was observed in glandular epithelium of disease samples (A) compared to no disease samples (B). Human placenta was used as positive control (C) and nonimmune isotype as negative control (D). Magnification, ×20. Scale bar, 40 µm.
THRA protein
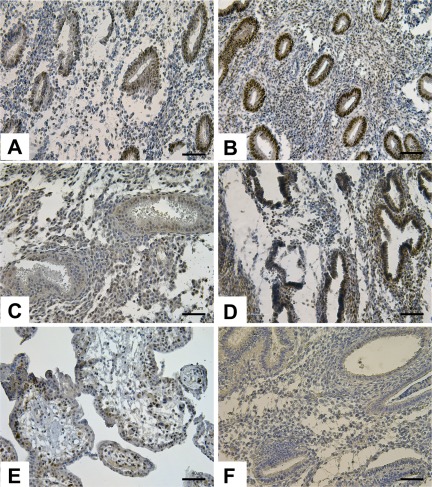
In glandular epithelium, there was stronger THRA protein immunoreactivity in control samples in the PE and MSE phases, compared to ESE (Figure 3B and D, respectively), confirming earlier findings.20 In endometriosis samples, THRA immunoreactivity was the highest in glandular epithelium of the proliferative phase and lowest in the mid-secretory phase (Figure 3A and E, respectively). Stronger immunostaining was observed in the PE phase of the control samples, and no significant differences were observed in the ESE phase between endometriosis and control samples (Figure 3C and D). Luminal epithelium had variable protein expression throughout all phases of the cycle in disease and no disease. Stronger immunoreactivity in the control samples compared to the disease samples is consistent with the downregulation of THRA gene expression in the proliferative phase of disease samples and activation of TR/RXR pathway in the mid-secretory versus proliferative phase, identified by IPA analysis.
Figure 3.
Results of immunostaining for thyroid hormone receptor alpha (THRA) protein in disease and no disease endometrium. In the proliferative phase, THRA protein expression was localized mainly to the glandular epithelium of both disease (A) and no disease (B) samples, with stronger expression in no disease samples. In the mid-secretory phase, stronger expression was observed in the glandular epithelium of no disease samples (D) compared to disease (C) samples. Human placenta was used as positive control (E) and nonimmune isotype as negative control (F). Magnification, ×20. Scale bar, 40 µm.
Discussion
Nuclear Receptors and Coregulators in Human Endometrium Without Endometriosis
The current study underscores the involvement of key signaling pathways of NRs and coregulators and other transcription factors in human endometrium during the P4-dominant phase of the menstrual cycle, as well as abnormalities in some of these in endometrium from women with endometriosis.
ESR, AR, NCOAs, NCORs
In endometrium, E2 induces cell proliferation; whereas P4 inhibits estrogen action and induces glandular and stromal cell differentiation in the secretory phase of the cycle.21 Steroid receptors such as ESR, AR, and PGR recruit NCORs and NCOAs to mediate gene expression.22 Downregulation of ESR and AR in secretory versus PE of women without endometriosis, reported herein, is consistent with the findings of others.22 However, there are conflicting data regarding NCOA1 and NCOR1 in human endometrium. Some studies have found that their messenger RNA (mRNA) levels are not cycle dependent22; whereas others found lower expression of NCOA1 and NCOR1 proteins in the secretory, compared to the proliferative, phase.23 Our results demonstrate an increase of NCOA1 mRNA in ESE and an increase of NCOR1 in MSE, suggesting roles for them in steroid hormone action in endometrium during the P4-dominant phase of the cycle. Nuclear coactivator 1 interacts with ESR1 and PGR in a ligand-dependent manner to enhance hormone-dependent transcriptional activities.24 We speculate that the NCOA pathway may play a general role in facilitating P4 action in the ESE.
Thyroid hormone
The thyroid hormone-signaling pathway consists of many proteins that regulate thyroid hormone synthesis and activation, as well as activation of gene transcription by NRs. Only recently has this family been identified in human endometrium, suggesting participation in endometrial function.20,25 We confirmed herein earlier findings of THRA protein expression in human endometrium across the cycle from women without endometriosis.20 Our findings and those of Aghajanova et al20 are in contrast to the findings using microarray analysis, by Catalano et al,25 of THRA1 and THRB1 mRNA expression significantly increased from the proliferative to the secretory phase. The reasons for the differences are unclear, although they may be due to the different methodologies used.
Chromatin-remodeling mediators
Histone deacetylases, HATs, and DNA methyltransferases (DNMTs) have been minimally investigated in human endometrium. Class I HDACs, HDAC1, 2, and 3 play an important role in steroid hormone-dependent gene expression by directly interacting with steroid hormone receptor proteins and other members of the transcriptional complex after ligand binding.26 Treatment of endometrial adenocarcinoma cells with the HDAC inhibitor, valproic acid, blocks estrogen-induced cellular proliferation, suggesting a potential link between endometrial proliferation and histone acetylation.27 Krusche et al28 found that HDAC 1, 2, and 3 mRNAs were constitutively expressed in human endometrium, while the proteins were expressed variably with a trend toward higher HDAC2 in the secretory phase and a reduction of HDAC3 in surface epithelium. In our transcriptome study of human endometrium across the menstrual cycle in normo-ovulatory women without endometriosis, we found about a 2-fold significant downregulation of HDAC2, Histone H2A histone family, member V (H2aV), and DNMT 1, 3A, and 3B in the mid-secretory phase (peak P4) compared to the proliferative phase (peak E2).29 Recently, preliminary data were presented30 on the total acetylation (ac) level in normal endometrium of specific lysines of histones 2A, 2B, 3, and 4, by Western blotting. A significant progressive decline of H2AK5ac was found across the proliferative phase, a decline in H3K9ac between the early and late proliferative phases, and a decline in H4K8ac progressively across the proliferative phase and a peak in the mid-secretory phase. In contrast, there were no significant differences in H2BK12ac, H3K14/18ac, or H4K5ac across the cycle,30 suggesting selectivity to the acetylome changes. In the current study, we observed a decrease of HDAC3 mRNA in the mid-secretory phase of the menstrual cycle compared to the proliferative phase, which correlates well with the immunostaining results of HDAC3 conducted by Krusche et al.28 Furthermore, similar results were observed in the immunostaining of HDAC1 in stromal cells in the cycling endometrium, herein, and in the study by Krusche et al.28 Importantly, overall these findings support a role for chromatin remodeling in different hormonal milieu across the menstrual cycle in human endometrium.
Endometrium in the Setting of Endometriosis
The results herein demonstrate the dysregulation of a number of NRs, coregulators, and signaling pathways in endometrium in women with endometriosis versus those without disease that have relevance to the pathophysiology of the disorder and are potential targets for therapy.
NCORs and NCOAs
Steroid receptor cofactors play an important role in steroid receptor signaling. A recent study found higher NCOR expression in the epithelium of ovarian endometriomas in the secretory phase, compared to normal eutopic endometrium,31 and NCOR expression is higher in endometrial hyperplasia compared to normal and neoplastic endometrial cells.32 Herein, we found higher levels of NCOR1 in secretory phase eutopic endometrium of women with versus without disease, suggesting a role for this coregulator in repressing P4 action and increased E2 signaling in the setting of endometriosis. Nuclear corepressor represses transcription by recruiting HDACs, and overexpression of NCOR represses the activity of ligand-bound PGR and ESR.33–35 In the setting of endometriosis, NCOR most likely downregulates genes targeted by P4. This correlates to the findings in the baboon model of endometriosis, where PGRA immunolocalization in glandular epithelial cells was observed to decrease, while PGRB levels remained unchanged in the eutopic endometrium.36 No difference in PGRA or PGRB immunoreactivity was observed in stromal cells; however, the ability of these cells to respond to PGR hormone and cyclic adenosine monophosphate (cAMP) stimulation is diminished.36 Jackson et al,36 observed a decrease of a chaperone immunophilin, 52 kDa FK506 binding protein (FKBP52), which regulates P4 action. Furthermore, a decrease of HIC5 expression in stromal cells from eutopic endometrium of endometriosis patients reduces PGR signaling.11 Overall, the observed decrease of P4 response could be attributed to the effects of the NCORs or PGR chaperones. Herein, we observed no significant changes in PGR mRNA levels, which may suggest that the NCOAs and NCORs affect the functional activity of PGR in the regulation of target genes and not expression per se of PGR in the setting of endometriosis.
The NCOA family members enhance the transcriptional activity of various NRs, including ESR and PGR.37 Interestingly, in NCOA1 null mice, endometrial stromal cell decidualization is compromised in response to E2 and P4.38 Herein we found upregulation of NCOA1 in endometrium from women with endometriosis compared to no disease. We speculate that NCOA1 and NCOR1 are important players, competing for binding to ESR and PGR for transcriptional activation and repression—resulting in P4 resistance and persistence of the proliferative phenotype observed in endometrium from women with endometriosis.12 The functional role of NOCA1 in endometriosis warrants further investigation.
Thyroid hormone
Since thyroid hormone action in endometrium may be P4 dependent,20,25 decreased expression of THRA in women with endometriosis may reflect P4 resistance in this tissue in the setting of disease. Thyroid hormone receptors (THRA and THRB) mediate T3 inhibitory effects on ras/mitogen-activated protein kinase (MAPK)-mediated proliferation in neuroblastoma cells and block induction of cyclin D1 expression.39 Whether THRs are involved in the observed persistent cyclin D1 expression in endometrial cells from women with endometriosis warrants further investigation.40
DDX5 and chromatin remodeling mediators
The most regulated canonical pathway in eutopic endometrium of women with endometriosis compared to controls was DNA methylation and transcriptional repression signaling involving gene and protein upregulation of DDX5 and HDAC1 in the early secretory phase. DDX5, also known as nuclear protein 68, is a member of the DEAD-box family of RNA helicases.41 Its functions include pre-mRNA splicing, ATPase and helicase activity, and RNA degradation and export.41,42 It functions as a transcriptional regulator, especially as a coactivator for ERα43 and AR.44 In the context of its function as a coactivator, DDX5 may enhance E2 action in the endometrium of women with endometriosis. However, DDX5 also acts as a repressor of transcription in a promoter-specific manner19 and interacts with HDAC1 to promote transcriptional repression.41,45–47 This hypothesis is supported by the results from both the PCR array and immunohistochemistry, due to the similar HDAC1 and DDX5 gene and protein expression observed herein. It is important to note that both DDX5 and HDAC1 increased equivalently and in parallel in the proliferative to the early secretory transition, strengthening this conclusion. Histone deacetylation, along with DNA methylation, generates a feedback loop to promote long-term transcriptional repression,3 and thus upregulation of HDAC1, HDAC2, and DDX5 genes in endometriosis in the proliferative and early secretory phases of the menstrual cycle could in fact promote DNA methylation and in turn transcriptional repression of associated genes leading to endometrial dysfunction. These findings support the suggestion of endometriosis as an epigenetic disorder,48 with multiple sites of gene transcriptional regulation by epigenetic modifications altering cellular function in the setting of disease.
Summary
Steroid hormones impart their ultimate effects through their NRs, which interact with chromatin and initiate diverse transcriptional programs. The genomic localization and functions of these receptors crucially depend on the local epigenetic state of their binding sites, and, in turn, transcription factors can induce chromatin remodeling and modifications. Both E2 and P4 thus have intricate interactions with the epigenome of targeted cells. Enforcing these considerations, epigenetic alterations have been linked directly to endometriosis48 and associated reduced fertility.49 The present study demonstrates dysregulation of signaling, chromatin remodeling, and gene expression regulatory pathways in eutopic endometrium of women with endometriosis, with the most profound dysregulation occurring within the proliferative and early secretory phases of the menstrual cycle, where the majority of those genes are dysregulated, especially in the early secretory phase. This is in striking parallel to our previous findings using gene expression analysis that demonstrated a blunted proliferative-to-secretory transition in early secretory phase endometrium.12 These and data from others7 have led to the conclusion of P4 resistance in the endometrium of women with endometriosis. Overall, the data support the involvement of the epigenome and chromatin remodeling in the steroid hormone response of normal endometrium throughout the cycle and also in abnormalities in the endometrium of women with endometriosis. These findings are significant in understanding steroid hormone action in endometriosis due to the coupling of the players in chromatin remodeling and gene transcription and silencing and underscoring the relevance of epigenetics in endometriosis, warranting further studies.
Acknowledgments
The authors thank Dr. David Erikson for assistance during manuscript preparation.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH through cooperative agreement U54HD055764-04 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (LCG).
References
- 1. Auboeuf D, Dowhan DH, Dutertre M, Martin N, Berget SM, O'Malley BW. A subset of nuclear receptor coregulators act as coupling proteins during synthesis and maturation of RNA transcripts. Mol Cell Biol. 2005;25(13):5307–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzpatrick DR, Wilson CB. Methylation and demethylation in the regulation of genes, cells, and responses in the immune system. Clin Immunol. 2003;109(1):37–45 [DOI] [PubMed] [Google Scholar]
- 3. Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15(5):490–495 [DOI] [PubMed] [Google Scholar]
- 4. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258 [DOI] [PubMed] [Google Scholar]
- 6. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279 [DOI] [PubMed] [Google Scholar]
- 7. Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28(1):51–58 [DOI] [PubMed] [Google Scholar]
- 8. Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol. 2007;69:201–220 [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1-2):94–103 [DOI] [PubMed] [Google Scholar]
- 10. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111 [DOI] [PubMed] [Google Scholar]
- 11. Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150(8):3863–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826 [DOI] [PubMed] [Google Scholar]
- 13. Aghajanova L, Giudice LC. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci. 2011;18(3):229–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuang HC, Chang CW, Chang GD, Yao TP, Chen H. Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucleic Acids Res. 2006;34(5):1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakai A, Seino S, Sakurai A, Szilak I, Bell GI, DeGroot LJ. Characterization of a thyroid hormone receptor expressed in human kidney and other tissues. Proc Natl Acad Sci U S A. 1988;85(8):2781–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Fujii H, Knipp GT. Expression of PPAR and RXR isoforms in the developing rat and human term placentas. Placenta. 2002;23(8-9):661–671 [DOI] [PubMed] [Google Scholar]
- 17. Kurooka H, Honjo T. Functional interaction between the mouse notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J Biol Chem. 2000;275(22):17211–17220 [DOI] [PubMed] [Google Scholar]
- 18. Leo C, Chen JD. The SRC family of nuclear receptor coactivators. Gene. 2000;245(1):1–11 [DOI] [PubMed] [Google Scholar]
- 19. Wilson BJ, Bates GJ, Nicol SM, Gregory DJ, Perkins ND, Fuller-Pace FV. The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Mol Biol. 2004;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aghajanova L, Stavreus-Evers A, Lindeberg M, Landgren BM, Sparre LS, Hovatta O. Thyroid-stimulating hormone receptor and thyroid hormone receptors are involved in human endometrial physiology. Fertil Steril. 2011;95(1):230–237 e232. [DOI] [PubMed] [Google Scholar]
- 21. Hess A, Nayak N, Giudice L. Oviduct and endometrium: cyclic changes in primate oviduct and endometrium. In: Knobil E, Neill J, eds. The Physiology of Reproduction. San Diego: Academic Press; 2005:337–381 [Google Scholar]
- 22. Vienonen A, Miettinen S, Blauer M, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11(2):104–112 [DOI] [PubMed] [Google Scholar]
- 23. Shiozawa T, Shih HC, Miyamoto T, et al. Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J Clin Endocrinol Metab. 2003;88(2):871–878 [DOI] [PubMed] [Google Scholar]
- 24. Wieser F, Schneeberger C, Hudelist G, et al. Endometrial nuclear receptor co-factors SRC-1 and N-CoR are increased in human endometrium during menstruation. Mol Hum Reprod. 2002;8(7):644–650 [DOI] [PubMed] [Google Scholar]
- 25. Catalano RD, Critchley HO, Heikinheimo O, et al. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod. 2007;13(9):641–654 [DOI] [PubMed] [Google Scholar]
- 26. Liu XF, Bagchi MK. Recruitment of distinct chromatin-modifying complexes by tamoxifen-complexed estrogen receptor at natural target gene promoters in vivo. J Biol Chem. 2004;279(15):15050–15058 [DOI] [PubMed] [Google Scholar]
- 27. Hodges-Gallagher L, Valentine CD, Bader SE, Kushner PJ. Inhibition of histone deacetylase enhances the anti-proliferative action of antiestrogens on breast cancer cells and blocks tamoxifen-induced proliferation of uterine cells. Breast Cancer Res Treat. 2007;105(3):297–309 [DOI] [PubMed] [Google Scholar]
- 28. Krusche CA, Vloet AJ, Classen-Linke I, von Rango U, Beier HM, Alfer J. Class I histone deacetylase expression in the human cyclic endometrium and endometrial adenocarcinomas. Hum Reprod. 2007;22(11):2956–2966 [DOI] [PubMed] [Google Scholar]
- 29. Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121 [DOI] [PubMed] [Google Scholar]
- 30. Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310 [DOI] [PubMed] [Google Scholar]
- 31. Suzuki A, Horiuchi A, Oka K, Miyamoto T, Kashima H, Shiozawa T. Immunohistochemical detection of steroid receptor cofactors in ovarian endometriosis: involvement of down-regulated SRC-1 expression in the limited growth activity of the endometriotic epithelium. Virchows Arch. 2010;456(4):433–441 [DOI] [PubMed] [Google Scholar]
- 32. Uchikawa J, Shiozawa T, Shih HC, et al. Expression of steroid receptor coactivators and corepressors in human endometrial hyperplasia and carcinoma with relevance to steroid receptors and Ki-67 expression. Cancer. 2003;98(10):2207–2213 [DOI] [PubMed] [Google Scholar]
- 33. Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol Endocrinol. 1997;11(6):693–705 [DOI] [PubMed] [Google Scholar]
- 34. Li H, Leo C, Schroen DJ, Chen JD. Characterization of receptor interaction and transcriptional repression by the corepressor SMRT. Mol Endocrinol. 1997;11(13):2025–2037 [DOI] [PubMed] [Google Scholar]
- 35. Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11(6):657–666 [DOI] [PubMed] [Google Scholar]
- 36. Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14(2):137–150 [DOI] [PubMed] [Google Scholar]
- 37. DeMayo FJ, Zhao B, Takamoto N, Tsai SY. Mechanisms of action of estrogen and progesterone. Ann N Y Acad Sci. 2002;955:48-59; discussion 86-48, 396-406 [DOI] [PubMed] [Google Scholar]
- 38. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279(5358):1922–1925 [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Silva S, Aranda A. The thyroid hormone receptor is a suppressor of ras-mediated transcription, proliferation, and transformation. Mol Cell Biol. 2004;24(17):7514–7523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3',5'-cyclic adenosine 5'-monophosphate inhibition of cyclin D1. Endocrinology. 2009;150(10):4701–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacobs AM, Nicol SM, Hislop RG, Jaffray EG, Hay RT, Fuller-Pace FV. SUMO modification of the DEAD box protein p68 modulates its transcriptional activity and promotes its interaction with HDAC1. Oncogene. 2007;26(40):5866–5876 [DOI] [PubMed] [Google Scholar]
- 42. Fuller-Pace FV. DExD/H box RNA helicases: multifunctional proteins with important roles in transcriptional regulation. Nucleic Acids Res. 2006;34(15):4206–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Endoh H, Maruyama K, Masuhiro Y, et al. Purification and identification of p68 RNA helicase acting as a transcriptional coactivator specific for the activation function 1 of human estrogen receptor alpha. Mol Cell Biol. 1999;19(8):5363–5372 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Clark EL, Coulson A, Dalgliesh C, et al. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res. 2008;68(19):7938–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carter CL, Lin C, Liu CY, Yang L, Liu ZR. Phosphorylated p68 RNA helicase activates Snail1 transcription by promoting HDAC1 dissociation from the Snail1 promoter. Oncogene. 2010;29(39):5427–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santos-Rosa H, Caldas C. Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer. 2005;41(16):2381–2402 [DOI] [PubMed] [Google Scholar]
- 47. Yoo EJ, Chung JJ, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006;281(10):6608–6615 [DOI] [PubMed] [Google Scholar]
- 48. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15(10):587–607 [DOI] [PubMed] [Google Scholar]
- 49. Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest. 1998;101(7):1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]