Abstract
Gold nanorods (GNRs) have emerged as promising nanomaterials for biosensing, imaging, photothermal treatment and therapeutic delivery for several diseases, including cancer. We have generated poly(amino ether)-functionalized gold nanorods (PAE-GNRs) using a layer-by-layer deposition approach; polymers from a poly(amino ether) library recently synthesized in our laboratory were employed to generate the PAE-GNR assemblies. PAE-GNR assemblies demonstrate long-term colloidal stability as well as the capacity to bind plasmid DNA by means of electrostatic interactions. Sub-toxic concentrations of PAE-GNRs were employed to deliver plasmid DNA to prostate cancer cells in vitro. PAE-GNRs generated using 1,4C-1,4Bis, a cationic polymer from our laboratory demonstrated significantly higher transgene expression and exhibited lower cytotoxicities when compared to similar assemblies generated using 25 kDa poly(ethylene imine) (PEI25k-GNRs), a current standard for polymer-mediated gene delivery. The roles of polyelectrolyte chemistry and zeta-potential in determining transgene expression efficacies of PAE-GNR assemblies were investigated. Our results indicate that stable and effective PAE-GNR assemblies are a promising engineered platform for transgene delivery. PAE-GNRs also have the potential to be used simultaneously for photothermal ablation, photothermally enhanced drug and gene delivery, and biological imaging, thus making them a powerful theranostic platform.
Keywords: gold nanorods, non-viral gene delivery, nanoassemblies, polyaminoethers, theranostics
Introduction
Several novel nanomaterials, including gold nanoparticles, are currently being investigated as carriers for delivery of therapeutic agents (Chinnaiyan et al. 2006; Cho et al. 2008; Ghosh et al. 2008; Heath and Davis 2008; Huang et al. 2011; Jabr-Milane et al. 2008; Smith et al. 2008). The plasmonic properties of gold nanorods make them attractive for application in biosensing (Castellana et al. 2011; Guo et al. 2011), imaging (Ding et al. 2007; Ha et al. 2011; Pan et al. 2010), photothermal therapy (Choi et al. 2011; Huang et al. 2006; von Maltzahn et al. 2009), and gene and drug delivery(Braun et al.; Chen et al. 2006; Salem et al. 2003). Effective delivery of exogenous genes using appropriate vehicles is a promising approach towards correction of diseases that are genetic in origin. Despite their high efficacies as delivery vectors, viruses suffer from several limitations including immunogenicity, insertional mutagenesis, and high production costs. Non-viral strategies, particularly those that can simultaneously enable delivery and imaging (‘theranostics’), are attractive alternatives to viral vectors in gene therapy applications (Garnett 1999; Glover et al. 2005; Yi et al. 2005).
Gold nanoparticles are promising vectors for non-viral transgene delivery due to their unique optical properties and the ability to modify their surfaces using facile methods (Huang et al. 2009b). In addition, gold nanoparticles possess high surface area-to-volume ratios which can facilitate high payload (transgene) carrying capabilities. Surface functionalization of gold nanoparticles allows for tuning of hydrophobicity and charge, which can enhance transgene expression and decrease cytotoxicity (Ghosh et al. 2008). Polymers have been investigated for functionalization of gold nanoparticles in several applications, including transgene delivery (Bonoiu et al. 2009; Ow Sullivan et al. 2003; Sandhu et al. 2002; Thomas and Klibanov 2003). Polymer-functionalized gold nanoparticles demonstrate increased stability, controllable surface properties, and the capacity for additional surface functionalization which further increases their potential for use in therapeutic applications (Wei et al. 2008).
We previously employed a cationic polymer synthesized in our laboratory (Barua et al. 2009; Kasman et al. 2009) primarily for enhancing the colloidal stability of gold nanorods in biological media (Huang et al. 2010). In this study, we describe an investigation into polymer type, plasmid DNA loading, and physicochemical properties of poly(amino ether)-functionalized gold nanorods in determining the efficacy of transgene delivery and expression using these vehicles. Poly(amino ether) or PAE-functionalized GNR (PAE-GNR) assemblies were generated by depositing polyelectrolyte multilayers on cetyltrimethyl ammonium bromide based gold nanorods (CTAB-GNRs); candidates from a cationic poly(amino ether) library recently synthesized in our laboratory were employed in the current study (Barua et al. 2009). PAE-GNRs are stable in cell culture media, and are also able to bind plasmid DNA by means of electrostatic interactions. PAE-GNRs were employed to successfully deliver plasmid DNA expressing the luciferase reporter protein to human prostate cancer cells; PAE-GNRs based on 1,4C-1,4Bis, a cationic polymer generated in our laboratory demonstrated higher transgene (luciferase) expression efficacies, and lower or comparable cytotoxicities compared to assemblies generated using 25 kDa poly(ethylene imine) (PEI25k), a current standard for polymer mediated gene delivery.
Materials and Methods
Gold Nanorod (GNR) Synthesis
The seed-mediated method (Nikoobakht and El-Sayed 2003) was used for the synthesis of the gold nanorods (CTAB-GNRs). Briefly, a seed solution was prepared by adding 5 ml of 0.2 M cetyltrimethyl ammonium bromide (CTAB) to 5 ml of 0.0005 M auric acid (HAuCl4.3H20). The addition of 0.6 ml of iced water-cooled 0.01 M sodium borohydride was used to reduce the solution. The growth solution was prepared by adding 5 ml of 0.001 M auric acid to 5 ml of 0.2 M CTAB containing 250 μl of 0.004 M silver nitrate. The growth solution was reduced by the addition of 70 μl of 0.0788 M L-ascorbic acid. Seed solution (12 μl) was added to the growth solution and continuously stirred for four hours to allow for the generation of the gold nanorods. CTAB-GNRs, with absorbance maxima at different wavelengths between 750-900 nm, were generated by modulating the silver nitrate concentration in the growth solution.
Polymer Synthesis
Polymer synthesis was carried out as described previously (Barua et al. 2009). Briefly 1,4-cyclohexanedimethanol diglycidyl ether (1,4C) and 1,4-bis(3-aminopropyl) piperazine (1,4Bis), neopentylglycol diglycidyl ether (NPDGE) and diethylenetriamine (DT), and 1 4 butanediol diglycidyl ether (1,4BDGE) and 1,4Bis were reacted in equimolar amounts at room temperature in order to generate 1,4C-1,4Bis, NPGDE-DT, and 1,4BDGE-1,4Bis cationic polymers, respectively. The polymerization reaction was carried out in 20 ml glass scintillation vials for 16 hours. Following the reaction, polymers were dissolved at a concentration of 10 mg/mL in phosphate-buffered saline (0.01 X PBS) and the solution pH was adjusted to 7.4 using 30% hydrochloric acid in deionized (DI) water in order to compensate for the basicity of the cationic polymer. The extent of polymerization was determined by comparing reactive amine concentrations at initial mixing of monomer reagents (time – 0h) and after 16 hours polymerization using the ninhydrin assay as described previously (Barua et al. 2009; Kasman et al. 2009).
Polymer Molecular Weight Determination
Molecular weights (MWs) of polymers were measured by gel permeation chromatography (GPC) system (Waters 1515) with a refractive index detector (Waters 2414) with ultrahydrogel column (Waters linear) at a flow rate of 1 ml/min at 35°C. Water containing 0.1% trifluoroacetic acid and 40% acetonitrile was used as a mobile phase. Poly (2-vinylpyridine) standards (MW: 3000; 7,000; 12,000; 35,000; 70,000) were used to calibrate the GPC instrument for molecular weight determination.
Generation of Poly(amino ether)-functionalized Gold Nanorod (PAE-GNR) Assemblies
Dispersions of GNRs with an optical density of 0.5 in 1.5 mL microcentrifuge tubes were centrifuged at 6000 rcf for 10 minutes using a Microfuge 18 centrifuge (Beckman Coulter) in order to remove excess CTAB surfactant. The supernatant was removed and the GNRs were redispersed in 100 μL of a poly(styrene sulfonate) (PSS) solution (10 mg/mL in 0.01XPBS; ∼1.5 mM salt concentration). This dispersion was immediately sonicated for 30 minutes to allow for the formation of PSS-coated GNRs (PSS-CTAB-GNRs). Excess PSS was removed by centrifugation at 6000 rcf for 10 minutes. PSS-CTAB-GNRs were then redispersed in 300 μL of nanopure water and 200 μL of different poly (amino ethers) (10 mg/mL in 0.01XPBS) were added to these dispersions which were immediately sonicated for 30 min to allow for the formation of the PAE-coated-PSS-CTAB-GNRs or PAE-GNRs. The PAE-GNRs were finally resuspended in serum-free media in order to monitor their stability and for use in in vitro experiments.
Determination of PAE-GNR Stability in Media
PAE-GNRs were prepared as described above and dispersed in serum-free media (SFM). Absorption spectra from 400-999 nm were determined at different times using a temperature-controlled plate reader (BioTek Synergy 2) for up to 48 hours.
Generation and Characterization of Plasmid DNA-loaded PAE-GNR Assemblies
Plasmid DNA
The pGL3 control vector (Promega Corp., Madison, WI), which encodes for the modified firefly luciferase protein under the control of an SV40 promoter, was used for transgene expression studies. E.coli (XL1 Blue) cells containing the pGL3 plasmid DNA were cultured overnight (16 h, 37 °C, 150 rpm) in 15 mL tubes (Fisher) in 5 mL of Terrific Broth (MP Biomedicals, LLC). The cultures were then centrifuged at 5400g and 4°C for 10 min. Plasmid DNA was purified according to the QIAprep Miniprep Kit (Qiagen) protocol and DNA concentration and purity were determined based on absorbance at 260 and 280 nm, determined using a NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies). Plasmid DNA concentrations of 200-300 ng/μL were typically obtained and volumes were adjusted in order to load between 10-200 ng of plasmid DNA on PAE-GNRs prior to transfections.
Plasmid DNA Loading on PAE-GNRs
PAE-GNRs (optical density 0.25 a.u.) were incubated with different amounts (10-200 ng) of pGL3 plasmid DNA in the presence of serum-free media for 30 min leading to the formation of PAE-GNR-pGL3 assemblies. These were centrifuged at 6000 rcf for 10 min, redispersed in serum-free media, and monitored for colloidal stability using the GNR near infrared (longitudinal) absorption peak for up to 6 h (duration of transfection experiments described below) as previously described. The amount of plasmid DNA remaining in the supernatant after centrifugation was determined using ethidium bromide, a DNA intercalating dye. Ethidium Bromide (1 μg of 0.5 mg/ml; Sigma-Aldrich) was added to each sample. Solutions were transferred to a black 96 well plate and fluorescence was measured with excitation at 320 nm and emission at 600 nm using a plate reader (BioTek Synergy 2), similar to methods previously described (Boger et al. 2001; Rege et al. 2004; Rege et al. 2005). Known plasmid DNA amounts in solution were used as standards for calibration of the assay.
Determination of Zeta Potential of PAE-GNR Assemblies
Stable PAE-GNR dispersions in SFM were prepared as described above and set to an optical density of 0.25 and co-incubated with 0, 50, or 100 ng of pGL3 plasmid DNA. Dispersions were transferred into folded capillary zeta cells (Malvern). Zeta potential values were determined using a dynamic light scattering machine (Malvern).
PAE-GNR Mediated Transgene Expression in Human Cancer Cell Lines
Cell Culture
The PC3 human prostate cancer cell line was obtained from the American Type Culture Collection (ATCC, VA). PC3-PSMA human prostate cancer cells (Gong et al. 1999), were a generous gift from Dr. Michel Sadelain of the Memorial Sloan Cancer Center, New York, NY and were used as received. RPMI 1640 with L-glutamine and HEPES (RPMI-1640 medium), Pen-Strep solution: 10000 units/mL penicillin and 10000 μg/mL streptomycin in 0.85% NaCl, and fetal bovine serum (FBS) were purchased from Hyclone. Serum-Free medium (SFM) consists of RPMI-1640 medium plus 1% Pen-Strep (1000 units/mL penicillin and 1000 μg/mL). Serum containing medium (SCM) consists of SFM plus 10% FBS. Cells were cultured in a 5% CO2 incubator at 37°C using RPMI-1640 medium containing 10% heat-inactivated FBS and 1% antibiotics (Pen-Strep).
PAE-GNR Cytotoxicity
Stable PAE-GNR dispersions in SFM were prepared as described above and set to an optical density of 0.25. PC3 or PC3-PSMA human prostate cancer cells were seeded in 24 well plates at a density of 50,000 cells/well and allowed to attach overnight in a 37°C, 5% CO2 incubator. Volumes ranging from 5 μL to 100 μL of the PAE-GNRs were added to each well and the final well volume was brought up to 500 μL with serum-free media, such that the final GNR O.D. in each well ranged from 0.0025 to 0.125 A.U, in order to carry out a dose-response study. The cells were then incubated for 6 hours after which the SFM was replaced with SCM. After incubation, cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit (ATCC CA# 30-1010k). This assay involves the enzymatic conversion of the MTT substrate to purple-colored formazan in metabolically active cells. This activity is widely employed as an indicator of cell viability and proliferation (Hayon et al. 2003); loss of metabolic activity was used as an indirect indicator of loss of cell viability upon PAE-GNR treatment. Following addition of the MTT reagent (2 h at 37 °C), cells were treated with a lysis buffer from the kit and kept at room temperature in the dark for 2 h in order to lyse cells and solubilize the MTT product. The absorbance of each well was measured using a plate reader (BioTek Synergy 2) at 570 nm to assay for the blue MTT product. For data analysis, absorbance readouts were normalized to the live (untreated) and dead (5 μL of 30% hydrogen peroxide treated) controls.
Transfections
PC3 and PC3-PSMA human prostate cancer cells were cultured as described above. Cells were seeded in 24-well plates (Costar) at a density of 50,000 cells/well and allowed to attach overnight. Sub-toxic amounts of PAE-GNR-pGL3 plasmid assemblies were added to each well in the presence of serum-free media for 6 h. The media was then replaced with serum-containing media for 48 h following which, cells were permeabilized with 150 μL of cell lysis buffer (Promega, Madison, WI). The luciferase activity in cell lysates was measured using a luciferase assay kit (Promega, Madison, WI) in a plate reader (BioTek Synergy 2). The relative light unit (RLU) readouts determined from the assay were normalized with respect to protein concentration in the respective cell lysates, measured using the Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Transgene expression efficacy was based on luciferase activity in cell lysates, which was expressed as relative light units (RLU) per milligram (mg) (RLU / mg) of protein. Transfection experiments were performed at least in triplicate.
Results and Discussion
We have previously developed a library of cationic polymers and evaluated them for plasmid DNA delivery and transgene expression in cancer cell lines (Barua et al. 2009; Barua and Rege 2010). Interfacing these poly(amino ethers) with gold nanorods can eventually lead to multifunctional nanoassemblies that can be employed in transgene delivery, optical / X-Ray CT imaging, and photothermal treatment to cancer cells. In the current work, we describe the generation of poly(amino ether)-gold nanorod (PAE-GNR) assemblies using a layer-by-layer deposition approach (Huang et al. 2010). Three recently developed cationic polymers, 1,4C-1,4Bis (Mw=5,076 g/mol), 1,4BDGE-1,4Bis (Mw=16,381 g/mol), and NPGDE-DT (Mw=12,146 g/mol) were employed to generate the assemblies in this study. We chose the 1,4C-1,4Bis polymer since it demonstrated significantly higher transgene expression efficacies compared to 25 kDa poly(ethylene imine) when delivered as polymer:plasmid DNA complexes (polyplexes) (Barua et al. 2009; Barua and Rege 2010). A previously untested but related polymer, 1,4BDGE-1,4Bis, was synthesized and investigated, since the amine monomer (1,4 Bis) in this polymer is the same as that in the 1,4C-1,4Bis polymer and the cross-linking diglycidyl ethers are different between the two. A third polymer, NPGDE-DT, was also included for generating PAE-GNR assemblies; the polymer was used as an unrelated control, since the two constituent monomers are not related to the 1,4C-1,4Bis polymer. We investigated the stability, plasmid DNA loading capacity, and zeta potential of these assemblies followed by an evaluation of their cytotoxicity and transgene expression efficacies in prostate cancer cell lines. PEI-25k-GNR assemblies were investigated as controls in this current study, since this polymer is widely used as a standard for polymeric gene delivery. In the following studies, the maximum longitudinal (NIR) optical density (O.D.) for the PAE-modified gold nanorod assemblies is used as an indication of concentration. Using inductively coupled plasma optical emission spectrometry (ICP-OES) we found a linear relationship between O.D. and gold concentration (supplementary figure 1) for gold nanorods with a maximum longitudinal absorption peak at both 750 and 800 nm, indicating that the peak NIR O.D. was an excellent surrogate for gold / GNR concentration.
Stability of PAE-GNRs
Previous results indicated that GNR-based assemblies were less stable in phosphate-buffered saline (PBS) and serum-free medium (SFM) compared to DI water and serum-containing media (Huang et al. 2010). The optical stability of different PAE-GNRs dispersed in serum-free media (SFM, RPMI-1640) was therefore investigated. As seen in Figure 1, minimal loss of the longitudinal peak in the NIR region was observed for all PAE-GNR dispersions following the six-hour incubation period in media, which is particularly promising since cells are transfected over this period of time. Less than 20% loss was observed in the longitudinal peak after incubating PAE-GNRs for 48h, indicating that PAE-GNRs were generally stable in dispersion over this duration; it is possible that a small fraction of PAE-GNR assemblies aggregates and precipitates over the 48 h duration.
Figure 1.
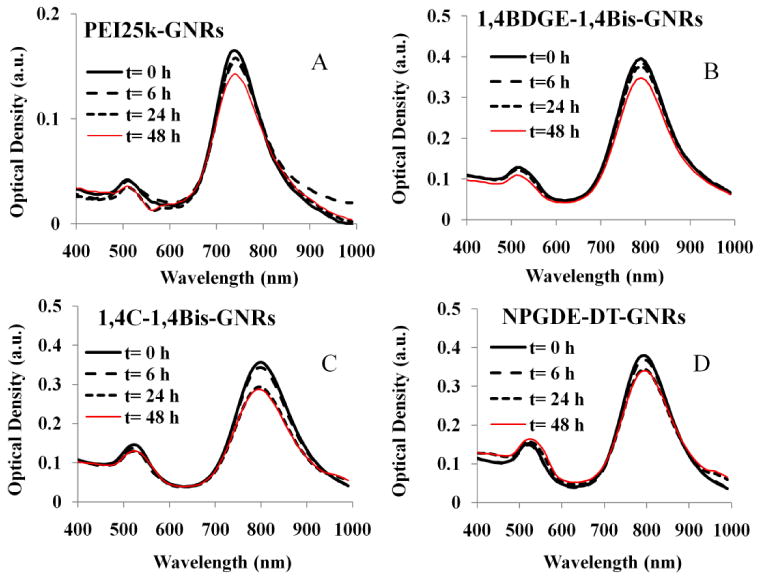
Optical stability of (A) PEI25k-GNRs, (B) 1,4BDGE-1,4Bis-GNRs, (C) 1,4C-1,4BisGNRs (D) NPGDE-DT-GNRs dispersed in serum-free media. Absorbance spectra were monitored from 400 to 999 nm for up to 48 h.
PAE-GNR Assemblies Exhibit Similar or Reduced Cytotoxicities Compared to PEI25k-GNR Assemblies
The cytotoxicities of the different PAE-GNRs were investigated with PC3 and PC3-PSMA human prostate cancer cells; PEI25k-GNR assemblies were used as controls. It was found that 1,4C-1,4Bis-GNRs and 1,4BDGE-1,4Bis-GNRs resulted in lower loss of PC3 cell viability compared to those generated with PEI25k (Figure 2a). NPGDE-DT-GNRs exhibited a greater loss of cell viability compared to PEI25k-GNRs. A similar observation was seen with the PAE-GNRs in PC3-PSMA cells at lower PAE-GNR concentrations. At lower concentrations, 1,4C-1,4Bis-GNRs and 1,4BDGE-1,4Bis-GNRs had similar or less loss of cell viability as compared to PEI25k-GNRs (Figure 2b), indicating some variability based on the cell line under investigation. As was seen in PC3 cells, NPGDE-DT-GNRs exhibited the highest loss of cell viability of the PAE-GNR assemblies that were tested. It is important that some polymers chosen from our library possess similar if not lesser cytotoxicities than PEI25k (Vicennati et al. 2008), since it may be possible to deliver higher amounts of plasmid DNA with higher doses of these PAE-GNRs. For purpose of use in further experiments in this study, a loss of cell viability of 30% was deemed as the upper limit of cytotoxicity that could be tolerated.
Figure 2.

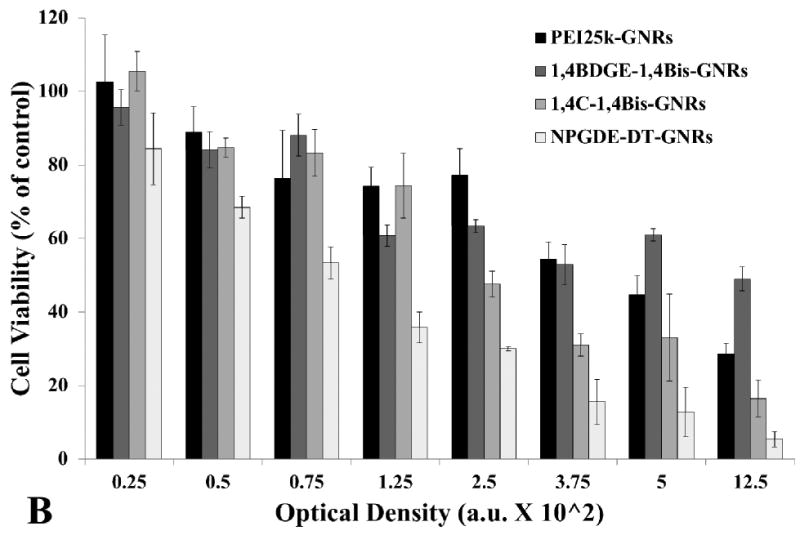
Cytotoxicity of PEI25k-GNRs and PAE-GNRs towards (A) PC3 and (B) PC3-PSMA cells as determined using the MTT assay. Cell viability is reported as percentage of untreated cells (control).
Plasmid DNA Loading onto PAE-GNRs
Cationic polymers have been increasingly used as non-viral vehicles for delivering nucleic acids into a variety of mammalian cells (Kulkarni et al. 2005; Lou et al. 2009). Given their ability to deliver plasmid DNA into cells (Barua et al. 2009; Barua and Rege 2010), we hypothesized that poly(amino ethers) generated in our laboratory would exhibit similar efficacies following functionalization on GNRs. The pGL3 plasmid DNA was incubated with PAE-GNRs for 6 h in order to determine plasmid DNA binding efficacies of the assemblies. Following centrifugation, the supernatant was tested for plasmid DNA content using an ethidium bromide-based fluorescence assay. Ethidium bromide is a DNA intercalating dye which is commonly used as a stain for nucleic acids and to determine DNA content (Geall and Blagbrough 2000). As seen in Table 1, all PAE-GNRs were found to bind and retain greater than or equal to 97% of the initially loaded plasmid DNA for plasmid DNA loadings up to 100 ng. These results indicate that poly(amino ether)-based PAE-GNRs strongly bind plasmid DNA and minimal, if any, losses of loaded plasmid DNA are anticipated for the duration of the transgene delivery experiments with PAE-GNR assemblies. As a result of these high loadings (>97%) in all cases, the amount of pGL3 plasmid DNA loaded on PAE-GNRs was approximated to be the same as that used initially in the loading experiments (i.e. 100% loading was assumed).
Table 1.
Percentage of pGL3 plasmid DNA loading on PEI25k-GNRs and PAE-GNRs for initial DNA amounts of 50 ng and 100 ng. Data are presented as mean values ± one standard deviation.
| Polymer-Modified GNRs | pGL3 Plasmid DNA Loaded (% of initial) |
|
|---|---|---|
| 50 ng | 100 ng | |
| PEI25k-GNRs | 97.0 ± 1.3 | 98.4 ± 0.3 |
| 1,4BDGE-1,4Bis-GNRs | 98.1 ± 1.1 | 99.2 ± 0.9 |
| 1,4C-1,4Bis-GNRs | 99.0 ± 0.8 | 96.9 ± 1.2 |
| NPGDE-DT-GNRs | 100 ± 0.7 | 98.3 ±1.8 |
1,4C-1,4Bis-GNRs and 1,4BDGE-1,4Bis-GNRsPAE-GNRs, loaded with different amounts of plasmid DNA, were dispersed in SFM and the absorption spectra of the assemblies were monitored for 6 h in order to determine their colloidal stability over the period that they are incubated with cells for transgene delivery. Minimal changes were observed in the longitudinal peak of PAE-GNRs. In the cases of 1,4C-1,4Bis-GNRs and 1,4BDGE-1,4Bis-GNRs after loading of 50 ng (Figure 3c and b respectively) and 100 ng plasmid DNA (Supplementary figure 2c and b respectively), the longitudinal peak was slightly higher by approximately 0.01 A.U. at 6 h compared to 0 h. This increase in the longitudinal peak is likely within limits of experimental error and may be explained by some evaporation of the media during experimental conditions. In contrast, the maximal longitudinal absorption peak decreased and the spectrum broadened in case of PEI25k-GNRs and NPGDE-DT-GNRs upon incubation for six hours after loading 50 ng (Figure 3a and d respectively) and 100 ng (Supplementary figure 2a and d respectively) of plasmid DNA. This is most likely due to aggregation of these assemblies following plasmid DNA loading, which leads to a reduction in the stabilizing cationic charge due to the surface PAE layer. Despite these modest changes in the absorption spectrum following addition of plasmid DNA, the stability is sufficient for these assemblies to be useful for transgene delivery.
Figure 3.
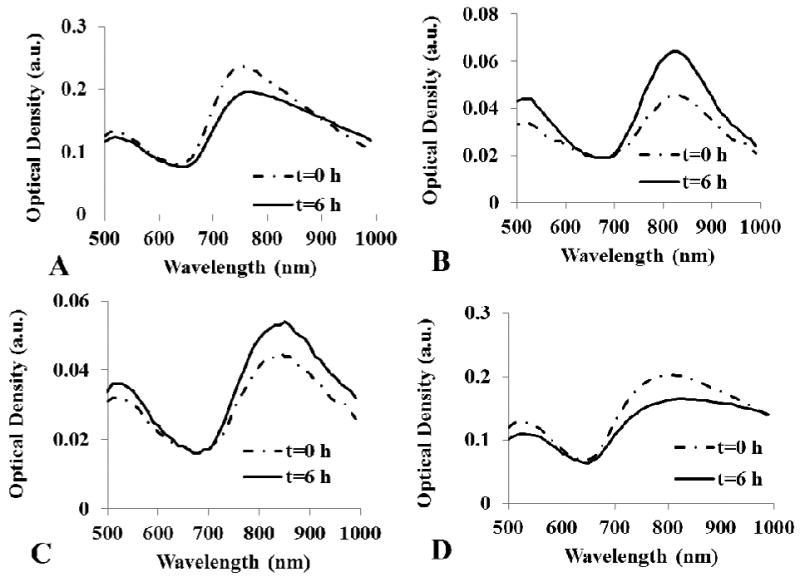
Optical stability of plasmid DNA-loaded PAE-GNR assemblies. pGL3 plasmid DNA (50 ng) were loaded on (a) PEI25k-GNR (b) 1,4BDGE-1,4Bis-GNR, (c) 1,4C-1,4Bis-GNR, and (d) NPGDE-DT-GNR assemblies. Stability data with 100 ng pGL3 plasmid DNA loading are shown in Supplementary Figure 2.
Zeta potentials of PEI25k-GNRs and PAE-GNR assemblies with and without plasmid DNA were determined (Figure 4) in order to further investigate their stability. In the absence of plasmid DNA, all PAE-GNRs possessed a positive zeta potential greater than 10 mV, with 1,4C-1,4Bis-GNR assemblies demonstrating the highest value of approximately 17 mV. The positive zeta potential value indicates that the cationic polymer coating is in fact on the outermost layer of the PAE-GNRs. Addition of 50 ng plasmid DNA resulted in a reduction of zeta potential of all assemblies except those based on PEI25k-GNRs. This decrease can be explained by the shielding of the initial positively charged coating polymer by negatively charged DNA. Further addition of plasmid DNA (100 ng) caused a charge reversal in all PAE-GNRs, except for those based on the 1,4C-1,4Bis-polymer which decreased but remained positive. This observation suggests that increased coverage of the PAE-GNR surface with plasmid DNA leads to a charge reversal of these assemblies. As shown previously, 1,4C-1,4Bis-GNR assemblies bind and retain over 98% of plasmid DNA. Although we did not test this further, the lack of charge reversal most likely indicates that higher amounts of plasmid DNA can be loaded onto this PAE-GNR. However, it is desirable to have the PAE-GNRs retain a net positive charge after addition of plasmid DNA, since excess positive charge can facilitate interactions with negatively charged cell surfaces, leading to efficacious cellular uptake of the PAE-GNR-pGL3 assemblies and expression of the delivered transgene.
Figure 4.
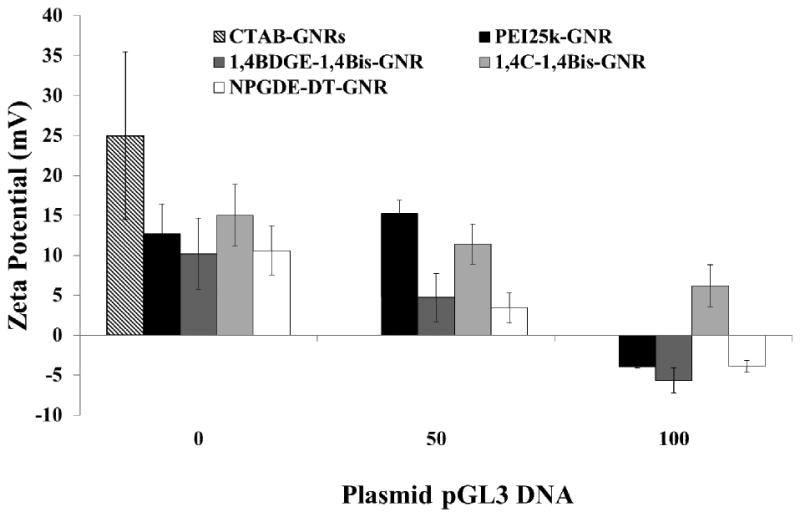
Zeta potential measurements of PAE-GNRs and PEI25k-GNRs at an optical density of 0.0025 a.u. with and without pGL3 plasmid DNA. As the loaded plasmid DNA amount increases zeta potential decreases due to the anionic nature of DNA.
PAE-GNR Mediated Delivery of Plasmid DNA to Prostate Cancer Cells in vitro
PC3 and PC3-PSMA human prostate cancer cells were treated with sub-toxic concentrations of PAE-GNRs and PEI25k-GNRs loaded with 10-200 ng of pGL3 plasmid DNA, in order to determine the efficacy of different PAE-GNRs for transgene expression. The final GNR O.D. was maintained at 0.0025 in each well since PAE-GNRs were not toxic to cells under these conditions. This was carried out to ensure that only plasmid DNA amounts changed, while maintaining the same amount of GNRs across these experiments.
A dose-dependent trend was observed for luciferase expression (absolute RLU/mg values) in both PC3 (Figure 5a) and PC3-PSMA cells (Figure 5b). Luciferase expression was found to be maximal typically between 40 ng and 60 ng of loaded pGL3 DNA, after which, it declined slightly and leveled off to a lower value. This observed trend is likely due to an optimized condition in which both, the amount of plasmid DNA, as well as net positive surface charge of the PAE-GNRs allow for sufficient cellular interaction and uptake for transgene expression. This is supported by the previously described observation; at a loading of 50 ng pGL3 plasmid, all PAE-GNRs still exhibited a positive zeta potential. The decline in luciferase expression with pGL3 plasmid DNA loadings greater than 60 ng correlates with zeta potential data, which indicated reduced but positive zeta potential for 1,4C-1,4Bis-GNRs and negative zeta potential values for PEI25k-GNRs, 1,4BDGE-1,4Bis-GNRs and NPGDE-DT-GNRs (e.g. at 100 ng plasmid DNA in Figure 4). This can result in repulsion of PAE-GNR-pGL3 assemblies from negatively charged cell surfaces leading to reduced uptake and lower levels of transgene expression. It is likely that further increase in the amount of plasmid DNA on PAE-GNRs may completely shield the positive charges present on the outermost layer of the PAE-GNRs resulting in further decline of transgene expression. Although PAE-GNRs are able to bind significant amounts of plasmid DNA, increasing loading results in decrease in the zeta potential of these nanoassemblies, which reduces their efficacies for transgene expression.
Figure 5.
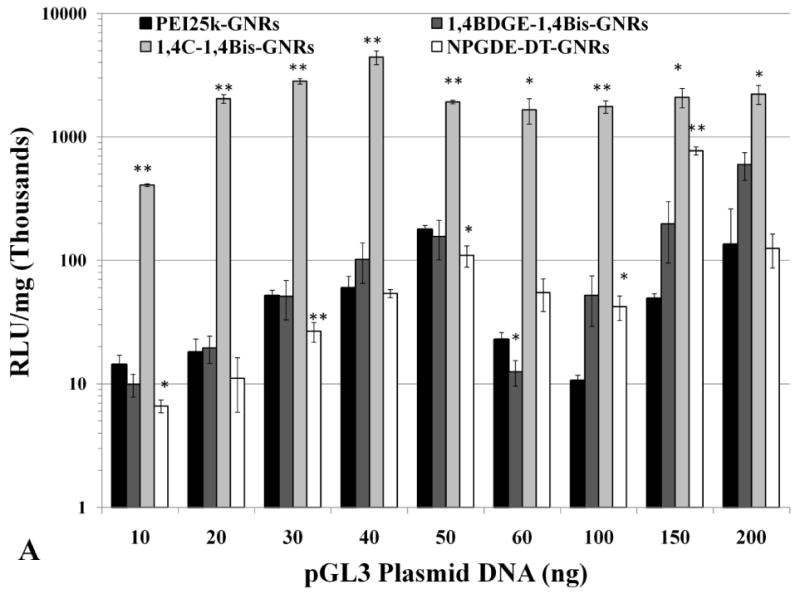
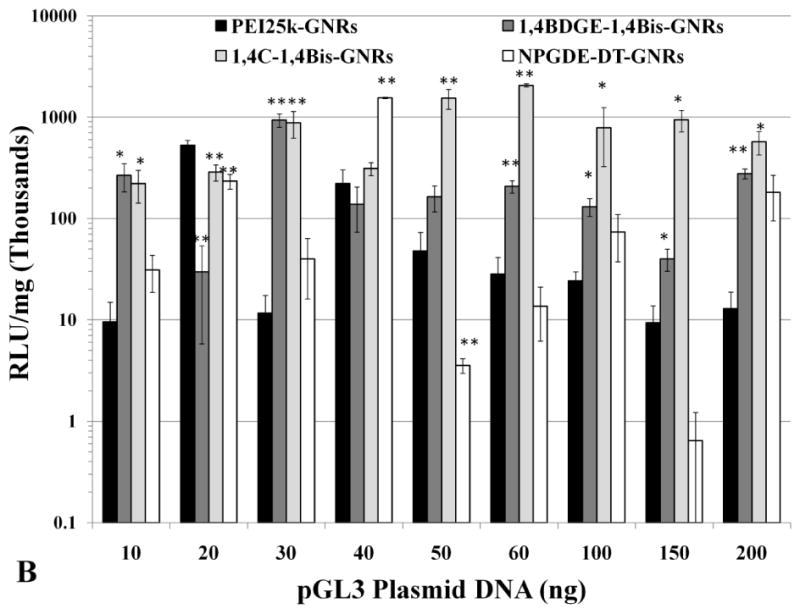
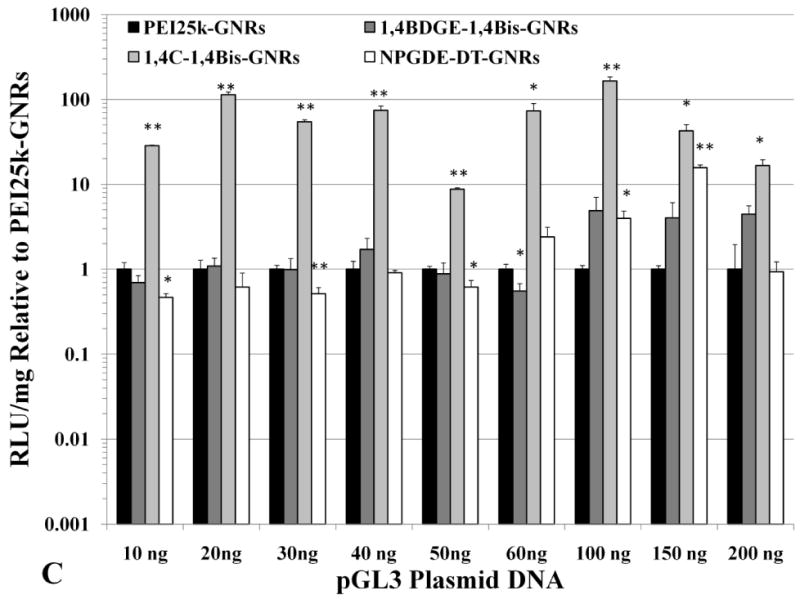
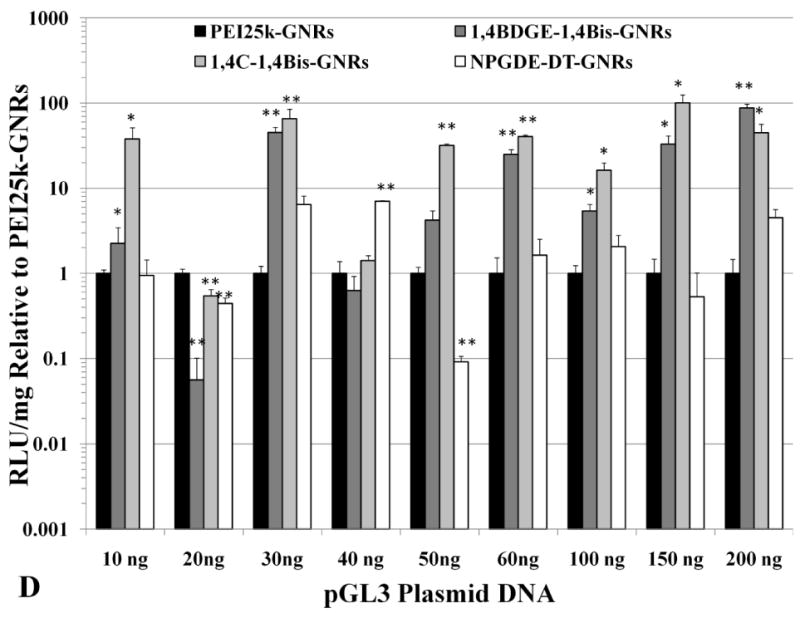
Absolute values of transgene (luciferase) expression in (A) PC3 and (B) PC3-PSMA cells with PEI25k-GNRs and PAE-GNRs at an optical density of 0.0025 a.u. loaded with different amounts of pGL3 plasmid DNA (ng). Transgene (luciferase) expression in (C) PC3 and (D) PC3-PSMA reported relative to PEI25k-GNRs (* p<0.05; ** p<0.01; Student t-test). Luciferase expression, in relative luminescence units (RLU) was analyzed 48 h post transfection and normalized to total protein content (mg) resulting in RLU /mg values.
It was observed that 1,4C-1,4Bis-GNR-pGL3 assemblies exhibited up to 165-fold enhanced luciferase expression in PC3 cells (Figure 5c) and up to 100-fold enhanced luciferase expression in PC3-PSMA cells (Figure 5d) compared to those based on PEI25k-GNRs. The highest enhancements compared to 25kDa pEI were observed for plasmid DNA loadings of 100 ng and 150 ng for PC3 and PC3-PSMA cells, respectively. These statistically significant levels of enhancement in luciferase expression can be partially attributed to the zeta potentials of the respective PAE-GNRs. For example, in case of PC3 cells, 1,4C-1,4Bis-GNR-plasmid DNA assemblies retain a net positive charge compared to those for PEI25k, which exhibit a charge reversal (Figure 4). Furthermore, these findings are consistent with previous observations that transgene expression with 1,4C-1,4Bis-pGL3 polyplexes (polymer-plasmid DNA complexes) was higher than that observed with PEI25k (Barua et al. 2009; Barua and Rege 2010). These results are significant since polymers which demonstrate high efficacies for transgene expression as polyplexes can be interfaced with gold nanorods without compromise of efficacy. Other PAE-GNRs exhibited similar or statistically significant higher levels of luciferase expression compared to PEI25k-GNRs in both, PC3 and PC3-PSMA cell lines, indicating that polymer candidates from our library result in significantly higher or at least comparable efficacies to that seen with PEI25k.
Higher doses of plasmid DNA-loaded 1,4C-1,4Bis-GNR and PEI25k-GNR assemblies were employed in order to investigate if higher levels of luciferase expression could be achieved by increasing the dose of plasmid DNA-loaded PAE-GNRs delivered. Doses of up to 0.01 a.u. PAE-GNR optical densities and those that exhibited less than 30% loss of cell viability in both, PC3 and PC3-PSMA cells, were employed. As previously mentioned, the linear relationship between maximum longitudinal O.D. and gold concentration (supplementary figure 1) facilitates the use of O.D. as a surrogate for GNR concentration under these conditions. A ratio of plasmid DNA to PAE-GNR of 50 ng to 0.0025 a.u. (GNR optical density in absorbance units) was chosen based on the optimal pGL3 plasmid range between 40ng to 60 ng described previously. The 1,4C-1,4Bis-GNR and PEI25k-GNR concentration and plasmid DNA loaded used were both doubled to 0.005 a.u. and 100 ng, respectively and quadrupled to 0.01 a.u. and 200 ng plasmid DNA, respectively in order to maintain the same optimal PAE-GNR to DNA ratio, while simultaneously increasing the amounts of plasmid DNA as well as PAE-GNRs. It was observed that increased PAE-GNR concentrations and pGL3 plasmid amount did not result in increased luciferase expression for cells treated with 1,4C-1,4Bis-GNRs. However, in case of PEI25k-GNR-pGL3 assemblies, luciferase expression rose up by approximately 12- and 18-fold in PC3 (Figure 6a) and PC3-PSMA (Figure 6c) cells, respectively compared to PEI25k-GNR-pGL3 assemblies with 50 ng plasmid DNA and O.D. of 0.0025 a.u. As a result, the expression of 1,4C-1,4Bis-GNRs relative to PEI25k-GNRs showed lower values (Figure 6b and d). It is likely that transgene expression with PAE-GNRs based on the 1,4C-1,4Bis polymer is already at a maximum at the previously used lower concentrations, and therefore, higher plasmid DNA amounts do not enhance this efficacy. Although higher concentrations of PEI25k-GNRs, resulted in increased transgene expression, lower amounts of plasmid DNA were required in case of 1,4C-1,4Bis-GNR assemblies compared to PEI25k-GNR assemblies in order to obtain similar levels of transgene expression.
Figure 6.
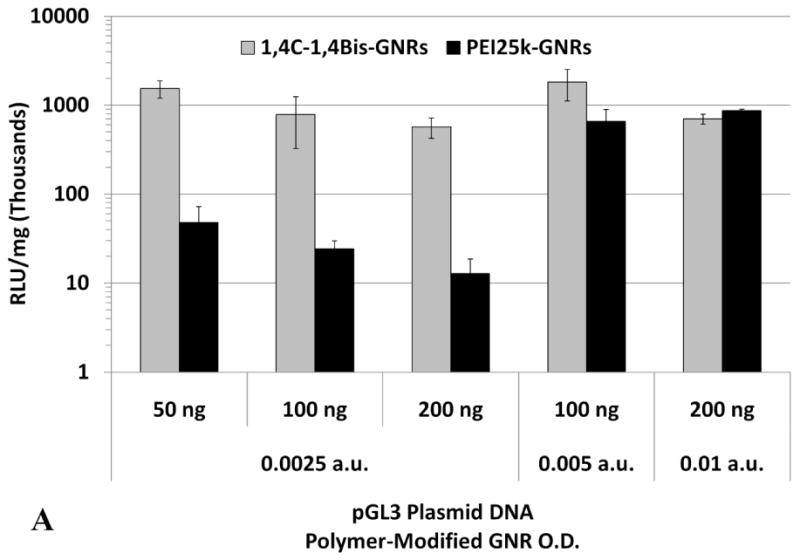
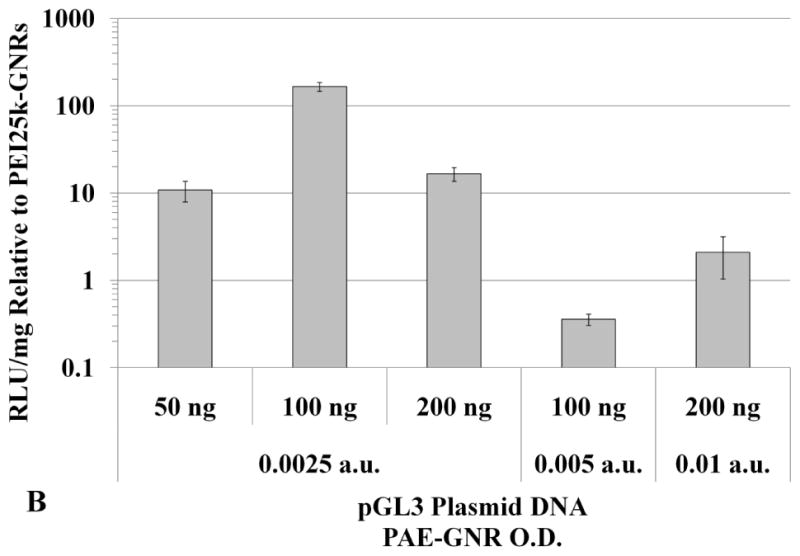
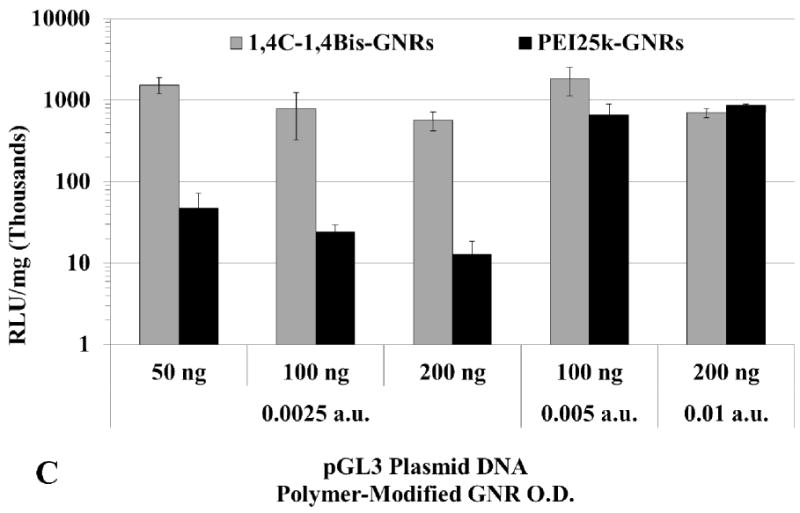
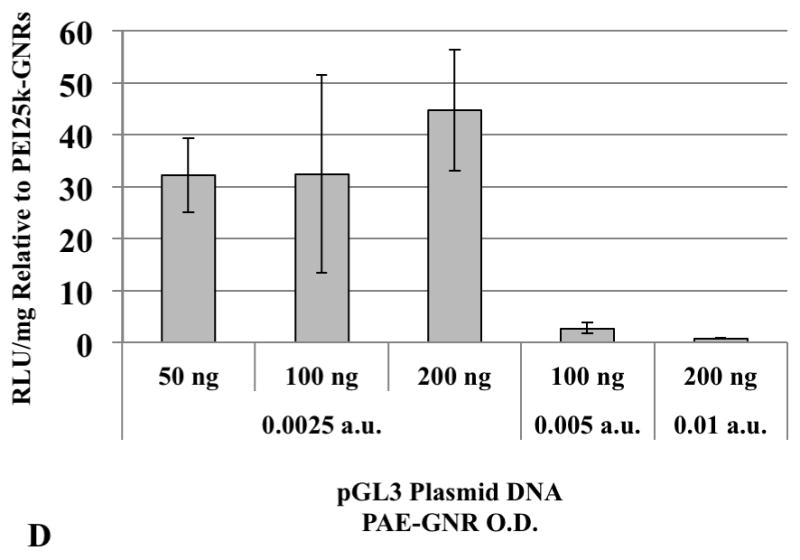
Transgene expression with higher concentrations of PAE-GNRs used for transfection. Luciferase expression values for 1,4C-1,4Bis-GNRs are reported as absolute values for (A) PC3 and (C) PC3-PSMA human prostate cancer cells and relative to PEI25k-GNRs for (B) PC3 and (D) PC3-PSMA cells. Luciferase expression, in relative luminescence units (RLU) was analyzed 48 h post transfection and normalized to protein content (RLU /mg).
Plasmid DNA (50 ng pGL3)-loaded 1,4C-1,4Bis-GNRs were evaluated for efficacy of transgene expression in serum-containing media. It was found that the absolute RLU/mg expression values in SCM decreased by approximately 10-fold of that observed in PC3 cells (Figure 7a) and by approximately 3-fold in PC3-PSMA cells (Figure 7b) in the absence of serum. A drop in transgene expression can be expected since interactions of these assemblies with serum proteins can result in lower efficacies (Huang et al. 2009a). However, the observed levels transgene expression in presence of serum indicate that PAE-GNRs may be viable candidates for further investigations for combined imaging and transgene delivery in vivo.
Figure 7.
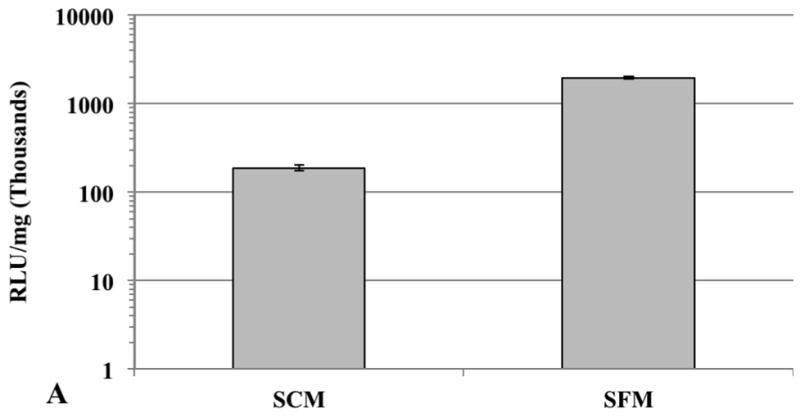
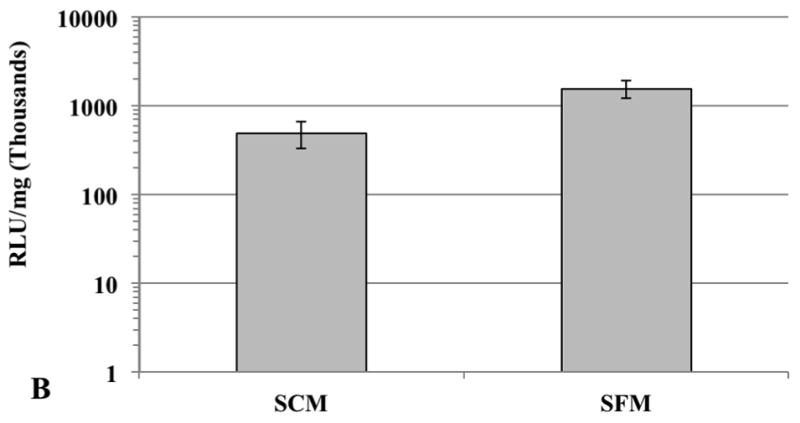
Comparison of 1,4C-1,4Bis-GNR mediated luciferase expression in (A) PC3 and (B) PC3-PSMA cells in serum-containing media (SCM) and serum-free media (SFM). The optical density of gold was set at 0.0025 and PAE-GNRs loaded with 50 ng pGL3 DNA were employed in all experiments. Values are reported in relative luminescence units (RLU) 48 h post transfection and normalized to protein content (RLU /mg).
Conclusions
We have synthesized PAE-GNRs using select candidates from a cationic polymer library recently synthesized in our laboratory. These PAE-GNRs are stable in media and demonstrate the capacity to bind to plasmid DNA. Subtoxic concentrations of PAE-GNRs demonstrated the ability to mediate transgene delivery and expression in two prostate cancer cell lines. PAE-GNRs based on one of our cationic polymers, the 1,4C-1,4Bis polymer, demonstrated higher transgene expression efficacy and lower cytotoxicity when compared to PEI25k-GNR assemblies. Our results indicate that engineering PAE-GNRs leads to a gold-based nanoassembly that combines high stabilities, low cytotoxicities, and the ability to deliver transgenes to cancer cells. These PAE-GNRs also have the potential to be used for photothermal ablation, photothermally enhanced delivery, optical imaging, further surface functionalization, and for carrying other anionic (bio)molecules for delivery to cells, making them a powerful theranostic platform.
Supplementary Material
Supplementary Figure 1. Linear relationship between GNR optical density and concentration determined by ICP-OES
Supplementary Figure 2. Optical stability of plasmid DNA-loaded PAE-GNR assemblies. pGL3 plasmid DNA (50 ng) were loaded on (a) PEI25k-GNR (b) 1,4BDGE-1,4Bis-GNR, (c) 1,4C-1,4Bis-GNR, and (d) NPGDE-DT-GNR assemblies.
Acknowledgments
The authors gratefully acknowledge financial support of the National Science Foundation (Grant CBET-082198). The authors thank Dr. Thrimoorthy Potta for help with the GPC experiments for molecular weight determination, Huang-Chiao Huang for help with the ICP-OES analyses, and Dr. Yanqing Tian, Dr. Roger Johnson, and Professor Deirdre Meldrum, Center for Biosignatures Discovery Automation (CBDA), Biodesign Institute, Arizona State University for access to the GPC instrument.
References
- Barua S, Joshi A, Banerjee A, Matthews D, Sharfstein ST, Cramer SM, Kane RS, Rege K. Parallel synthesis and screening of polymers for nonviral gene delivery. Mol Pharm. 2009;6(1):86–97. doi: 10.1021/mp800151j. [DOI] [PubMed] [Google Scholar]
- Barua S, Rege K. The influence of mediators of intracellular trafficking on transgene expression efficacy of polymer-plasmid DNA complexes. Biomaterials. 2010;31(22):5894–902. doi: 10.1016/j.biomaterials.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Boger DL, Fink BE, Brunette SR, Tse WC, Hedtick MR. A Simple, High-Resolution Method for Establishing DNA Binding Affinity and Sequence Selectivity. Journal of the American Chemical Society. 2001;123:5878–5891. doi: 10.1021/ja010041a. [DOI] [PubMed] [Google Scholar]
- Bonoiu AC, Mahajan SD, Ding H, Roy I, Yong KT, Kumar R, Hu R, Bergey EJ, Schwartz SA, Prasad PN. Nanotechnology approach for drug addiction therapy: Gene silencing using delivery of gold nanorod-siRNA nanoplex in dopaminergic neurons. Proc Natl Acad Sci USA. 2009;106(14):5546–5550. doi: 10.1073/pnas.0901715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun GB, Pallaoro A, Wu G, Missirlis D, Zasadzinski JA, Tirrell M, Reich NO. Laser-Activated Gene Silencing via Gold Nanoshell-siRNA Conjugates. 3(7):2007–2015. doi: 10.1021/nn900469q. [DOI] [PubMed] [Google Scholar]
- Castellana E, Gamez R, Russell D. Label-free biosensing with lipid funcionalized gold nanorods. J Am Chem Soc. 2011;133(12):4182–4185. doi: 10.1021/ja109936h. [DOI] [PubMed] [Google Scholar]
- Chen C, Lin Y, Wang C, Tzeng H, Wu C, Chen Y, Chen C, Chen L, Wu Y. DNA-Gold Nanorod Conjugates for Remote Control of Localized Gene Expression by near Infrared Irradiation. J Am Chem Soc. 2006;128(11):3709–3715. doi: 10.1021/ja0570180. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan P, Varambally S, Tomlins SA, Ray S, Huang SM, Chinnaiyan AM, Harari PM. Enhancing the antitumor activity of ErbB blockade with histone deacetylase (HDAC) inhibition. International Journal of Cancer. 2006;118(4):1041–1050. doi: 10.1002/ijc.21465. [DOI] [PubMed] [Google Scholar]
- Cho K, Wang X, Nie S, Chen Z, Shin D. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- Choi WI, Kim JY, Kang C, Byeon CC, Kim YH, Tae G. Tumor Regression In Vivo by Photothermal Therapy Based on Gold-Nanorod-Loaded, Functional Nanocarriers. ACS Nano. 2011;5(3):1995–2003. doi: 10.1021/nn103047r. [DOI] [PubMed] [Google Scholar]
- Ding H, Yong KT, Roy I, Pudavar HE, Law WC, Bergey EJ, Prasad PN. Gold Nanorods Coated with Multilayer Polyelectrolyte as Contrast Agents for Multimodal Imaging. The Journal of Physical Chemistry C. 2007;111(34):12552–12557. [Google Scholar]
- Garnett MC. Gene-delivery systems using cationic polymers. Crit Rev Ther Drug Carrier Syst. 1999;16(2):147–207. [PubMed] [Google Scholar]
- Geall A, Blagbrough I. Rapid and sensitive ethidium bromide fluorescence quenching assay of polyamine conjugate-DNA interaction for the analysis of lipoplex formation in gene therapy. Journal of Pharmaceutical and Biomedical Analysis. 2000;22(5):849–59. doi: 10.1016/s0731-7085(00)00250-8. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Han G, De M, Kyu Kim C, Rotello VM. Gold nanoparticles in delivery aplications. Advanced Drug Delivery Reviews. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nature Reviews Genetics. 2005;6(4):299–U29. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer Patient T Cells Genetically Targeted to Prostate-Specific Membrane Antigen Specifically Lyse Prostate Cancer Cells and Release Cytokines in Response to Prostate-Specific Membrane Antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhou X, Kim D. Facile fabrication of distance-tunable Au-nanorod chips for single nanoparticle plasmonic biosensors. Biosens Bioelectron. 2011;26(5):2246–2251. doi: 10.1016/j.bios.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Ha S, Carson A, Agarwai A, Kotov N, Kim K. Detection and monitoring of the multiple inflammatory responses by photoacoustic molecular imaging using selectively targeted gold nanorods. Biomed Opt Express. 2011;2(3):645–657. doi: 10.1364/BOE.2.000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayon T, Dvilansky A, Shpilberg O, Nathan I. Appraisal of the MTT-based assay as a useful tool for predicting drug chemosensitivity in leukemia Leuk. Lymphoma. 2003;44:1957–1962. doi: 10.1080/1042819031000116607. [DOI] [PubMed] [Google Scholar]
- Heath J, Davis M. Nanotechnology and cancer. Annu Rev Med. 2008;59:251–265. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Barua S, Kay DB, Rege K. Simultaneous Enhancement of Photothermal Stability and Gene Delivery Efficacy of Gold Nanorods using Polyelectrolytes. ACS Nano. 2009a;3(10):2941–2952. doi: 10.1021/nn900947a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155(3):344–57. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Huang HC, Rege K, Heys JJ. Spatiotemporal temperature distribution and cancer cell death in response to extracellular hyperthermia induced by gold nanorods. ACS Nano. 2010;4(5):2892–900. doi: 10.1021/nn901884d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Neretina S, El-Sayed M. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Advanced Biomaterials. 2009b;27:1–31. doi: 10.1002/adma.200802789. [DOI] [PubMed] [Google Scholar]
- Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. Journal of the American Chemical Society. 2006;128(6):2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- Jabr-Milane L, van-Vlerken L, Devalapally H, Shenoy D, Komareddy S, Bhavsar M, Amiji M. Multi-functional nanocarriers for targeted delivery of drugs and genes. J Control Release. 2008;130(2):121–128. doi: 10.1016/j.jconrel.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Kasman LM, Barua S, Lu P, Rege K, Voelkel-Johnson C. Polymer-Enhanced Adenoviral Transduction of CAR-Negative Bladder Cancer Cells. Mol Pharm. 2009;6(5):1612–9. doi: 10.1021/mp9000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RP, Mishra S, Fraser SE, Davis ME. Single Cell Kinetics of Intracellular, Nonviral, Nucleic Acid Delivery Vehicle Acidification and Trafficking. Bioconjugate Chemistry. 2005;16:986–994. doi: 10.1021/bc050081u. [DOI] [PubMed] [Google Scholar]
- Lou YL, Peng YS, Chen BH, Wang LF, W LK. Poly(ethylene imine)-g-chitosan using EX-810 as a spacer for nonviral gene delivery vectors. Journal of Biomedical Materials Research Part A. 2009;88(4):1058–1068. doi: 10.1002/jbm.a.31961. [DOI] [PubMed] [Google Scholar]
- Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chemistry of Materials. 2003;15(10):1957–1962. [Google Scholar]
- Ow Sullivan MM, Green JJ, Przybycien TM. Development of a novel gene delivery scaffold utilizing colloidal gold-polyethylenimine conjugates for DNA condensation. Gene Ther. 2003;10(22):1882–1890. doi: 10.1038/sj.gt.3302083. [DOI] [PubMed] [Google Scholar]
- Pan D, Pramanik M, Senpan A, Wickline S, Wang L, Lanza G. A facile synthesis of novel self-assembled gold nanorods designed for near-infrared imaging. J Nanosci Nanotechnol. 2010;10(12):8118–8123. doi: 10.1166/jnn.2010.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege K, Hu S, Moore JA, Dordick JS, Cramer SM. Chemoenzymatic Synthesis and High-Throughput Screening of an Aminoglycoside-Polyamine Library: Identification of High-Affinity Displacers and DNA-Binding Ligands. Journal of the American Chemical Society. 2004;126:12306–12315. doi: 10.1021/ja049437n. [DOI] [PubMed] [Google Scholar]
- Rege K, Ladiwala A, Hu S, Breneman CM, Dordick JS, Cramer SM. Investigation of DNA-binding properties of an aminoglycoside-polyamine library using quantitative structure-activity relationship (QSAR) models. J Chem Inf Model. 2005;45(6):1854–63. doi: 10.1021/ci050082g. [DOI] [PubMed] [Google Scholar]
- Salem AK, Searson PC, Leong KW. Multifunctional nanorods for gene delivery. Nat Mater. 2003;2(10):668–671. doi: 10.1038/nmat974. [DOI] [PubMed] [Google Scholar]
- Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. Gold nanoparticle-mediated Transfection of mammalian cells. Bioconjugate Chemistry. 2002;13(1):3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- Smith A, Duan H, Mohs A, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60(11):1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(16):9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicennati P, Giuliano A, Ortaggi G, Masotti A. Polyethylenimine In Medicinal Chemistry. Current Medicinal Chemistry. 2008;15:2826–2839. doi: 10.2174/092986708786242778. [DOI] [PubMed] [Google Scholar]
- von Maltzahn G, Park JH, Agrawal A, Bandaru NK, Das SK, Sailor MJ, Bhatia SN. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69(9):3892–900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Ji J, Shen J. Synthesis of Near-Infrared Responsive Gold Nanorod/PNIPAAm Core/Shell Nanohybrids via Surface Initiated ATRP for Smart Drug Delivery. Macromol Rapid Commun. 2008;29:645–650. [Google Scholar]
- Yi Y, Hahm SH, Lee KH. Retroviral gene therapy: Safety issues and possible solutions. Current Gene Therapy. 2005;5(1):25–35. doi: 10.2174/1566523052997514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Linear relationship between GNR optical density and concentration determined by ICP-OES
Supplementary Figure 2. Optical stability of plasmid DNA-loaded PAE-GNR assemblies. pGL3 plasmid DNA (50 ng) were loaded on (a) PEI25k-GNR (b) 1,4BDGE-1,4Bis-GNR, (c) 1,4C-1,4Bis-GNR, and (d) NPGDE-DT-GNR assemblies.


