Abstract
Introduction: Trophoblast cells in vivo form a 3-dimensional structure that promotes complex cell-to-cell interactions that cannot be studied with traditional monolayer culture. We describe a 3-dimensional trophoblast bioreactor to study cellular interactions. Methods: Nonadhesive agarose hydrogels were cast from molds using computer-assisted prototyping. Trophoblast cells were seeded into the gels for 10 days. Morphology, viability, and vesicle behavior were assessed. Results: Trophoblast cells formed uniform spheroids. Serial sectioning on days 3, 7, and 10 revealed central vacuolization with a consistent outer rim 12.3-μ thick. The vesicle configuration has been confirmed with confocal imaging. Electron Microscopic (EM) imaging revealed its ultrastructure. The vesicles migrate across a fibronectin-coated surface and invaded basement membrane. Conclusions: Trophoblast cells cultured in a novel substrate-free 3-dimensional system form trophoblast vesicles. This new cell culture technique allows us to better study placental cell-to-cell interactions with the potential of forming microtissues.
Keywords: trophoblast cell, blastocyst, 3-dimensional culture
Human cellular differentiation begins 5 days postconception in the blastocyst that is composed of inner cell mass (ICM) cells and trophoblast cells. The ICM ultimately forms the embryo.1 The trophoblast cells, the focus of this research, further differentiate into the cells required to attach the embryo to the uterine wall, invade the endometrium and the maternal vasculature,2 and form the maternal-fetal interface and the hormone producing placental cells.3 Surprisingly, little is known about the molecular mechanisms that guide the process of early differentiation.
A wealth of information about placental cell differentiation has been gained from traditional 2-dimensional (2D) monolayer culture. However, traditional 2D cell culture does not allow the study of cell-to-cell interactions. Furthermore, monolayer culture prevents analysis of the gene programs required to form complex cellular structures such as trophoblast vesicles and the placenta. We have recently developed a method to culture trophoblast cells into self-assembled symmetric 3D vesicles using nonadhesive micromold bioreactors.4
Methods
Wax molds were designed in computer-assisted design (Solid Works Corporation, Concord, Massachusetts) and produced with a ThermoJet rapid prototyping machine (3D Systems Corporation, Valencia, California) as described by Dean et al.3 Each mold contained a matrix of 822 cylindrical bottom pegs 200 m in diameter. Agarose gel bioreactors were cast directly from wax molds. Aliquots of 2 g Ultra Pure Agarose (American Bioanalytical, Natick, Massachusetts) were autoclaved as a powder, 200 mL of sterile dH2O was added and the agarose was dissolved by heating and mixing on a hot plate. The solution was cooled and approximately 2.75 mL was pipetted into each wax mold in a sterile dish. After setting, the bioreactors were separated from the mold using a spatula, transferred to a 6-well plate, and equilibrated overnight with 3 mL of RPMI 1640 media containing 10% FBS, 2 mmol/L/mL of l-glutamine, and 100 U/mL of penicillin/streptomycin.
Cell Culture
TCL-1 trophoblast cells (a generous gift from Dr James Padbury) were maintained in a monolayer in culture with medium as described above. The TCL-1 cells are immortalized chorionic trophoblast cells that express markers of both extravillous and villous trophoblast cells.5 Cells were passaged at 90% confluency and discarded after 20 passages. For experiments, cell suspensions of 800 000 cells per 100 L were created and the suspension was added to the rectangular recess of the bioreactor. Spheroids were removed by inverting the bioreactor in the culture well and centrifuging them at 300 RPM for 5 minutes.
Histology
To maintain architecture, the spheroids were fixed in 10% neutral buffered formalin for 10 minutes at room temperature. They were pelleted by centrifugation at 1200 RPM for 5 minutes, suspended in OCT compound (Tissue-Tek, Torrance, California), and frozen at −80°C until processed. The spheroids were cryosectioned at 15 m and collected on poly-l-lysine coated slides (Electron Microscopy Science, Hatfield, Pennsylvania). The slides were stained with hematoxylin and eosin following standard protocol6 and imaged on a Nikon Eclipse 80i microscope. Microscopic photographs were acquired with a Spot Insight 2 CCD (Diagnostic Instruments, Sterling Heights, Michigan). Once the structure was noted with cryostat sections, vesicles were produced, fixed, imbedded in resin, and sectioned at 6 m. These sections were stained with hematoxylin and eosin and visualize the cellular morphology.
Confocal Microscopy
Spheroids were formed for 10 days and then removed from the wells as described. The spheroids were immersed in 10 mol/L 5-bromo-2′-deoxyuridine (BrdU; Sigma, St Louis, Missouri) in medium overnight. Confocal images were acquired with a Nikon C1si confocal (Nikon Inc, Mellville, New York) using diode lasers 402 and 488. Serial optical sections were performed with EZ-C1 computer software (Nikon Inc). Serial z sections were collected at 1.0 µm with a 20× Plan Apo lens and a scan zoom of 1×. Image projections were done in Elements (Nikon Inc) computer software.
Live: Dead Assay
Spheroids were washed 3 times with 1 mL of phosphate buffered-saline (PBS) to remove the culture media. They were then incubated with a solution containing 5 µmol/L of 5-chloromethylfluorescein diacetate (CMFDA) dye and 5 µmol/L of propidium iodide at 37°C for 45 minutes. The cells were then imaged with 640-nm long pass filter.
Transmission Electron Microscopy
The Electron Microscopy Core Research Laboratory at Rhode Island Hospital processed electron microscopy samples. In brief, the spheroids were fixed in glutaraldehyde and sodium cacodylate buffer for 1 hour. The specimens were then dehydrated in a graded series of ethanol and embedded in resin overnight. Thin sections (silver-gold) were mounted on nickel grids and examined with a Phillips 300 electron microscope at 60 kV.
Invasion Assay
Vesicles were allowed to form for 7 days. Matrigel invasion chambers (Becton Dickinson, Franklin Lakes, New Jersey) were prepared as described by the manufacturer. Briefly, the transwells were rehydrated with RPMI for 5 hours at 37°C. Ten discrete vesicles or 5 × 104 cells from a monolayer (control) were suspended in 400 mL of serum-free media and placed in the upper chamber of the transwell; the lower chamber was filled with 600 mL of complete media. After a 48-hour incubation, the matrigel and noninvaded cells were removed from the upper chamber with a cotton swab. The membranes were then removed from the chamber, fixed with methanol at −20°C for 20 minutes and stained with 5% hematoxylin for 10 minutes.
Results
Trophoblast Cells Consistently Form Symmetric Spheroids
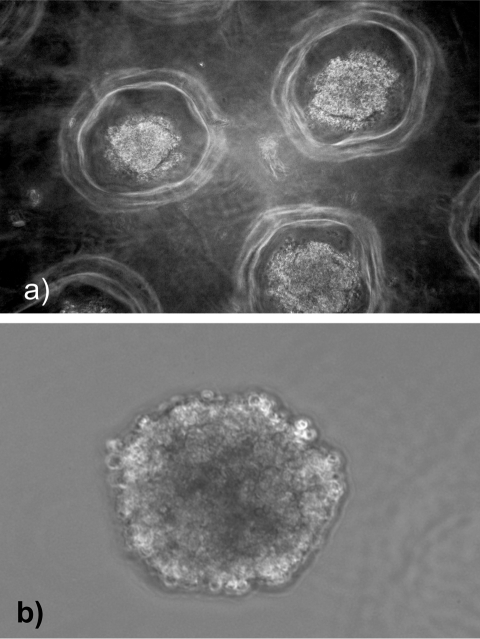
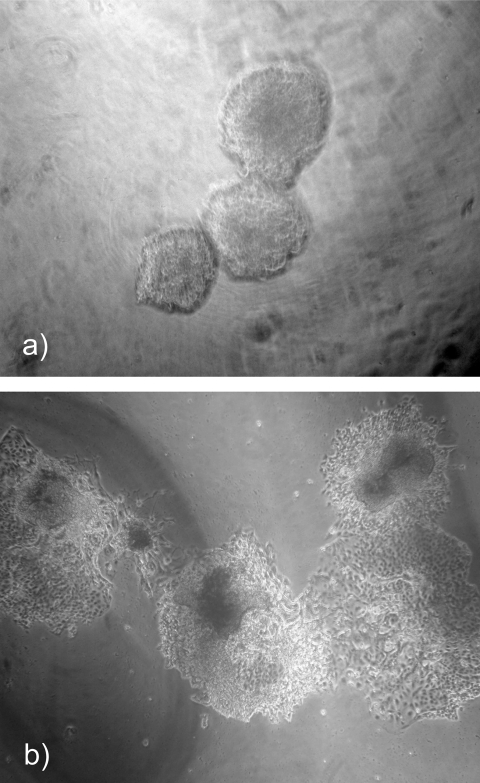
TCL-1-immortalized trophoblast cells were seeded into nonadhesive micromolded bioreactors for 3 to 10 days. At various time points, the micromolds were inverted and the cells were easily removed from the wells. The cells consistently formed spheroids measuring 187 m (±4.6 m) in diameter (Figure 1 ). The spheroid size was established by 72 hours in culture and did not change significantly when cultured for longer periods of time in the bioreactor. Each spheroid contained approximately 100 cells as predicted by the number of recesses and the plating density. Once removed from the bioreactor wells, these spheroids maintained their structure in culture and were easily manipulated.
Figure 1.
Trophoblast cells from speroids. a, Cells cultured in nonadhesive round-bottom agarose hydrogels readily formed spheroids. b, Spheroids removed from the hydrogels remain intact and are manipulatable in culture.
Self-Assembled Vesicles Have Central Vacuolization to Form Trophoblastic Vesicles
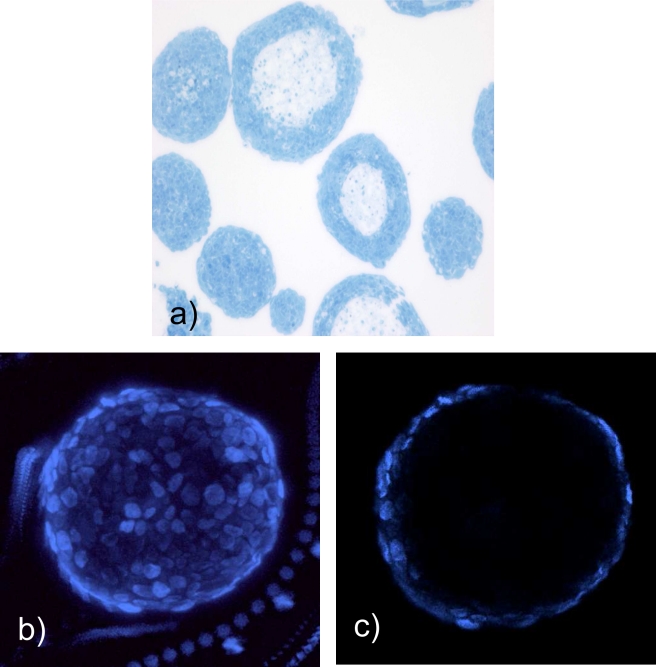
Trophoblast spheres were cultured for 7 to 10 days, collected in pellets, cryosectioned, and stained with hemotoxylin and eosin. Surprisingly, the trophoblast spheroids had acellular centers (Figure 2a), a shape not previously observed by our experience with 3D spheroids using similar bioreactors. The vesicle structure was highly consistent with a cellular rim measuring 12.3 m (±1 m). This morphology was maintained for at least 20 days of culture in the bioreactor. To assure that the vesicle morphology was not an artifact of cryosectioning, intact spheroids were stained with BrdU and imaged with confocal microscopy (Figure 2b and c). These studies confirmed the presence of central vacuolization and consistent rim size.
Figure 2.
Spheroids form vesicle structures. Spheroids were formed in nonadherent bioreactors for 7 to 10 days. They were removed from the bioreactor, fixed, sectioned, and stained with hematoxylin and eosin (H&E; a). The hollow structure is confirmed by confocal microscopy through the edge (b) and center (c) of the spheroid.
Vesicles Remain Viable and Proliferative
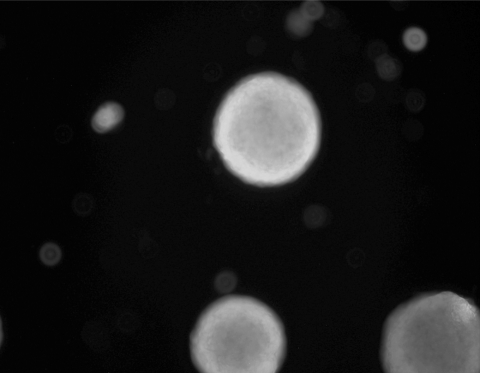
To assess cellular viability, cells were cultured in the bioreactor for 10 days and trophoblastic vesicles were then removed and treated with 5-chloromethylfluorescein diacetate (CMFDA) and propidium iodide or vehicle. Virtually, all the cells were noted to be viable (Figure 3a).
Figure 3.
Vesicles are viable in long-term culture. Vesicles labeled with 5-chloromethylfluorescein diacetate (CMFDA) demonstrate viability for at least 20 days in culture.
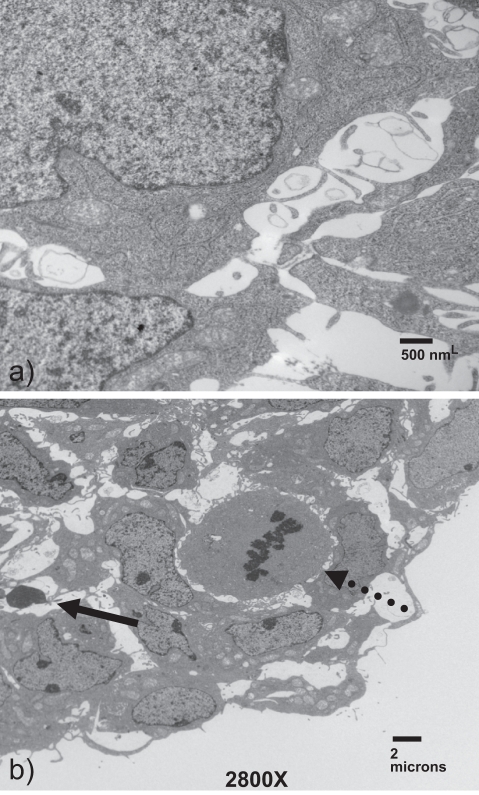
Transmission electron microscopy was performed to evaluate cellular morphology within the vesicles. The cells within the rim are replete with organelles, including mitochondria and endoplasmic reticulum, demonstrating that the cells are viable and metabolically active (Figure 4a). Readily detectable mitotic figures confirm that the cells are continuing to proliferate and interestingly, these mitotic cells (Figure 4b, dashed arrow) are often seen adjacent to apparent apoptotic cells (Figure 4b, yellow arrow). The apoptotic cells may explain how the vesicle maintains its constant size in the presence of actively dividing cells. Clearly, cellular communication is occurring among the vesicle cells.
Figure 4.
Trophoblast vesicles are bioactive and proliferative. Vesicles were formed for 10 days, then fixed and stained. Imaging on transmission electron microscopy illustrated that the cells were replete with organelles suggesting high viability (a). Throughout the vesicle, numerous mitotic figures (dotted arrow) are identified. These can often be seen alongside apparent apoptotic figures (solid arrow).
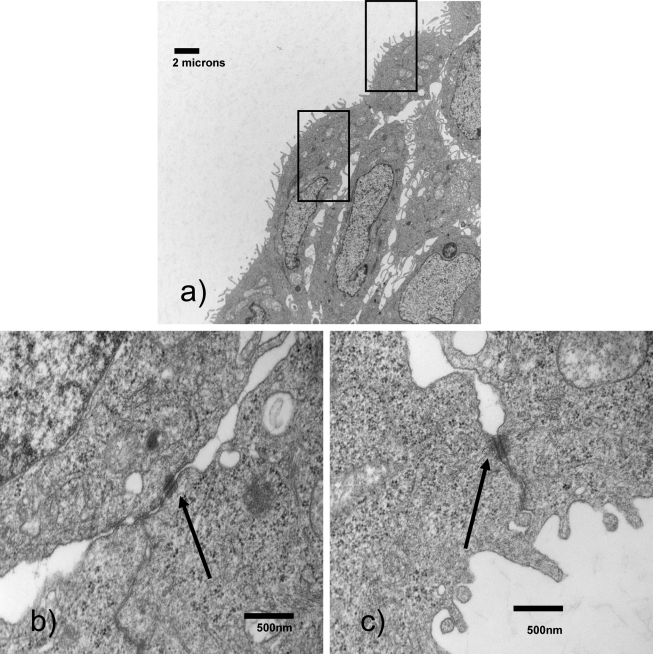
Intercellular Junctions Form Between Cells in Bioengineered Trophoblast Vesicle
High resolution inspection of the trophoblast vesicle demonstrates the presence of numerous intercellular junctions increasing in number across the diameter of the rim (Figure 5 ). These junctions demonstrate the presence of cell-to-cell communication, which is absent in monolayer cell culture. Importantly, the development of junctions is a critical step in trophoblast vesicle formation.
Figure 5.
Cells in the rim of the vesicle form cellular junctions. Cellular junctions can be noted between cells in the vesicle rim.
Vesicles are Highly Adhesive and Invasive
Trophoblast vesicles were cultured for 10 days, removed from the micromold, and placed in 35-mm cell culture dishes coated with 1% agarose. Free-floating vesicles made contact with other vesicles and the vesicles attached to each other demonstrating their highly adherent surfaces (Figure 6a). The vesicles fused and, after several days, formed one large vesicle.
Figure 6.
Trophoblast cell spheroids are highly adherent. Spheroids were formed in the nonadherent bioreactor. After 7 days, the spheroids were removed and placed on (a) a nonadherent agarose surface and noted to rapidly fuse or (b) placed in cell culture-treated dishes, the spheroids bind to the plate and proliferate.
Vesicles were placed on fibronectin-coated cell culture dishes to assess whether they will migrate across the basement membrane. This test has been standardized as an implantation assay for blastocysts.7 Within 1 hour, all vesicles were attached to the plates. The cells flattened out and developed invasive-like microvilli within 24 hours (Figure 6b). These processes covered the cell culture dish by 72 hours after plating.
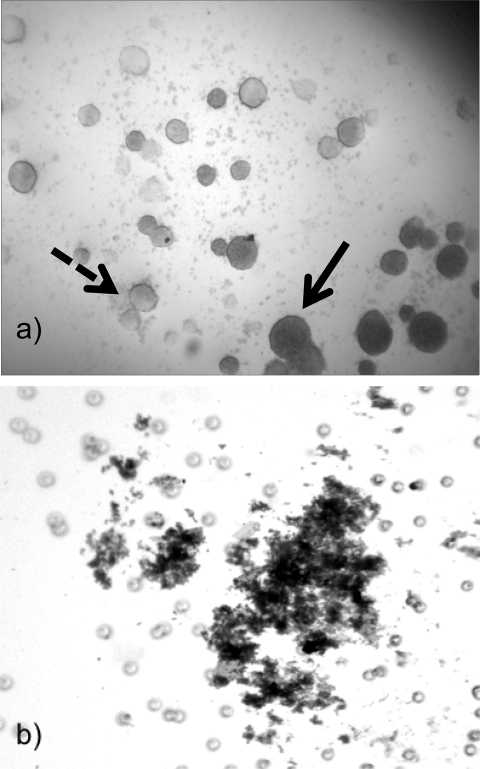
Vesicles were placed on matrigel-coated cell culture dishes to assess their invasive potential. Within 24 hours, stereo microscopy revealed that the cells invaded the matrix (Figure 7a). Cellular invasion was assessed by placing vesicles and cells on matrigel invasion chambers. Cells from the vesicles were identified on the posterior side of the membrane clearly identifying their invasive potential ( Figure 7b). In contrast, because the TCL-1 cells are not invasive in monolayer, no cells from the monolayer culture were noted to invade the matrigel.
Figure 7.
Trophoblast vesicles are invasive. Vesicles for formed in the bioreactor for 7 days. The spheroids were then placed on a matrigel-coated cell culture dish for 36 hours. a, Vesicles can be seen at various positions throughout the matrix. The vesicles in focus (solid arrow) are closest to the objective while the vesicles varying in densities are farther away from the objective (dashed arrow). b, Cells left the vesicle and invaded through the membrane.
Cells Demonstrated Differential Differentiation
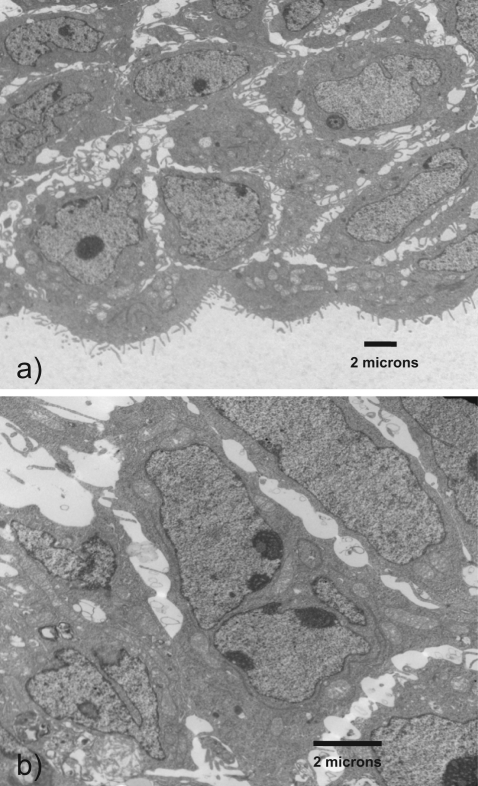
Morphologic differentiation was assessed with electron microscopy. Cells on the outer rim of the vesicle have numerous microvillous projections. In contrast, cells on the inner portion of the rim are larger and do not contain microvilli. Furthermore, several multinucleated cells developed on the inner portion of the rim (Figure 8 ). These observations illustrate the cellular polarity within the rim. They also parallel the differentiation of cells into both extravillous and villous pathways as would be observed in trophoblast vesicles in vivo.
Figure 8.
The vesicular rim contains differentiated cells. Inner cells are noted to form giant cells (a) while the outer cells are cuboidal in appearance and contain numerous microprojections (b).
Discussion
Three-dimensional cell culture can maximize the formation of complex cell-to-cell interactions not seen in traditional monolayer culture and can allow the formation of cellular micro-tissues.8 Under appropriate culture conditions, mono-dispersed cells can self-assemble into a 3D spheroid in a process that appears to mimic natural processes that occur during embryogenesis.9,10 These processes have led to the formation of several different micro-tissue spheroids including bile canniculi11 and beating cardiomyocytes.12,13 LaMarca et al created 3D trophoblast aggregates of the SGHPL-4 cell line and demonstrated that the 3D configuration induced the invasive properties of these cells. This important study illustrated that trophoblast cells cultured in 3D have functional behavior strikingly different from those in monolayer.14
We have demonstrated that trophoblast cells placed into a nonadhesive micromold bioreactor will self-assemble into a vesicular structure. This structure is not readily predicted by the current theory of cellular self-assembly.15 According to this theory, called the differential adhesion hypothesis, monodispersed cells self-assemble via surface adhesion molecules (eg, cadherins) to form spheroids to maximize cell-to-cell adhesion and to minimize surface free energy. The trophoblast cells of this study formed spheroids, but these were hollow structures with a consistent rim size and clear cell-to-cell communication. Thus, our nonadhesive micromolds facilitate not only the cell-to-cell interactions needed to form a 3D microtissue, they also enable the cell-to-cell interactions necessary to form a hollow vesicular structure. This in vitro vesicular structure of trophoblast cells is predicted by the in vivo behavior of these cells. During embryogenesis, trophoblast cells polarize and create a vesicle by pumping fluid into the center of the embryo.16,17 Intercellular tight junctions enable this fluid to organize the trophoblast cells into a vesicle similar to that present in our model.18 This in vivo-like organization of our spheroids is further strengthened by the presence of the cellular junctions noted on electron microscopy. Therefore, these data suggest that the trophoblast vesicles are forming a functional micro-tissue.
The importance of 3D trophoblast cell culture has been described by Korff et al.19 This group created densely packed spheroids from cytotrophoblast cell suspensions placed into 96-well round bottom plates for 24-hours. Their model demonstrates phenotypic and functional differences among cells from first, third, and preeclamptic placenta. This model is not incongruent with our hollow sphere model because the vesicular structure did not form until after 3 days in culture.
A primary role of early first trimester trophoblast cells is to facilitate implantation.20 Initially, the trophoblast cells make contact with the uterus and attach to the uterine epithelium. Attachment and implantation require that the cells differentiate into an adhesive phenotype.21 The TCL-1 cells in monolayer culture are not invasive. However, our trophoblastic vesicles are highly adhesive, easily attach to a cell culture dish, and are migratory. Furthermore, they migrate across a treated cell culture plate and invade across a basement membrane in a manner similar to that seen with mammalian blastocysts in vivo.
The morphologic development of our model also mimics in vivo blastocyst development. Human trophoblast cells differentiate along 1 of 2 distinct pathways to form either syncytiotrophoblast cells or extravillous trophoblast cells.22 The invasive extravillous trophoblast cells, which mediate embryo implantation and placental invasion, are the predominant cell type. The syncytiotrophoblast cells are multinucleated giant cells, formed by cell fusion, responsible for the synthesis of critical growth factors and hormones. There was morphologic evidence of both of these cell types in the trophoblast vesicles formed in vitro.
The development of an anembryonic blastocyst will provide investigators with an ideal model to study the molecular mechanisms involved in human trophoblast differentiation, blastocyst formation, and implantation. The majority of our understanding of these events has been derived from traditional monolayer cell culture and murine tissue models. As mentioned earlier, monolayer culture is inadequate to study the critical cell-to-cell interactions involved in these complicated processes. The murine model is also a poor model because trophoblast differentiation is along 3 potential pathways (as compared to the 2 pathways in the human).23 Furthermore, while both the human and the rodent have chorioallantoic placentas (blood vessels are derived from the fetal allantois and interact with the maternal endometrium), the rodent has an additional yolk sac−derived placenta that plays an important supportive function prior to chorioallantoic placenta formation.24,25 Because this yolk sac−derived placenta protects the fetus from nutritional and oxidative stress, the placental gene programming is likely to be highly disparate between human and murine placentas. Because of these clear distinctions, particularly during early embryologic events, the mouse is a poor model for human blastocyst formation and implantation research. The trophoblast culture technique described in this paper may provide investigators with a valuable model to study the early molecular mechanisms involved in trophoblast differentiation, blastocyst formation, and implantation.
In summary, we have developed a nonadhesive micromold bioreactor for the formation of micro-tissues in culture. Human trophoblast cells cultured in this reactor form trophoblastic vesicles. This structure, while not predicted by the current theories on cellular self-assembly, is the functional biological structure anticipated for this micro-tissue. The cells in 3D undergo differentiation and become invasive, behaviors not seen by these cells in monolayer. We believe this 3D structure is a better model to study the molecular events involved in early trophoblast differentiation and blastocyst formation.
These data were presented, in part, at the 2008 annual meeting of the Society for Gynecologic Investigation, San Diego, CA.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
This work was funded by grants from the NIH/NCRR P20 RR108728 and from the National Center for Women's Health at Women and Infants Hospital, Warren Alpert School of Medicine.
References
- 1. Veeck L, Zaninovic N. An Atlas of Human Blastocysts. New York, NY: The Parthenon Publishing Group; 2003 [Google Scholar]
- 2. Graham CH, Lala PK. Mechanisms of palcental invasion of the uteris and their control. Biochem Cell Biol. 1992;70(10-11):867–874 [DOI] [PubMed] [Google Scholar]
- 3. Conley AJ, Mason JL. Placental steroid hormones. Baillieres Clin Endocrinol Metab. 1990;4(2):249–272 [DOI] [PubMed] [Google Scholar]
- 4. Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the self-assembly of complex cellular aggregates on micromolded nonadhesive hydrogels. Tissue Eng. 2007;13(8):2087–2094 [DOI] [PubMed] [Google Scholar]
- 5. Lewis MP, Clements M, Takeda S, et al. Partial characterization of an immortalized human trophoblast cell-line, TCL-1, Which possesses a CSF-1 auotcrine loop. Placenta. 1996;17(2-3):137–146 [DOI] [PubMed] [Google Scholar]
- 6. Lynch MJ, Raphael SS, Mellor LD, Spare PD, Inwood MJ. Medical Laboratory Technology and Clinical Pathology.2nd ed.Philadelphia: WB Saunders Co; 1969 [Google Scholar]
- 7. Stachecki JJ, Yelian FD, Leach RE, Armant DR. Mouse blastocyst outgrowth and implantation rates following exposure to ethanol or A23187 during culture in vitro. J Reprod Fertil. 1994;101(3):611–617 [DOI] [PubMed] [Google Scholar]
- 8. Kelm JM, Ehler E, Nielsen LK, Schlatter S, Perriard JC, Fussenegger M. Design of artificial myocardial microtissues. Tissue Eng. 2004;10(1-2):201–214 [DOI] [PubMed] [Google Scholar]
- 9. Dean DM, Napolitano AP, Youssef J, Morgan JR. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 2007;21(14):4005–4012 [DOI] [PubMed] [Google Scholar]
- 10. Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424(6951):870–872 [DOI] [PubMed] [Google Scholar]
- 11. Yamashita Y, Shimada M, Tsujita E, et al. High metabolic function of primary human and porcine hepatocytes in a polyurethane foam/spheroid culture system in plasma from patients with fulminant hepatic failure. Cell Transplant. 2002;11(4):379–384 [PubMed] [Google Scholar]
- 12. Blan NR, Birla RK. Design and fabrication of heart muscle using scaffold-based tissue engineering. J Biomed Mater Res A. 2008;86(1):195–208 [DOI] [PubMed] [Google Scholar]
- 13. Bartholomä P, Gorjup E, Monz D, Reininger-Mack A, Thielecke H, Robitzki A. Three-dimensional in vitro reaggregates of embryonic cardiomyocytes: a potential model system for monitoring effects of bioactive agents. J Biomol Screen. 2005;10(8):814–822 [DOI] [PubMed] [Google Scholar]
- 14. LaMarca HL, Ott CM, Bentrup KH, et al. Three-dimensional growth of extravillous cytotrophoblasts promotes differentiation and invasion. Placenta. 2005;26:709–720 [DOI] [PubMed] [Google Scholar]
- 15. Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278(1):255–266 [DOI] [PubMed] [Google Scholar]
- 16. Kidder GM, Watson AJ. Roles of Na, K-ATPase in early development and trophectoderm differentiation. Semin Nephrol. 2005;25(5):352–325 [DOI] [PubMed] [Google Scholar]
- 17. Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778(3):770–793 [DOI] [PubMed] [Google Scholar]
- 18. Eckert JJ, Fleming TP. Tight junction biogenesis during early development. Biochim Biophys Acta. 2008;1778(3):717–728 [DOI] [PubMed] [Google Scholar]
- 19. Korff T, Krauss T, Augustin HG. Three-dimensional spheroidal culture of cytotrophoblast cells mimics the phenotype and differentiation of cytotrophoblasts from normal and prreclamptic pregnancies. Exp Cell Res. 2004;297(2):415–423 [DOI] [PubMed] [Google Scholar]
- 20. Bischof P, Campana A. Molecular mediators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):801–814 [DOI] [PubMed] [Google Scholar]
- 21. Armant DR, Kaplan HA, Lennarz WJ. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev Biol. 1986;116(2):519–523 [DOI] [PubMed] [Google Scholar]
- 22. Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39(1-2):179–195 [DOI] [PubMed] [Google Scholar]
- 23. Niwa H, Toyooka Y, Shimosato D, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123(5):917–929 [DOI] [PubMed] [Google Scholar]
- 24. Jollie WP. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 1990;41(4):361–381 [DOI] [PubMed] [Google Scholar]
- 25. Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(suppl A):S41–S47 [DOI] [PubMed] [Google Scholar]