Abstract
We tested the hypothesis that interleukin 1 (IL-1) mediates intra-amniotic lipopolysaccharide (LPS)-induced chorioamnionitis in preterm fetal sheep. Time-mated Merino ewes with singleton fetuses received IL-1α, LPS, or saline (control) by intra-amniotic injection 1 to 2 days before operative delivery at 124 ± 1 days gestational age (N = 5-9/group; term = 150 days). Recombinant human IL-1 receptor antagonist (rhIL-1ra) was given into the amniotic fluid 3 hours before intra-amniotic LPS or saline to block IL-1 signaling. Inflammation in the chorioamnion was determined by histology, cytokine messenger RNA (mRNA), protein expression, and by quantitation of activated inflammatory cells. Intra-amniotic IL-1 and LPS both induced chorioamnionitis. However, IL-1 blockade with IL-1ra did not decrease intra-amniotic LPS-induced increases in pro-inflammatory cytokine mRNAs, numbers of inflammatory cells, myeloperoxidase, or monocyte chemotactic protein-1-expressing cells in the chorioamnion. We conclude that IL-1 and LPS both can cause chorioamnionitis, but IL-1 is not an important mediator of LPS-induced chorioamnionitis in fetal sheep.
Keywords: fetal inflammatory response syndrome, prematurity, innate immunity, IL-1 receptor antagonist
Introduction
Chorioamnionitis, defined as inflammation of the fetal membranes, complicates up to 70% of preterm deliveries before 30 weeks of gestation.1 The epidemiological associations of chorioamnionitis are fetal inflammation,2,3 a decreased incidence of respiratory distress syndrome,4,5 and an increased risk of brain injury, necrotizing enterocolitis, and bronchopulmonary dysplasia.2,5–7 Although multiple inflammatory cytokines and chemokines are increased in amniotic fluid with chorioamnionitis, interleukin 1 (IL-1) is postulated to play a central role in the progression of preterm labor and of the fetal inflammatory response.1,8
The hierarchy of activation cascade of the cytokines and other mediators in inflammatory conditions including chorioamnionitis remain to be defined. Broadly speaking, 2 classes of inflammatory agents exist: exogenous or microbial components and endogenous pro-inflammatory signals (also called as sterile inflammation).9 Microbial components can also activate endogenous pro-inflammatory ligands. Recent studies demonstrate that IL-1 signaling is critically required to mediate inflammation induced by a variety of endogenous pro-inflammatory ligands for example uric acid, adenosine triphosphate (ATP), reactive oxygen species, heat-shock proteins, and others.10 The normally cell-associated IL-1α is one of the common final pathways mediating inflammation in response to cellular necrosis.11 The importance of IL-1 in diverse diseases is highlighted by the use of IL-1 receptor antagonist in rheumatoid arthritis, gout, type II diabetes, and a group of multisystem inflammatory diseases.12–14
Elevated levels of IL-1 are detected in the amniotic fluid of women with chorioamnionitis15,16 and IL-1 as well as IL-1 receptor antagonist (IL-1ra) are colocalized in the placenta of both normal and inflamed placentas.17 In primates, intra-amniotic IL-1β induced preterm labor,18 and anti-inflammatory therapy (betamethasome, indomethacin, or IL-10) blocked IL-1β-mediated uterine contractions.19,20 In mice, IL-1 and tumor necrosis factor (TNF) receptors are necessary for preterm labor induced with heat-killed Escherichia coli.21 Intra-amniotic injection of IL-1α or IL-1β causes lung inflammation but not preterm labor in sheep.22 These observations in the human and experimental models reinforce the notion that IL-1 is a central mediator in the pathogenesis of chorioamnionitis and its associated inflammatory responses.
In fetal sheep, intra-amniotic injection of lipopolysaccharide (LPS) causes chorioamnionitis, lung inflammation, and systemic inflammation,23,24 mimicking human pathology. Intra-amniotic LPS greatly induces IL-1β expression in the chorioamnion and fetal lung.23 Inhibition of IL-1 signaling decreases LPS-induced lung and systemic inflammation in the preterm sheep.24 We therefore hypothesized that IL-1 is an important mediator of intra-amniotic LPS-induced inflammation in the chorioamnion. We used both gain of function and loss of function strategies to test the hypothesis. Recombinant sheep IL-1α was given by intra-amniotic injection. In separate experiments using intra-amniotic LPS as the pro-inflammatory agonist, the IL-1 receptor was blocked with recombinant human IL-1 receptor antagonist (rhIL-1ra), which inhibits both IL-1α and IL-1β signaling.25 Inflammatory markers and cytokines were assessed in the fetal membranes and amniotic fluid.
Materials and Methods
Animals, Intra-Amniotic Injections
All animals were studied in Western Australia with approval from the animal ethics/care and use committees of the Cincinnati Children’s Hospital, OH and the University of Western Australia. In separate protocols, time-mated Merino ewes with singleton fetuses were randomly assigned to groups of 5 to 9 animals, to receive by intra-amniotic injections: (a) Recombinant sheep IL-1α 100 µg (Protein Express, Cincinnati, Ohio)22 or (b) LPS (Escherichia coli 055:B5; Sigma, St. Louis, Missouri) 10 mg, or an equivalent 2 mL volume of saline (control). Interleukin 1-injected animals were surgically delivered 1 day and 2 days after injection and LPS or rhIL-1ra + LPS-injected animals were delivered 2 days after injection at 124 ± 1 days gestational age. All injections were given by the intra-amniotic route using ultrasound guidance and after verification of the amniotic compartment by fluid electrolyte analysis of aspirated samples.26 The ewes were killed with a penetrating captive bolt or heavily anesthetized with an intravenous injection of ketamine (12 mg/kg) and medetomidine (0.12 mg/kg) followed by a spinal injection of 2% lignocaine hydrochloride (60 mg, 3 mL). The fetus was then surgically delivered via a caesarean section. At delivery, rolls of fetal chorioamnion membranes were snap-frozen for RNA analysis and a roll was fixed in 10% buffered formalin (pH 7.4) for histology. Amniotic fluid was snap-frozen for cell analysis and cytokine proteins.
Recombinant Human IL-1 Receptor Antagonist
During an initial dose finding experiment, the rhIL-1ra injection dose was 20 mg intra-amniotic+ 20 mg fetal intramuscular (Figure 1). Fetal intravenous administration used doses ranging from 50 to 200 mg.24 In contrast to the plasma, the half-life of rhIL-1ra was about 3-fold longer in the amniotic fluid after an intra-amniotic injection (online section of the article24). Since fetal systemic injection of the inhibitor with rapid clearance resulted in low inhibitor levels, a higher dose and the intra-amniotic-only route was used for the definitive experiments. To block IL-1 signaling, and to ensure adequate time for the antagonist to bind the IL-1 receptor, 100 mg rhIL-1ra was injected into the amniotic fluid 3 hours before intra-amniotic LPS or saline. The tissues from the LPS only and the LPS + rhIL-1ra animals in the present study were from animals in which we reported efficacy of rhIL-1ra in the lung and systemic compartments,24 while the IL-1-only exposed animals, and some controls are previously unreported.
Figure 1.
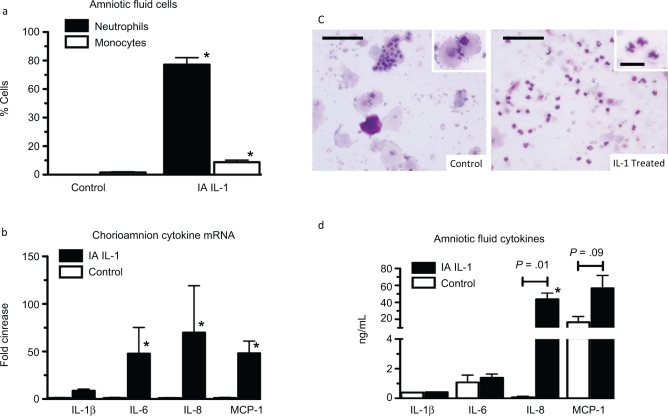
Interleukin 1 (IL-1) induced chorioamnionitis in preterm fetal sheep. Assessments were made 1 day after intra-amniotic injections. a, Differential counts of inflammatory cells in the amniotic fluid. b, Representative photomicrographs of amniotic fluid cytospin stained with hematoxylin and eosin. The insets show a higher magnification of inflammatory cell morphology. c, Quantification of IL-1β, IL-6, IL-8, and monocyte chemotactic protein-1 (MCP-1) messenger RNAs (mRNAs) in the fetal chorioamnion. The mRNA quantitations were performed using real-time PCR with Taqman probe assays and values were normalized to 18S ribosomal protein mRNA (internal control), and levels for each animal group were expressed relative to controls. d, Amniotic fluid cytokine protein analysis by ELISA (n = 3-6 animals/group; scale bar is 50 μm for larger frame and 20 µmol/L for the inset, * P < .05 compared to controls).
Cytokine messenger RNA Quantitation
Total RNA was isolated from the frozen chorioamnion samples using a modified Chomzynski method.23 The quantitations of messenger RNA (mRNA) for animals exposed to IL-1 were performed using real-time polymerized chain reaction (PCR). The mRNA was reverse transcribed to yield a single-strand complementary DNA (cDNA), which was used as a template with primers and Taqman probes (Applied Biosystems, Carlsbad, California) specific to sheep sequences. The values for each cytokine were normalized to the internal 18S ribosomal RNA (rRNA) value. Final expression data were represented as fold increase over the control value.
Amniotic Fluid Protein Analysis
Cytokine protein quantification was performed using a sandwich enzyme-linked-immunosorbent assay (ELISA) assay as described.27,28 The following antibody sets were used: for IL-1β (coating antibody—rabbit anti-ovine IL-1β and primary antibody guinea pig anti-ovine IL-1β [Seven Hills Bioreagents, Cincinnati, Ohio]), IL-6 (coating antibody—mouse anti-ovine IL-6 [Chemicon # MAB1004] and primary antibody rabbit anti-ovine IL-6 [Chemicon #AB1839]), IL-8 (coating antibody—mouse anti-ovine IL-8 [Chemicon # MAB10445] and primary antibody rabbit anti-ovine IL-8 [Chemicon # AB1840]), monocyte chemotactic protein-1 (MCP-1; rabbit anti-sheep MCP-1 coating antibody, and primary antibody guinea pig anti-sheep MCP-1 detection antibody [Seven Hills Bioreagents]). The detection antibody in all the assays was an appropriate species-specific HRP-conjugated antibody. The lowest detection limits of this assay (dynamic range) was IL-1β—0.195 ng/mL (0.195-12.5 ng/mL), IL-6—0.195 ng/mL (0.195-12.5 ng/mL), IL-8—0.39 ng/mL (0.39-25 ng/mL), and MCP-1 0.1 ng/mL (0.1-80 ng/mL). The correlation coefficient was 0.94-0.99 for all assays.
Immunohistochemistry and Scoring of Inflammation
Amniotic fluid cytospins were stained by DiffQuick and inflammatory cell differential counts were performed using a hemocytometer. Inflammatory cells in the fetal membranes were quantified using hematoxylin and eosin (H&E)-stained sections and by immunohistochemistry for markers of inflammatory cell activation. Briefly, formalin was removed from the fixed tissue within 48 hours by washing in phosphate buffered saline (pH 7.4) and then transferred to 70% ethanol and embedded in paraffin. Sections were cut (5 µm), then deparaffinized and rehydrated, and antigen-retrieval was carried out using citric acid buffer, pH 6.0 with microwave boiling. Endogenous peroxidase activity was blocked with methyl alcohol/hydrogen peroxide. Nonspecific binding was inhibited with 2% goat serum during both primary and secondary antibody incubation. Sections were incubated with antimyeloperoxidase (MPO) antibody (Cell marque, Rocklin, California, catalogue # CMC028; 1:400) or a guinea-pig polyclonal anti-MCP-1 (1:1000) antibody generated at our institution (Seven Hills Bioreagents). Following incubation with the primary antibody at 4°C overnight, sections were incubated with the appropriate secondary antibody for 30 minutes at room temperature (1:200). Immunostaining was visualized using a Vectastain ABC peroxidase Elite kit to detect the antigen: antibody complexes (Vector Laboratories Inc, Burlingame, CA). Antigen detection was enhanced with nickel-diaminobenzidine, followed by incubation with TRIS-cobalt to give a black precipitate. Nuclei were counterstained with Nuclear Fast Red for photomicroscopy. Blind scoring of inflammation in the chorioamnion was done by counting MCP-1 or MPO-positive inflammatory cells in 10 comparable nonoverlapping high-power fields of each animal.
Data Analysis
Results are given as mean ± SEM. Comparisons between 3 or more groups were performed by analyses of variance with Student-Newman-Keuls tests used for post hoc analyses. Comparison of 2 groups was done by a nonparametric t-test (Mann-Whitney U-test) for data not distributed normally and with a student t-test for normally distributed data. Statistical significance was accepted at P < .05.
Results
Interleukin 1 Induced Chorioamnionitis
Within 1 day of intra-amniotic IL-1 exposure, the inflammatory cell counts in the amniotic fluid increased (7.3 × 105 ± 2.1 × 105/mL vs 0.3 × 105 ± 0.08 × 105/mL in controls, P < .05). While the controls had no neutrophils, 77% of the cells in the IL-1 exposed fetuses were neutrophils (Figure 2a and b). Consistent with the inflammatory cell recruitment in the IL-1-exposed fetal chorioamnion, IL-1β, IL-6, IL-8, and MCP-1 mRNA increased (9- to 70-fold; Figure 2c). Interestingly, IL-1β and IL-6 protein levels did not increase in the amniotic fluid, however IL-8 protein increased from 0.07 ± 0.07 ng/mL in controls to 44 ± 7 ng/mL in IL-1 exposed fetuses. MCP-1 protein tended to increase from levels of 16.6 ± 6.7 ng/mL in controls to 56.7 ± 15.1 ng/mL in IL-1-exposed animals (P = .09; Figure 2d). At 2 days after intra-amniotic IL-1α, cytokine mRNAs in the chorioamnion for IL-1β, IL-6 and MCP-1 remained increased compared to controls (Figure 1a, b, d). However, IL-8 mRNA returned to control levels (Figure 1c).
Figure 2.
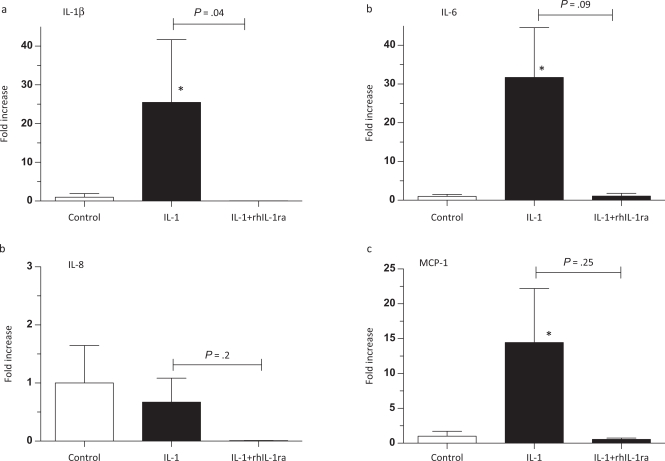
Interleukin 1 (IL-1) increased chorioamnion cytokine messenger RNA (mRNA) in preterm fetal sheep. Assessments were made 2 days after intra-amniotic injections. Quantification of (a) IL-1β, (b) IL-6, (c) IL-8, or (d) monocyte chemotactic protein-1 (MCP-1) mRNAs in the chorioamnion. The mRNA quantitations were performed using real-time PCR with Taqman probe assays and values were normalized to 18S ribosomal protein mRNA (internal control), and levels for each animal group were expressed relative to controls (n = 8 for controls, n = 5 for IL-1 only, and n = 3 for IL-1 + rhIL1ra groups; * P < .05 compared to controls).
Recombinant human IL-1 receptor antagonist Inhibited Intra-Amniotic IL-1α-Induced Increases in Chorioamnion Cytokine mRNAs
In our previous study of IL-1 blockade, the biological efficacy of the dose and route of administration of rhIL-1ra in the sheep was verified by demonstrating that the same lot and dosage of rhIL-1ra completely inhibited intra-amniotic IL-1α-induced lung and systemic inflammation. Further, the 50 mg rhIL-1ra dose effectively prevented intravascular IL-1α-induced mortality (reported in the online section of reference 24). To understand pharmacodynamic availability of rhIL-1ra and to evaluate efficacy in the chorioamnion, we analyzed cytokine mRNAs in the chorioamnion after intra-amniotic IL-1α, 2 days later using animals previously evaluated for lung inflammation.24 In contrast to the increased cytokine mRNAs after IL-1 exposure only, the mRNAs for IL-1β, IL-6, IL-8, and MCP-1 after the combined rhIL-1ra + IL-1 exposure at 2 days were no different from controls (Figure 1a-d). While IL-1β mRNA in the rhIL-1ra + IL-1 animals was significantly lower compared to IL-1-only animals (Figure 1a), the decreases for IL-6 and MCP-1 (Figure 1b and d) did not achieve statistical significance, most likely because of small numbers of animals used in the dose-finding/efficacy study.
Recombinant human IL-1 Receptor Antagonist Did Not Inhibit Intra-Amniotic LPS-Induced Inflammatory Cytokine Expression in the Chorioamnion
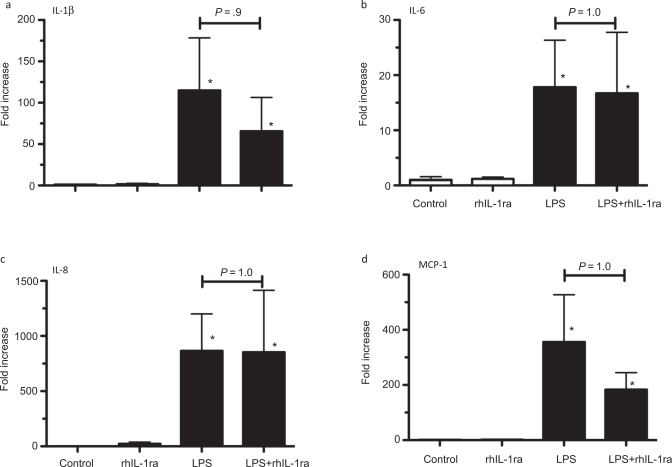
We previously demonstrated that cytokine mRNA expression in the fetal chorioamnion was the highest 5 to 15 hours after intra-amniotic LPS with expression returning to baseline values 4 days later.23 In this study, with measurements made 2 days after LPS exposure, intra-amniotic LPS increased IL-1β mRNA (110-fold), IL-6 mRNA (18-fold), IL-8 mRNA (860-fold), and MCP-1 mRNA (356-fold; Figure 3a-d). Intra-amniotic LPS also increased the acute-phase reactant serum amyloid A3 (SAA3) mRNA 92 ± 28-fold (P = .06). Pretreatment with rhIL-1ra did not significantly inhibit cytokine or acute phase reactant mRNA expression induced by LPS, and the inhibitor alone had no inflammatory effects.
Figure 3.
Interleukin 1 (IL-1) signaling did not decrease lipopolysaccharide (LPS)-induced chorioamnion pro-inflammatory messenger RNA (mRNA) expression in preterm fetal sheep. Assessments were made 2 days after exposures. Quantification of (a) IL-1β, (b) IL-6, (c) IL-8, or (d) MCP-1 mRNAs in the chorioamnion. The mRNA quantitations were performed using real-time PCR with Taqman probe assays and values were normalized to 18S ribosomal protein mRNA (internal control), and levels for each animal group were expressed relative to controls. Inhibition of IL-1 signaling did not change LPS-induced increases in the inflammatory genes (n = 4-5 animals/group; * P < .05 compared to controls).
Recombinant human IL-1 Receptor Antagonist Did Not Inhibit Intra-Amniotic LPS-Induced Inflammatory Cell Recruitment to the Chorioamnion
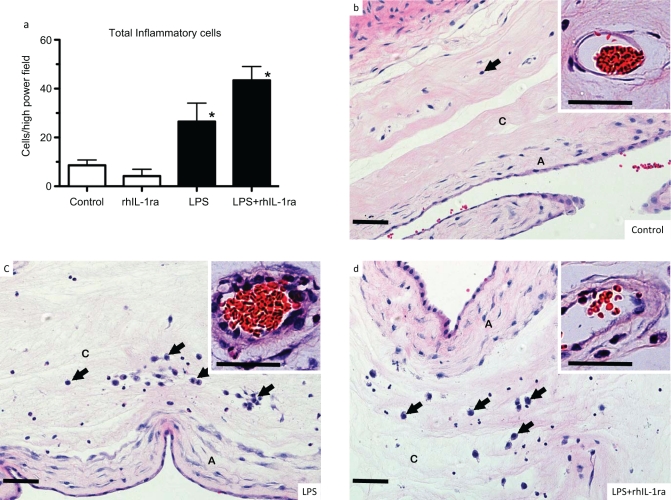
Next, we asked whether blockade of IL-1 reduced LPS-induced chorioamnionitis. Consistent with our previous results,23 intra-amniotic injection of LPS induced a 3-fold increase in inflammatory cells in the chorioamnion (Figure 4a). Intra-amniotic rhIL-1ra given 3 hours prior to the intra-amniotic LPS did not change the LPS-induced recruitment of inflammatory cells to the chorioamnion. In contrast to the minimal matrix deposition beneath the amniotic epithelium in controls, LPS-exposed animals had increased subepithelial matrix and fibroblasts (compare Figure panels 4b with c). A majority of the inflammatory cells had the morphologic appearance of neutrophils or monocytes. Intra-amniotic rhIL-1ra did not change the histological appearance of the chorioamnionitis induced by LPS (Figure 4d). Consistent with the cytokine mRNA data, the inhibitor alone did not recruit inflammatory cells to the chorioamnion.
Figure 4.
Interleukin 1 (IL-1) signaling did not decrease lipopolysaccharide (LPS)-induced histologic chorioamnionitis in preterm fetal sheep. Assessments were made 2 days after intra-amniotic injections. a, Quantitation of inflammatory cells in the chorioamnion. b-d, Representative photomicrographs of chorioamnion stained with hematoxylin and eosin in fetuses exposed to (b) saline (controls), (c) intra-amniotic LPS, or (d) LPS + recombinant human IL-1 receptor antagonist (rhIL-1ra). The insets show a higher magnification of chorionic blood vessels. Exposure to LPS increased inflammatory cells (arrows) in the chorion, increased subepithelial mesenchyme thickness in the amnion, and induced vasculitis (chorion arteriolar thickness in inset). Inhibition of IL-1 signaling did not modify LPS-induced changes (n = 4 animals/group; A indicates amnion; C = chorion; Scale bar 50 μm; * P < .05 compared to controls).
Recombinant human IL-1 Receptor Antagonist Did Not Inhibit Intra-Amniotic LPS-Induced Inflammatory Cell Activation in the Chorioamnion
Monocyte chemotactic protein-128 and MPO expression increase in activated inflammatory cells in fetal sheep.29 We therefore evaluated the expression of these proteins by immunohistology. Lipopolysaccharide induced a 10-fold increase in MCP-1-expressing cells—primarily the monocytes (Figure 5) and a 5-fold increase in MPO-positive cells—predominantly neutrophils (Figure 6) in the chorioamnion. Pretreatment with intra-amniotic rhIL-1ra did not change the numbers of inflammatory cells expressing these activation markers induced by LPS, and the inhibitor alone did not increase MCP-1 or MPO expression. Interestingly, exposure to LPS did not increase expression of inducible nitric oxide synthase (iNOS; NOSII), another activation marker in the inflammatory cells (data not shown).
Figure 5.
Interleukin 1 (IL-1) signaling did not decrease lipopolysaccharide (LPS)-induced monocyte chemotactic protein-1 (MCP-1) expression in inflammatory cells in preterm fetal sheep. Assessments were made 2 days after intra-amniotic injections. a, Quantitation of MCP-1-expressing cells in the chorioamnion. b-d, Representative photomicrographs of chorioamnion immunostained with an anti-sheep MCP-1 antibody in fetuses exposed to (b) saline (controls), (c) intra-amniotic LPS, or (d) LPS + recombinant human IL-1 receptor antagonist (rhIL-1ra). The insets in panels c and d show a higher magnification of inflammatory cells (note the monocytic morphology). Exposure to LPS increased MCP-1-expressing monocytes (arrow) in the chorion. Inhibition of IL-1 signaling did not modify LPS-induced changes (n = 4 animals/group; A indicates amnion; C = chorion; ND not detected; Scale bar 50 μm; * P < .05 compared to controls).
Figure 6.
Interleukin 1 (IL-1) signaling did not decrease lipopolysaccharide (LPS)-induced myeloperoxidase (MPO) expression in inflammatory cells in preterm fetal sheep. Assessments were made 2 days after intra-amniotic injections. a, Quantitation of MPO-expressing cells in the chorioamnion. b-d, Representative photomicrographs of chorioamnion immunostained with an anti-MPO antibody in fetuses exposed to (b) saline (controls), (c) intra-amniotic LPS, or (d) LPS + recombinant human IL-1 receptor antagonist (rhIL-1ra). The insets in panels c and d show inflammatory cells at a higher magnification (note the neutrophil morphology in most cells). Exposure to LPS increased MPO expressing inflammatory cells (arrow) in the chorion. Inhibition of IL-1 signaling did not modify LPS-induced changes (n = 4 animals/group; A indicates amnion; C = chorion; Scale bar 50 μm; * P < .05 compared to controls).
Discussion
We demonstrated that intra-amniotic injection of IL-1 was sufficient to cause chorioamnionitis. However, contrary to our hypothesis, IL-1 was not an important mediator of LPS-induced chorioamnionitis in the preterm fetal sheep model. Interleukin 1 receptor antagonist is an endogenous inhibitor of IL-1 signaling and the recombinant human IL-1ra (Anakinra) used in the present study is an approved anti-inflammatory drug used for rheumatoid arthritis and other inflammatory conditions.12–14 The intra-amniotic dose of rhIL-1ra used in the present study blocked IL-1 signaling, because the inhibitor effectively suppressed IL-1 induced increases in pro-inflammatory cytokine expression in the chorioamnion. The dosing regimen and the same lot of rhIL-1ra also completely blocked intra-amniotic IL-1-induced lung inflammation in the same animals.24 Furthermore, intra-amniotic rhIL-1ra effectively blocked intra-amniotic LPS-induced lung and systemic inflammation.24 Therefore, the experiment is informative for interpreting the role of IL-1 signaling in LPS-induced chorioamnionitis. We did not analyze inflammation in the placenta, because we previously demonstrated that placental inflammation is not a part of the inflammatory response of fetal sheep to chorioamnionitis induced by intra-amniotic LPS.23
Fetal inflammatory response syndrome (FIRS) in the human is a unique systemic inflammatory response defined as chorioamnionitis associated with cord plasma IL-6 levels more than 11 pg/mL.2,3 Unlike the “cytokine storm” causing multi-organ dysfunction associated with the systemic inflammatory response syndrome in the adults,30 FIRS is a more subtle inflammatory response. Despite the modest inflammatory responses, FIRS was postulated to be the proximate cause of multiple adverse neonatal outcomes.31 Since less than 2% of the preterm infants exposed to chorioamnionitis have early onset bacteremia,32 systemic inflammation in the fetus must be initiated by inflammatory responses in the fetal organs in contact with infected amniotic fluid. Indeed, we and others have demonstrated that the fetal chorioamnion, lung, gut, and the skin contribute to the systemic inflammation induced by chorioamnionitis.33–36 We previously demonstrated that IL-1 signaling mediated most of the pulmonary inflammation and part of the systemic inflammation caused by intra-amniotic LPS.24 Taken together, these reports along with the findings of the present study indicate that mechanisms underlying fetal inflammatory responses to chorioamnionitis can be different in different fetal organs. An implication of the study is that chorioamnionitis-induced FIRS may be variably modulated by inhibitors in different fetal organs.
Lipopolysaccharide signals via toll-like receptor 4 (TLR4) whereas IL-1 signals via IL-1R, but both agonists share similar intracellular signaling pathways.37 Subsequent to TLR4 activation, an intracellular signaling cascade is activated culminating in the nuclear translocation and activation of NF-κB, which induces transcription of cytokines and other inflammatory genes. Several studies have examined the interactions between IL-1 and LPS signaling. Lipopolysaccharide-induced preterm labor in mice was not prevented by rhIL-1ra38 or in IL-1β knockout mice.39 Conversely, an IL-1 receptor antagonist reduced systemically administered LPS-induced lethality in adult rabbits and mice.40,41 In a preterm labor model induced by bacterial inoculation of uterine horns in mice, IL-1β signaling was not required but the combined signaling via IL-1β and TNFα receptors was necessary for initiation of preterm labor.21,42 In a nonhuman primate model, Sadowsky et al demonstrated that intra-amniotic infusions of IL-1β and TNFα, but not IL-6 or IL-8, induced preterm labor.18 In preterm fetal sheep, we demonstrated that fetal inflammation induced by intra-amniotic LPS was greatly decreased by inhibition of IL-1 signaling, but not IL-8 signaling,24,27 and intra-amniotic TNFα did not induce fetal inflammation.43 Lipopolysaccharide induced IL-1β expression in the inflammatory cells in the chorioamnion in fetal sheep.44 Collectively, these studies along with the findings of the present experiment demonstrate that different inflammatory signals can initiate fetal inflammation and preterm labor in the setting of chorioamnionitis and that IL-1β can contribute to preterm labor but is not invariantly required to initiate preterm labor.
In addition to IL-1β, other cytokines contribute to the inflammatory response. Increased amniotic IL-17 and the presence of IL-17-positive cells were associated with more advanced chorioamnionitis in preterm deliveries.45 Using a proteomic approach, Buhimschi et al46 demonstrated that the innate immune proteins, defensins 1 and 2, and calgranulins A and C were bonafide biomarkers of fetal inflammation associated with intra-amniotic inflammation. Romero et al47 demonstrated 39 unique (mostly previously unreported) proteomic “fingerprints” associated with chorioamnionitis and preterm labor. In a mouse model of preterm labor induced by intrauterine injection of LPS, proteomic discovery identified the acute phase reactants, SAA1 and 2, as consistently associated with preterm labor.48 Lipopolysaccharide-induced SAA3 expression was not inhibited by rhIL-1ra in the chorioamnion in the present study, while previously we demonstrated that the liver but not lung expression of LPS-induced SAA3 expression was inhibited by rhIL-1ra.24 These identify IL-1-dependent and independent pathways of fetal inflammation associated with chorioamnionitis.
Histopathological evaluation remains the cornerstone of the diagnosis of chorioamnionitis. The severity is based on the anatomic localization of the inflammatory cells (chorion vs. amnion), the numbers of inflammatory cells, and whether the umbilical cord inflammation is present.49 However, predominance of neutrophils (diagnosed by morphologic criteria) is a prerequisite for the diagnosis of acute chorioamnionitis.49 We also observed a predominance of neutrophils in the amniotic fluid or chorioamnion membrane after exposure to LPS and IL-1. Because of limited availability of antibodies for the sheep, a high-resolution evaluation of leukocyte subsets was not possible. Despite the limitation, expression of myeloperoxidase and MCP-1 in the inflammatory cells suggested activation of the inflammatory cells.29 The use of these and other activation markers may be helpful in further exploring the associations of chorioamnionitis with outcomes in preterm infants.
In conclusion, IL-1 can cause chorioamnionitis but may not be an essential mediator of LPS-induced chorioamnionitis. These results have implications for the pathogenesis of fetal inflammatory response syndrome in infants exposed to chorioamnionitis.
Footnotes
This work was presented in part at the Pediatric Academic Societies Meeting on May 2, 2010, in Vancouver, BC, Canada.
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: NIH grants HD57869 (SGK) and HL97064 (AHJ and SGK).
References
- 1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507 [DOI] [PubMed] [Google Scholar]
- 2. Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202 [DOI] [PubMed] [Google Scholar]
- 3. Romero R, Gomez R, Ghezzi F, Yoon B, Mazor M, Edwin S, Berry S. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179(1):186–193 [DOI] [PubMed] [Google Scholar]
- 4. Watterberg K, Demers L, Scott S, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97(2):210–215 [PubMed] [Google Scholar]
- 5. Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808 [DOI] [PubMed] [Google Scholar]
- 6. Alexander J, Gilstrap L, Cox S, McIntire D, Leveno K. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol. 1998;91(5 pt 1):725–729 [DOI] [PubMed] [Google Scholar]
- 7. Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–1017 [DOI] [PubMed] [Google Scholar]
- 8. Genc MR, Gerber S, Nesin M, Witkin SS. Polymorphism in the interleukin-1 gene complex and spontaneous preterm delivery. Am J Obstet Gynecol. 2002;187(1):157–163 [DOI] [PubMed] [Google Scholar]
- 9. Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13(7):851–856 [DOI] [PubMed] [Google Scholar]
- 12. Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526 [DOI] [PubMed] [Google Scholar]
- 13. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 14. Waugh J, Perry CM. Anakinra: a review of its use in the management of rheumatoid arthritis. BioDrugs. 2005;19(3):189–202 [DOI] [PubMed] [Google Scholar]
- 15. Arntzen K, Kojllesdal A, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26(1):17–26 [DOI] [PubMed] [Google Scholar]
- 16. Baud O, Emilie D, Pelletier E, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol. 1999;106(1):72–77 [DOI] [PubMed] [Google Scholar]
- 17. Baergen R, Benirschke K, Ulich T. Cytokine expression in the placenta. The role of interleukin 1 and interleukin 1 receptor antagonist expression in chorioamnionitis and parturition. Arch Pathol Lab Med. 1994;118(1):52–55 [PubMed] [Google Scholar]
- 18. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–1589 [DOI] [PubMed] [Google Scholar]
- 19. Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2000;183(1):173–180 [DOI] [PubMed] [Google Scholar]
- 20. Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188(1):252–263 [DOI] [PubMed] [Google Scholar]
- 21. Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194(5):1334–1340 [DOI] [PubMed] [Google Scholar]
- 22. Willet KE, Kramer BW, Kallapur SG, et al. Intra-amniotic injection of IL-1 induces inflammation and maturation in fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L411–L420 [DOI] [PubMed] [Google Scholar]
- 23. Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L527–L536 [DOI] [PubMed] [Google Scholar]
- 24. Kallapur SG, Nitsos I, Moss TJ, et al. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med. 2009;179(10):955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dinarello C. Interleukin-1. Cytokine Growth Factor Rev. 1997;8(4):253–265 [DOI] [PubMed] [Google Scholar]
- 26. Jobe AH, Newnham JP, Willet KE, et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162(5):1656–1661 [DOI] [PubMed] [Google Scholar]
- 27. Kallapur SG, Moss TJ, Auten RL., Jr et al. IL-8 signaling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L512–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah TA, Hillman NH, Nitsos I, et al. Pulmonary and systemic expression of monocyte chemotactic proteins in preterm sheep fetuses exposed to lipopolysaccharide-induced chorioamnionitis. Pediatr Res. 2010;68(3):210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheah FC, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr Res. 2008;63(3):274–279 [DOI] [PubMed] [Google Scholar]
- 30. Matsuda N, Hattori Y. Systemic inflammatory response syndrome (SIRS): molecular pathophysiology and gene therapy. J Pharmacol Sci. 2006;101(3):189–198 [DOI] [PubMed] [Google Scholar]
- 31. Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–683 [DOI] [PubMed] [Google Scholar]
- 32. Stoll BJ, Gordon T, Korones SB, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):72–80 [DOI] [PubMed] [Google Scholar]
- 33. Kramer BW, Kallapur SG, Moss TJ, et al. Modulation of fetal inflammatory response on exposure to lipopolysaccharide by chorioamnion, lung, or gut in sheep. Am J Obstet Gynecol. 2010;202(1):77 e71–e79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49(5):506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolfs TG, Buurman WA, Zoer B, et al. Endotoxin induced chorioamnionitis prevents intestinal development during gestation in fetal sheep. PLoS One. 2009;4(6):e5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur S, Newnham JP. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci. 2011;18(1):88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li X, Qin J. Modulation of Toll-interleukin 1 receptor mediated signaling. J Mol Med. 2005;83(4):258–266 [DOI] [PubMed] [Google Scholar]
- 38. Fidel PL, Jr, Romero R, Cutright J, et al. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J Soc Gynecol Investig. 1997;4(1):22–26 [DOI] [PubMed] [Google Scholar]
- 39. Reznikov LL, Fantuzzi G, Selzman CH, et al. Utilization of endoscopic inoculation in a mouse model of intrauterine infection-induced preterm birth: role of interleukin 1beta. Biol Reprod. 1999;60(5):1231–1238 [DOI] [PubMed] [Google Scholar]
- 40. Ohlsson K, Bjork P, Bergenfeldt M, Hageman R, Thompson RC. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348(6301):550–552 [DOI] [PubMed] [Google Scholar]
- 41. Alexander HR, Doherty GM, Buresh CM, Venzon DJ, Norton JA. A recombinant human receptor antagonist to interleukin 1 improves survival after lethal endotoxemia in mice. J Exp Med. 1991;173(4):1029–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol. 2002;186(3):523–530 [DOI] [PubMed] [Google Scholar]
- 43. Ikegami M, Moss TJ, Kallapur SG, et al. Minimal lung and systemic responses to TNF{alpha} in preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L121–L129 [DOI] [PubMed] [Google Scholar]
- 44. Newnham JP, Kallapur SG, Kramer BW, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol. 2003;189(5):1458–1466 [DOI] [PubMed] [Google Scholar]
- 45. Ito M, Nakashima A, Hidaka T, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84(1):75–85 [DOI] [PubMed] [Google Scholar]
- 46. Buhimschi CS, Dulay AT, Abdel-Razeq S, et al. Fetal inflammatory response in women with proteomic biomarkers characteristic of intra-amniotic inflammation and preterm birth. BJOG. 2009;116(2):257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Romero R, Espinoza J, Rogers WT, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21(6):367–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang Q, Whitin JC, Ling XB, et al. Plasma biomarkers in a mouse model of preterm labor. Pediatr Res. 2009;66(1):11–16 [DOI] [PubMed] [Google Scholar]
- 49. Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–448 [DOI] [PubMed] [Google Scholar]