Abstract
Endometriosis is an estrogen-dependent disease. Steroidogenic factor 1 (SF-1), a transcription factor, is essential for the activation of multiple steroidogenic genes for estrogen biosynthesis in endometriosis-derived stromal cells. Objective: Unravel the mechanism for differential SF-1 expression in endometrial and endometriotic stromal cells. DESIGN: We identified a novel CpG island in the SF-1 gene, which spans from exon II to intron III. We evaluated the methylation status of this CpG island. PATIENTS: Eutopic endometrium from disease-free participants (n = 8) and the walls of cystic endometriosis lesions of the ovaries (n = 8). None of the patients had received any preoperative hormonal therapy. Stromal cells were isolated from these 2 types of tissues. Results: SF-1 messenger RNA (mRNA) levels in endometriotic stromal cells were significantly higher than those in endometrial stromal cells. Bisulfite sequencing showed strikingly increased methylation in endometriotic cells compared with endometrial cells (P < .001). A strong correlation between mRNA levels and percentage methylation of the exon II/intron III are observed. Specifically, the Pearson correlation coefficient was .98 (P < .001) for this association. Conclusions: We demonstrated that methylation of a coding exon/intron sequence in the SF-1 gene positively regulated its expression in endometriosis, whereas its hypomethylation in normal endometrium was associated with drastically lower SF-1 levels.
Keywords: SF-1, endometriosis, DNA methylation, intron, CpG island
Introduction
Endometriosis is an estrogen-dependent disease that affects 10% of women of reproductive age and is the most common cause of chronic pelvic pain.1,2 Endometriosis is a systemic disorder that is characterized by the presence of endometrium-like tissue in ectopic sites outside the uterus, primarily on pelvic peritoneum and ovaries. Only 50% of women with endometriosis achieve pain relief in response to existing hormonal treatments or conservative surgery.2 Thus, there is a clear need to understand the underlying mechanisms and develop novel and effective therapies for endometriosis.
The significance of estrogen biosynthesis in endometriosis is exemplified by the clinical observations that estrogen is essential for the growth of endometriosis. We and others demonstrated abundant aromatase expression and local estrogen production in endometriotic tissue.3–6 Aromatase catalyzes the final step of estrogen production via conversion of C19 steroids to estrogens7 and is expressed in the stromal cell compartment of endometriosis, whereas they are undetectable in eutopic endometrial stromal cells from disease-free women.8,9 Aromatase and some of the other key steroidogenic enzymes are regulated by a nuclear receptor termed steroidogenic factor 1 (SF-1), also called Ad4BP or NR5A1, which is a member of the nuclear receptor superfamily.10 In contrast to other nuclear receptors, which are activated by ligands, nuclear receptors 5A are orphan receptors because the existence of their ligands is still under debate.11,12 Steroidogenic factor 1 is a key regulator for steroid biosynthesis. Steroid hormones are synthesized in steroidogenic tissues such as adrenal gland, gonad, placenta, and brain. Steroidogenic factor 1 is involved in the regulation of adrenal and testicular steroidogenic genes such as StAR, hydroxysteroid dehydrogenase genes (HSD3B and HSD11B), MC2R, and those in the CYP family encoding cytochrome P450 enzymes.13 Steroidogenic factor 1, which is expressed in endometriosis but not in its normal counterpart tissue, eutopic endometrium, is the key activator of the aromatase gene promoter in endometriotic stromal cells.4
CpG islands are areas rich in CG dinucleotides that are found in the genome-wide scale. Abnormal CpG island methylation seems to be a frequent event in most malignancies.14,15 Hypermethylation of CpG islands in the 5’ regulatory region and first exon of genes is a potential mechanism for the loss of gene expression.
We previously reported that DNA methylation of promoter and first exon region is a major mechanism of SF-1 silencing in normal endometrial cells and its aberrant expression in endometriotic cells.16 Here, we characterize another CpG island, this time, in the coding region of the gene. We identified an approximately 600-bp CpG island that spans from exon II to intron III. Our results below are suggestive of a unique role of this new CpG island in differentially regulating SF-1 expression in endometriotic versus endometrial stromal cells.
Materials and Methods
Isolation and Culture of Endometrial and Endometriotic Stromal Cells
Eutopic endometrium from disease-free participants (n = 8) and ectoic endometrium from the walls of ovarian endometriomas (containing a dense brown chocolate-like fluid, n = 8) were obtained at the time of laparoscopy. Written informed consent was obtained before surgeries, including a consent form and protocol approved by the institutional review board at the Northwestern University. The average age of participants was 40.75 ± 3.37 year (endometrium) and 38.88 ± 2.95 year (endometriosis), and there were no statistically significant differences between the 2 groups with respect to age. None of the patients had received any preoperative hormonal therapy. All samples were histologically confirmed. Eutopic endometrial samples were obtained from premenopausal women undergoing hysterectomy for cervical dysplasia or uterine leiomyoma. The phase of the menstrual cycle was determined by preoperative history and histological examination. Half of the tissue samples were in the proliferative phase and the other half in the secretory phase in both groups. Stromal cells were isolated from these 2 types of tissues using a protocol previously reported by Ryan et al with minor modification8,17 and suspended in DMEM/F12 1:1 (GIBCO/BRL, Grand Island, New York) containing 10% fetal bovine serum.
RNA Extraction and Quantitative Analysis by Real-time Reverse Transcriptase Polymerase Chain Reaction
Total RNA was isolated from stromal cells with TRIzol (Sigma, St. Louis, MO), according to the manufacturer’s protocol. One microgram of total RNA was used to generate complementary DNA (cDNA) with the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, California). Real-time quantitative polymerase chain reaction (PCR) was performed using the ABI 7900 Sequence Detection system and the ABI Taqman Gene Expression system (Applied Biosystems, Foster City, California) to quantify SF-1 and human 18S RNA; 18S values were used for normalization. Relative quantification for all transcripts was analyzed by the comparative threshold cycle method described previously.18 The following primers were used for the SF-1 coding region: forward: 5′-CTGGAGCCGGATGAGGAC-3′, reverse: 5′-ACCTGGCGGTAGATGTGGT-3′. 18S primers were forward: 5′-AGGAATTCCCAGTAAGTGCG-3′, reverse: 5′-GCCTCACTAAACCATCCAA-3′.
Bisulfite Modification and Sequencing Analysis
Genomic DNA from all samples (500 ng) was subjected to sodium bisulfite modification using EZ DNA methylation—Gold kit following the manufacturer’s protocol (Zymo Research, Orange, California). The manufacturer’s recommended alternative reaction conditions were chosen for the modification reaction (98°C for 10 minutes, 53°C for 30 minutes, followed by 8 cycles at 53°C for 6 minutes and 37°C for 30 minutes).
Bisulfite DNA Sequencing Analysis
The bisulfite sequencing primers (forward: 5′-GAAGGTTAATGGTATTATTTTTTTAG-3′, and reverse: 5′-CACRTATAAAAACTACAAAATAAAC-3′) for SF-1 can amplify a 333 base pair (bp) product flanking 29 CpG dinucleotides. Polymerase chain reaction was carried out in a thermocycler with the following conditions: 95°C for 10 minutes followed by 40 cycles of denaturation at 95°C 30 seconds, annealing at 50°C for 2 minutes, and elongation at 72°C for 2 minutes, followed by an extension at 72°C for 7 minutes. Amplified PCR products were separated by electrophoresis employing 1.5% agarose gels and visualized by ethidium bromide staining. Then, 1 μL of PCR products were subcloned using the pGEM-Teasy vector (Promega, Madison, Wisconsin). After transformation, we randomly selected 10 to 20 individual clones for each sample assessed. Plasmid DNA was directly amplified without bacterial culture. Six to eight plasmids containing the right insert were then sequenced using an Applied Biosystems 377 instrument. The sequence data were compared with the University of California at Santa Cruz genome Ref sequence in order to assess the methylation status of each CpG site.
Statistical Analysis
Percentage methylation of each clone obtained from each of the 8 patients in each group was treated as a single value for the statistical analysis of bisulfite sequencing. The data were analyzed using Student t test with statistical significance at the level of P < .05 when comparing percentage methylation between the 2 groups of cells. Pearson coefficient was calculated for the correlation between SF-1 messenger RNA (mRNA) levels and percentage methylation.
Results
SF-1 Exon II to Intron III Region DNA Hypermethylation in the Endometriotic Stromal Cells
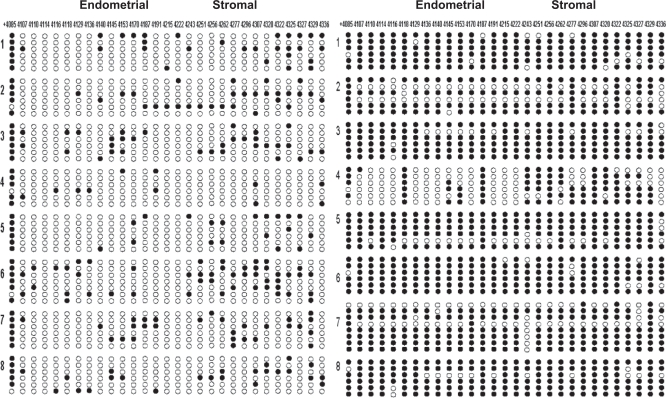
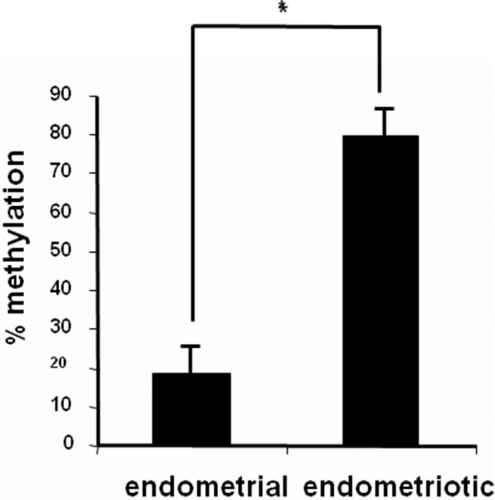
Methylation status of a total of 29 CpGs across this 333-bp region containing exon II, intron II, exon III, and intron III of the SF-1 gene (Figure 1) was characterized by bisulfite genomic sequencing, which is the gold standard for mapping methylation across CpG sites. In Figure 2, at the top, the first nucleotide (cystosine) of each CpG sequence located in the dense island between +4085 bp and +4337 bp is listed. After subcloning of PCR products generated from bisulfite-treated DNA using a pGEM-Teasy vector, sequencing of each selected clone provided the precise methylation status for 29 CpG sites in the CpG island of SF-1 gene (Figure 2). The endometriotic stromal cells that express high levels of SF-1 showed a dense methylation pattern at this region of SF-1 gene. In contrast, the majority of the CpG sites were not methylated in endometrial stromal cells, which do not express SF-1. There was a significant difference (P < .001, Student t test) in methylation status between the 2 groups of cells (Figure 3).
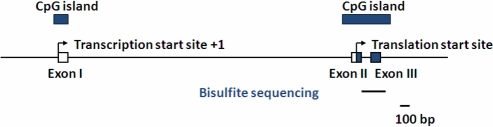
Figure 1.
A schematic diagram indicating the CpG island in SF-1 intron region. The transcription start site (TSS) is indicated as +1. Upper black bar predicted CpG island; lower black bar predicted bisulfite sequencing fragment containing the promoter region.
Figure 2.
DNA methylation status of 29 CpG sites in the CpG island flanking exon II to intron III region of SF-1 gene in endometrial and endometriotic stromal cells. Open and filled circles represent unmethylated and methylated cytosines, respectively. The numbers on the top indicate the positions of cytosine residues of CpGs relative to the transcription start site (+1); and the numbers 1 to 8 on each side represent participants, from whom primary stromal cells were obtained. Cells were obtained from a total of 16 participants.
Figure 3.
Percentage methylation of SF-1 intron region in endometrial and endometriotic cells. *P < .001.
Correlation Between SF-1 mRNA Levels and Percentage Methylation
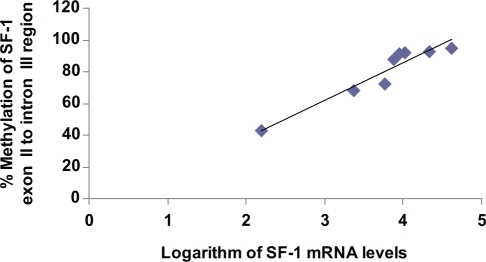
Previously, we reported a striking and statistically significant difference between endometrial and endometriotic stromal cells with respect to SF-1 mRNA levels, which were much higher in endometriotic stromal cells.16 Here, in order to characterize the effects of DNA methtylation specifically at the exon II/intron III region on SF-1 gene expression, we analyzed the correlation between SF-1 mRNA levels and percentage methylation of this region in endometriotic stromal cells. As shown in Figure 4, a remarkably strong and significant correlation between SF-1 mRNA levels and percentage methylation in the exon II/intron III region was observed. Specifically, Pearson correlation coefficient was .98 (P < .001) for this region. These data are suggestive that DNA methylation at this specific CpG island may suppress a silencer element that possibly regulates SF-1 expression.
Figure 4.
Significant correlation (Pearson correlation coefficient is .98; P < .001) between percentage methylation of SF-1 intron region and SF-1 mRNA expression (in logarithmc scale) among 8 endometriotic stromal cells.
Discussion
Recently, a number of pioneering publications revolutionized our understanding of gene expression and transcriptional factor binding sites.19 Using chromatin immunoprecipitation-on-chip, these groups found that the majority of the binding sites of transcription factors such as estrogen receptor-α (ERα) were remarkably distant from the transcriptional start sites of regulated genes.20 In fact, many functionally relevant binding sites for transcription factors likely exist in regions outside of gene promoters, particularly in introns.21–23 The binding sites located distal to genes or within introns might function through long-range interactions that involve looping of chromatin to bring the regulatory elements within proximity of gene promoters.21,24 Recent reports about intronic binding of other transcriptional factors, such as cyclic adenosine monophosphate cAMP-responsive element binding protein and BARX2 homeobox protein, provide further support that intronic binding may be an important mechanism of gene regulation.24,25 Our data also showed that the methylation status in exonic and intronic sequences within the coding region of the SF-1 gene was strongly associated with mRNA levels and possible regulation of gene transcription.
Data presented here are unique to define a mechanism whereby SF-1 gene expression is activated in endometriotic stromal cells. First, SF-1 is expressed in endometriosis, but not in its normal counterpart tissue, eutopic endometrium. Applying the EMBOSS CpG Plot identification analysis to the human SF-1 coding region (Figure 1), we demonstrated a positive association between increased methylation of the CpG island at the exon II/intron III region and SF-1 expression in endometriosis because this exact sequence is significantly hypomethylated in normal endometrial stromal cells that do not express SF-1.
Intriguingly, hypermethylation of this exon/intron region activates SF-1 mRNA expression in endometriotic cells, which is distinct from the 5′ promoter sequence, hypermethylation which classically silences gene expression. We previously demonstrated that differential methylation of a CpG island at the promoter and exon I region regulates SF-1 transcriptional activity in endometriosis or endometrium-derived stromal cells.16 This is consistent with a large body of literature showing that DNA methylation at the promoter region is generally associated with gene silencing.
Our results are consistent with other reports indicating that methylation outside the promoter lead to increased gene expression.26–28 We speculate that the CpG island at the exon II/intron III region may contain a silencer element. It follows then that the methylation of this element would suppress its silencer function giving rise to increased SF-1 expression. The significance of this CpG island in regulating SF-1 expression in the hypothalamus, adrenal, and gonads remains to be elucidated.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Natural Science Foundation of China (30973183), Science Foundation of First Hospital of Beijing University, and partly by the NIH/NICHD grant R37-HD38691.
References
- 1. Ryan IP, Taylor RN. Endometriosis and infertility: new concepts. Obstetr Gynecol Surv. 1997;52(6):365–371 [DOI] [PubMed] [Google Scholar]
- 2. Olive DL, Schwartz LB. Endometriosis. N Eng J Med. 1993;328(24):1759–1769 [DOI] [PubMed] [Google Scholar]
- 3. Kitawaki J, Noguchi T, Amatsu T, et al. Expression of aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biol Reprod. 1997;57(3):514–519 [DOI] [PubMed] [Google Scholar]
- 4. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol (Baltimore, Md. ) 1999;13(2):239–253 [DOI] [PubMed] [Google Scholar]
- 5. Bulun SE, Yang S, Fang Z, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79(1-5):19–25 [DOI] [PubMed] [Google Scholar]
- 6. Fang Z, Yang S, Gurates B, et al. Genetic or enzymatic disruption of aromatase inhibits the growth of ectopic uterine tissue. J Clin Endocrinol Metab. 2002;87(7):3460–3466 [DOI] [PubMed] [Google Scholar]
- 7. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12(1):49–56 [DOI] [PubMed] [Google Scholar]
- 8. Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–606 [DOI] [PubMed] [Google Scholar]
- 9. Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383 [DOI] [PubMed] [Google Scholar]
- 10. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–163 [DOI] [PubMed] [Google Scholar]
- 11. Krylova IN, Sablin EP, Moore J, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120(3):343–355 [DOI] [PubMed] [Google Scholar]
- 12. Wang W, Zhang C, Marimuthu A, et al. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA. 2005;102(21):7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocrine Rev. 1997;18(3):361–377 [DOI] [PubMed] [Google Scholar]
- 14. Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58(23):5489–5494 [PubMed] [Google Scholar]
- 15. Issa JP, Baylin SB, Belinsky SA. Methylation of the estrogen receptor CpG island in lung tumors is related to the specific type of carcinogen exposure. Cancer Res. 1996;56(16):3655–3658 [PubMed] [Google Scholar]
- 16. Xue Q, Lin Z, Yin P, et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5’ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92(8):3261–3267 [DOI] [PubMed] [Google Scholar]
- 17. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78(3):642–649 [DOI] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. San Diego, Calif). 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 19. Carroll JS, Liu XS, Brodsky AS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33–43 [DOI] [PubMed] [Google Scholar]
- 20. Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nature Genet. 2006;38(11):1289–1297 [DOI] [PubMed] [Google Scholar]
- 21. Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell. 2005;19(5):631–642 [DOI] [PubMed] [Google Scholar]
- 22. Stevens TA, Iacovoni JS, Edelman DB, Meech R. Identification of novel binding elements and gene targets for the homeodomain protein BARX2. J Biol Chem. 2004;279(15):14520–14530 [DOI] [PubMed] [Google Scholar]
- 23. Lin Z, Reierstad S, Huang CC, Bulun SE. Novel estrogen receptor-alpha binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res. 2007;67(10):5017–5024 [DOI] [PubMed] [Google Scholar]
- 24. Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods (San Diego, Calif). 2002;26(1):48–56 [DOI] [PubMed] [Google Scholar]
- 25. Impey S, McCorkle SR, Cha-Molstad H, et al. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119(7):1041–1054 [DOI] [PubMed] [Google Scholar]
- 26. Hu M, Yao J, Cai L, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nature Genet. 2005;37(8):899–905 [DOI] [PubMed] [Google Scholar]
- 27. Feinberg AP, Tycko B. The history of cancer epigenetics. Nature Rev. 2004;4(2):143–153 [DOI] [PubMed] [Google Scholar]
- 28. Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405(6785):482–485 [DOI] [PubMed] [Google Scholar]