Abstract
There is increasing evidence linking in utero infection and inflammation to preterm birth. Many commensal urogenital tract microorganisms, including the Mycoplasmas and Ureaplasmas, are commonly detected in association with preterm birth. Using an ovine model of sterile fetal inflammation, we demonstrated previously that the fetal skin generates a robust inflammatory response following in utero exposure to lipopolysaccharides from Escherichia coli. The fetal skin’s response to colonization of the amniotic fluid by viable microorganisms remains unstudied. We hypothesised that in utero infection with Ureaplasma parvum serovar 3 would induce a proinflammatory response in the fetal skin. We found that (1) cultured fetal keratinocytes (the primary cellular constituent of the epidermis) respond to U. parvum exposure in vitro by increasing the expression of the chemotactant monocyte chemoattractant protein 1 (MCP-1) but not interleukin 1β (IL-1β), IL-6, IL-8, or tumor necrosis factor-α (TNF-α); (2) the fetal skin’s response to 7 days of U. parvum exposure is characterized by elevated expression of MCP-1, TNF-α, and IL-10; and (3) the magnitude of inflammatory cytokine/chemokine expression in the fetal skin is dependent on the duration of U parvum exposure. These novel findings provide further support for the role of the fetal skin in the development of fetal inflammation and the preterm birth that may follow.
Keywords: preterm birth, uterine infection, inflammation
Introduction
Inflammation is predominantly a defensive physiological response to tissue injury or infection.1 The response is characterized by localized increases in cytokine and chemokine (interleukin 1 [IL-1], IL-6, tumor necrosis factor-α [TNF-α], inductible nitric oxide synthase [iNOS], and monocyte chemoattractant protein 1 [MCP-1]) expression in conjunction with immunocyte influx and activation and increased vascular permeability.1 Normal term birth is itself characterized by an inflammation of the maternal (decidua, cervix) tissues.2,3 Many cases of early preterm birth result from infection and inflammation.4–9
Fetal Inflammatory Response Syndrome (FIRS) is a term first proposed by Gomez and colleagues10 in 1998. Fetal inflammatory response syndrome is defined as elevated levels of cord-blood IL-6 (>11 pg/mL) and is often found in association with histological chorioamnionitis and funisitis.11–13 This inflammation occurring before birth shares features with adult systemic inflammatory response syndrome although the fetal response is more subtle.10
A large body of experimental evidence now exists to support the association between in utero infection and preterm birth.14–16 Utilizing a noninfectious ovine model of inflammation (induced by the intrauterine administration of lipopolysaccharides [LPS] from gram-negative Escherichia coli), we demonstrated previously that in utero exposure to bacterial agonist precipitates a robust, time-dependent, and modifiable inflammatory response.6,17 This response is evident in fetal (lung, gut, brain, and chorioamnion) and maternal (placenta and decidua) tissues and involves elevated cytokine/chemokine expression, and neutrophil influx.18–20 In the rhesus macaque, informative studies by Novy, Gravett, and Grigsby provided insight into the relative timing of inflammation in maternal tissues as a prelude to membrane rupture and preterm birth: a study employing chronically catheterized macaques demonstrated increased uterine contractility and elevated levels of cytokine and prostaglandin production in response to intra-amniotic infection with group B streptococci; and subsequent studies have shown that infusion of IL-1β and TNF-α are able to induce preterm labor.9,15,21,22 Murine studies, including those by Hirsch et al using single/double IL1r1/Tnfrsf1a knockout, have highlighted both functional redundancies in preterm birth-associated inflammatory signaling pathways and potential targets for therapeutic interventions.23,24
The fetal skin is entirely exposed to the amniotic milieu and, for a significant percentage of gestation (in humans), lacks a protective outer stratum corneum. We demonstrated previously that the fetal ovine skin possesses the ability to initiate a robust, locally derived proinflammatory response following exposure to E coli LPS involving distinct basophilic infiltration of the dermis and epidermis in conjunction with increases in cytokine expression.25 These findings are in concordance with the results of previous clinical studies reporting fetal dermatitis as a component of FIRS and in association with histological chorioamnionitis.26
In the present study, we tested the hypothesis that the fetal skin can initiate a proinflammatory response to the presence of in utero infection by Ureaplasma parvum serovar 3 (UP). To test this hypothesis, we examined the in vitro proinflammatory response of primary fetal ovine keratinocytes to UP serovar 3 in conjunction with in vivo studies with our ovine model of intra-amniotic UP infection. We present the first data (of which we are aware) demonstrating that exposure to UP elicits a marked inflammatory response in the fetal skin characterized by striking basophilic infiltration in conjunction with increases in cytokine and chemokine expression.
Materials and Methods
Animals and Tissue Collection
All procedures involving animals were performed in Western Australia following review and approval by the animal care and use committees of the Cincinnati Children’s Hospital (Cincinnati, Ohio) and The University of Western Australia. Date-mated Australian merino ewes (term = 148 ± 2 days) were bred to carry single pregnancies. At the conclusion of each experimental protocol, animals were heavily sedated with an intravenous injection of metedomidine (0.12 mg/kg) and ketamine (12 mg/kg; Provet, Perth, Australia). The fetus was then surgically delivered and both ewe and lamb were euthanized with pentobarbitone (100 mg/kg). Skin samples for RNA or histological analyses were collected from the left groin of each fetus.
Intra-amniotic Administration of UP
Intra-amniotic administration of UP was performed under ultrasound guidance with successful intra-amniotic targeting verified by electrolyte analysis of 2 mL of amniotic fluid.25 To investigate the proinflammatory effects of UP on the fetal skin, 5 to 7 animals were randomly assigned to receive (1) 7-day intra-amniotic UP (117 days of gestational age [GA] ± 2 days); (2) 69-day intra-amniotic UP (55 days GA ± 2 days); (3) 7-day intra-amniotic sterile UP culture media (117 days of GA ± 2 days; control, n = 5) or (4) 69-day intra-amniotic sterile UP culture media (55 days of GA ± 2 days; control). Exposure length (either 7 days or 69 days) was selected to allow us to model fetal skin inflammation subsequent to acute and chronic UP exposures. All animals were euthanized at 124 days of GA (±2 days) and necroscopy studies performed.
Ureaplasma Culture and Infection Screening
Ureaplasma parvum serovar 3 were cultured in 10B broth as published previously.5 DNA was extracted from 0.5 g of skin using a DNeasy blood and tissue extraction kit (Qiagen, Hilden, Germany), in accordance with the manufacturer’s instructions. Forward (CAA TCT GCT CGT GAA GTA TTA) and reverse (ACG ACG TCC ATA AGC AAC) primers targeting the Ureaplasma spp urease gene (NCBI reference AF085729.2) were used in a standard 20 μL polymerase chain reaction (PCR) in 1× reaction buffer (containing 20 mmol/L MgCl2) as follows: 50 ng template DNA/positive control DNA, 125 pmol of each primer, 0.5 U FastStart DNA polymerase (Roche, Basel, Switzerland), 2 μmol/L each deoxyribonucleotide triphosphate (dNTP).27 Nuclease-free water replaced DNA template as a negative control. Cycling conditions were as follows: 1 × 4 minutes at 95°C, 35 × 1 minutes at 94°C, 1 minutes at 55°C, 1 minutes at 72°C, and 1 × 5 minutes at 72°C, hold at 4°C. Reaction mixtures were loaded on a 1% Tris-acetate-EDTA (TAE) agarose gel for electrophoresis and visualized with ethidium bromide staining. The presence of UP in a sample was by the identification of a single band at 429 bp.
Quantitative PCR analysis of UP-infected tissues was performed with a Corbett Robotics Rotorgene 3000 (cycling conditions: 1 × 7 minutes initial denaturation (95°C), 45 × 5 seconds denaturation (95°C), 30 seconds annealing (55°C), 10 seconds extension (72°C), 1× final 0.5°C stepped temperature ramp 72°C to 95°C). Forward and reverse primers for UP MBA (F: TGCTGCACTTACATCAGTTGAA; R: TTCATTAGGTTTTGGTTCACGA) and universal 18s (F: TGCATGTCTAAGTACGCACG; R: TTGATAGGGCAGACGTTCGA) were used to calculate the fold-change differences in expression relative to control (sterile UP media exposed) tissues. DNA from fetal skin was amplified in a 10 μL PCR reaction in 1× ImmoBuffer (Bioline, London, UK), 25 pmol of each primer (Bioline), 0.5 U FastStart DNA polymerase (Roche), 2 μmol/L each dNTP (Bioline), and 0.5 U immolase (Bioline).
Primary Keratinocyte Cell Culture
Primary ovine keratinocytes were collected from normal fetuses (no in utero exposure to UP or LPS) immediately following surgical delivery and postmortem at 124-day gestation (term = 148 days ± 2 days), as described previously.25 Briefly, fetal skin was quickly shaved and dissected free of the underlying fascia before being stored at 4°C in 10 mL of serum-free DMEM and prior to processing. The epidermis was isolated by dispase treatment (16 hours at 4°C) and disrupted in a 10 mL solution of phosphate-buffered saline (PBS) containing 0.1% trypsin in 1 mmol/L EDTA. Keratinocytes were pelleted by centrifugation and resuspended in 5 mL of keratinocyte growth media (KGM) (Life Technologies, Carlsbad, California) and assessed for viability using trypan blue staining before being seeded into 25 cm2 flasks (total culture volume, 10 mL) and cultured at 37°C in a 5% CO2 atmosphere in serum-free KGM containing 5 mg/mL streptomycin and 5 mg/mL penicillin(Life Technologies). All fetal ovine keratinocytes used in this study were passage 3 and were taken from fetuses at 124 days of GA (term = 148 days ± 2 days).
In vitro UP Inflammation Assays
Primary ovine keratinocytes were seeded into 12-well culture plates at a density of 2 × 105 cells/well in 2 mL of serum-free keratinocyte growth media containing 5 mg/mL streptomycin and 5 mg/mL penicillin. Cells were grown until >95% confluent. Cells were exposed to 2 × 107 colony forming units (CFU)/mL UP diluted in 2 mL of keratinocyte growth media for 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, or 12 hours. Cells were exposed to an equivalent volume of sterile UP culture media diluted in 2 mL of keratinocyte growth media as a negative control. All time points and an additional mock infection (sterile 10B media diluted in keratinocyte growth media) were performed in triplicate.
RNA Isolation and Complementary DNA Generation
Total RNA was isolated from cells and frozen tissue using Trizol (Life Technologies). Frozen tissue was finely milled in liquid nitrogen, added directly to 1 mL of Trizol and processed in accordance with the manufacturer’s instructions. RNA pellets were resuspended in nuclease-free water and incubated at 50°C for 20 minutes to aid resuspension. RNA yield and purity was assessed using 260/280 nm absorbance readings. Complementary (cDNA) was generated from 600 ng RNA using BIOSCRIPT MMLV reverse transcriptase (Bioline), according to the manufacturer’s instructions.
Quantitative PCR
Primer pairs and reaction conditions were as previously published.25,28,29 Quantitative PCR analysis (cycling conditions: 1 × 5 minutes initial denaturation at 95°C, 35 × 30 seconds denaturation at 95°C, 30 seconds annealing at 60°C, 30 seconds extension at 60°C, and 1× final 0.5°C stepped temperature ramp 60°C-95°C) of cytokine and chemokine expression was performed on a Corbett Robotics Rotorgene 3000 using 2× power SYBR master mix (Life Technologies) in a final volume of 20 μL. All reactions were performed in triplicate. Primer specificity was confirmed by the presence of a stable, single peak in melt curve analyses and an absence of amplification in no-template controls. Primer efficiency (E) for each amplicon pair was calculated for amplification using cDNA generated from cultured keratinocytes and fetal skin using the log-linear slope (R2 ≥ .98) of fluorescence data from 6 randomly selected replicates (18 reactions in total). Calculated E values (keratinocytes/fetal skin) for each primer pair were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) 101%/94%; IL-1β 99%/92%; IL-8 96/92%; MCP-1 100%/92%; TNF-α 96%/92%; IL-10 97%/91%; and IL-6 90% (IL-6 analysis not performed using keratinocyte cDNA). Averaged Cq values for each target amplicon were normalized against averaged GAPDH Cq values. One-way analysis of variance (ANOVA) was used to compare individual replicate efficiencies for each amplicon. For each cDNA source, no significant differences (P > .05) were detected between amplicon efficiencies; accordingly, data were processed to generate the fold-change estimates using a 2−ΔΔCq method.30
Tissue Immunocytochemistry
Fetal ovine skin was dissected, embedded in optimal cutting temperature compound (OCT), and immediately frozen on dry ice. OCT-embedded skin was transversely orientated and serially sectioned with a Leica CM1900 cryostat to a thickness of 9 μm. Transverse skin sections were snap fixed in 500 μL of a 1:1 solution of ice-cold acetone:methanol, then blocked for 2 hours at room temperature in 1% newborn calf serum (FCS) in PBS containing 0.1% triton X-100. Three sections were analyzed for each animal. For immunofluorescence analysis, primary antibodies against IL-1β, IL-8, and TNF-α (MCA1658, AHP425, and AHP852Z, respectively; ABDSerotec, Kidlington, UK) were diluted 1:250 in PBS containing 1% FCS, 0.1% triton X-100, and applied to fixed sections. After overnight (16 hours) incubation at 4°C, sections were rinsed repeatedly in PBS followed by incubation with secondary antibody: Alexa fluor 488 antimouse/rabbit IgG (Biolegend, London, UK) diluted 1:600 in PBS containing 1% FCS, 0.1% triton X-100, and incubated for 2 hours at room temperature. Sections were rinsed repeatedly in PBS before being mounted using an aqueous mounting medium and sealed with hard-set mounting medium. Staining specificity was demonstrated by the absence of staining in sections prepared without primary antibody. Sections were imaged using a Nikon Eclipse Ti inverted fluorescent microscope set to a 400 ms (IL-8/TNF-α) or 600 ms (IL-1) exposure time at a constant aperture, distribution (greater/less) and staining intensity (greater/less) was assessed relative to control tissues.
Representative images were made following the analysis of 3 sections (per target/per animal) by an investigator blinded to sample groups. Representative images were assessed for staining distribution (greater/less) and staining intensity (greater/less) relative to control tissues. Sections for hemotoxylin and eosin staining were processed and imaged using an Olympus BX-51 bright-field microscope
Statistical Analysis
Analyses were performed using SigmaPlot 11.0 (Systat Software, Erkath, Germany). Assuming no difference in expression between groups, the analysis of apparent changes in transcript expression across 3 or more groups was assessed using 1-way ANOVA. Tukey test was used to perform posthoc analysis to investigate apparent differences between specific groups. A cutoff value of 95% (P < .05) was used to assess statistical significance. Ninety-five percent confidence intervals were constructed about mean fold-change (2⁁−(ddcq)) values to assess the variation of apparent changes in expression, relative to control, for each transcript assessed.
Results
Ureaplasma Infection Screening
All samples taken from control fetuses (exposed only to sterile UP media and sterile saline) tested negative for the presence of UP. Five of 6 samples taken from fetuses exposed to UP for 7 days and 5 of 5 samples taken from fetuses exposed to UP for 69 days tested positive for the presence of UP. No significant difference (P = .514; 1-way ANOVA) was determined in fetal weights following in vivo exposure to UP serovar 3. No significant difference (P = .455; 1-way ANOVA) was detected in UP genomic DNA (gDNA) load between the 7-day and 69-day UP-exposed tissues.
In Vitro Exposure to UP Induces MCP-1 but not Cytokine Expression in Primary Fetal Ovine Keratinocytes
The fidelity of the primary keratinocyte culture methodology employed in this study has previously been validated.25 All quantitative PCR (qPCR) primers were demonstrated to generate consistent, single peaks under the described reaction conditions. Cultures of primary fetal ovine keratinocytes were assessed for cytokine and chemokine expression following exposure to 2 × 107 CFU/mL UP. Of the targets measured, only MCP-1 was found to be increased following UP exposure (Figure 1). No statistically significant increase was identified in the expression of IL-1β, IL-8, TNF-α, or IL-10 (P > .05) at any time point. Relative to control, the expression of MCP-1 was found to be significantly increased at 1 hour (P < .001), 2 hours (P < .001), 4 hours (P = .003), and 6 hours (P = .008) post-exposure, returning to baseline after 8 hours (P = .206) exposure.
Figure 1.
Quantitative PCR analysis of cytokine/chemokine expression in cultured primary keratinocytes exposed to 2 × 107 UP in a time-course experiment at 30 minutes, 1, 2, 4, 6, and 8 hours postexposure. Graph represents mean fold change in infected cells ± SEM normalized to uninfected controls (red line). *Statistically significant increase in transcript expression relative to control. PCR polymerase chain reaction; SEM, standard error of the mean; UP, Ureaplasma parvum.
Acute and Chronic in Utero Exposure to UP Induces Basophilic Influx in the Fetal Ovine Skin
Hemotoxylin and eosin staining of skin from fetuses exposed to UP demonstrated a marked progressive basophilic infiltration (white arrows) of the epidermis and dermis at both 7 days and 69 days post-UP exposure (Figure 2). In contrast, skin from fetuses exposed to sterile media and sterile saline demonstrated a relative absence of basophilic epidermal infiltration. Dermal and epidermal infiltration was most distinct in animals exposed to UP for 69 days.
Figure 2.
Transverse fetal ovine skin sections demonstrating basophilic infiltration of the dermis and epidermis in representative samples taken from control; 7-day UP exposed; or 69-day UP exposed animals. H&E staining of 9 μm frozen sections. Scale bar = 10 μm. Total magnification 400×. H&E indicates hemotoxylin and eosin staining; UP, Ureaplasma parvum.
Cytokine and Chemokine Expression in the Fetal Skin is Dependent upon the Nature and Timing of Proinflammatory Agonist
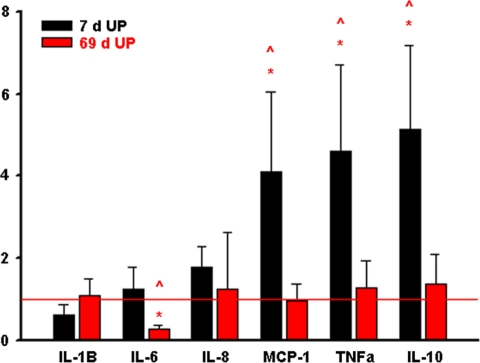
Quantitative PCR analysis of fetal skin from 7-day UP exposure animals demonstrated statistically significant increases in MCP-1 (P = .036), TNF-α (P = .027), and IL-10 (P = .015), relative to control (Figure 3). Interleukin 6 expression was significantly reduced (P = .010) in fetal skin exposed to 69-day UP. No significant change was detected in the expression of IL-1β, IL-8, MCP-1, TNF-α, or IL-10 in fetal skin following the 69-day UP exposure. Compared to the 69-day UP-exposed fetal skin, 7-day UP-exposed fetal skin demonstrated significant increases in IL-6 (P = .010), MCP-1 (P = .024), TNF-α (P = .05), and IL-10 (P = .040). No significant difference was detected between IL-1β (P = .462) and IL-8 (P = .898) messenger RNA (mRNA) expression. Ninety-five percent confidence intervals for changes in transcript expression are shown in Table 1.
Figure 3.
Quantitative PCR analysis of cytokine/chemokine expression in fetal ovine skin exposed UP in utero for either 7 days or 69 days. Bars represent mean fold change (relative to control) ± SEM. *, statistically significant increase in transcript expression relative to control; ⁁, statistically significant difference between 7-day and 69-day UP-exposed tissues. PCR indicates polymerase chain reaction; SEM, standard error of the mean; UP, Ureaplasma parvum.
Table 1.
Quantitative PCR Analysis (Mean Fold Change Relative to Control) of Cytokine/Chemokine Transcript Expression in Fetal Ovine Skin Exposed to UP in Utero for 7 Days or 69 Days
| Group | Transcript | Mean Fold Change | 95% CI− | 95% CI+ |
|---|---|---|---|---|
| 7-Day UP | IL-1β | 0.62 | 0.30 | 1.26 |
| IL-6 | 1.26 | 0.64 | 2.47 | |
| IL-8 | 1.77 | 1.07 | 2.92 | |
| TNF-α | 4.62 | 2.22 | 9.60 | |
| MCP-1 | 4.10 | 1.92 | 8.77 | |
| IL-10 | 5.14 | 2.68 | 9.86 | |
| 69-Day UP | IL-1β | 1.10 | 0.60 | 2.02 |
| IL-6 | 0.28 | 0.17 | 0.46 | |
| IL-8 | 1.24 | 0.28 | 5.388 | |
| TNF-α | 1.28 | 0.57 | 2.88 | |
| MCP-1 | 0.96 | 0.48 | 1.92 | |
| IL-10 | 1.37 | 0.59 | 3.20 |
Abbreviations: UP, Ureaplasma parvum; IL, interleukin; TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein 1; PCR, polymerase chain reaction; CI, confidence interval.
Immunohistochemical analyses of fetal skin for cytokine/chemokine expression after exposure to bacterial agonist demonstrated changes in protein expression in accordance with our qPCR analyses (Figure 4). Fluorescent staining for IL-1β in fetal skin exposed to UP for 7 or 69 days demonstrated equivalent intensity of dermal and epidermal staining relative to control, suggesting no change in protein expression subsequent to UP exposure. Fluorescent staining for IL-8 in fetal skin exposed to UP for 7 and 69 days demonstrated equivalent intensity of dermal and epidermal staining relative to control, suggesting no change in protein expression subsequent to UP exposure. Fluorescent staining for TNF-α demonstrated increased epidermal and dermal staining intensity relative to control, suggesting an increase in protein expression following UP exposure. The greatest increases in staining intensity were identified following 7-day UP exposure.
Figure 4.
Immunohistochemical analysis of cytokine expression in the fetal skin. Alexa 488 secondary immunofluorescence for IL-1β, IL-8, and TNF-α expression in fetal skin from control, 7-day UP, and 69-day UP exposed animals. UP indicates Ureaplasma parvum
Discussion
A growing body of data exists, providing a causal association between infection of the amniotic cavity, in utero inflammation and preterm birth.10,31–33 Ureaplasma species (notably U parvum and U urealyticum) are among the microorganisms most commonly identified in association with preterm birth.16,33–35
Due to the observation that a number of the microorganisms implicated in the etiology of preterm birth are fastidious with respect to nutrient and atmospheric culture conditions, there potentially exists a negative culture bias in the data relating to preterm birth and infection.36 Indeed, there is growing evidence that the utilization of dual culture—PCR-based screening methodologies for amniotic infections—may provide a more accurate picture of infection status than culture alone.36 Irrespective, a number of clinical cohort studies in preterm populations have identified significant associations between positive UP cultures and chorioamnionitis.35 Cases of spontaneous preterm labor were significantly more likely to be culture positive for UP than preterm delivery resulting from medically indicated intervention. Using a molecular approach, Kasper and colleagues recently reported a positive correlation between UP bacterial load and intrauterine inflammation in preterm infants.37
A number of investigators, ourselves included, have employed animal models to demonstrate an association between in utero infection with UP and fetal/uterine inflammation.38–40 Substantive data exist to provide a strong clinical and experimental link between inflammation and preterm birth12,32,34; however, despite these advances in our understanding, significant questions remain in relation to the relative spatial and temporal contributions of fetal and maternal tissues involved in the initiation and propagation of in utero inflammation.
Adult human keratinocytes express a wide array of Toll-like receptors (TLRs) including TLR-1, TLR-2, and TLR-6, which are presently believed to mediate the innate immune system’s response to UP.41 We assessed the ability of primary fetal ovine keratinocytes (the major cellular constituent of the developing epidermis) to respond to the presence of UP over a 12-hour time course. Analysis of mRNA expression demonstrated that exposure to UP increased expression of MCP-1 between 2 and 6 hours postexposure. In striking contrast to our earlier in vitro studies using E coli LPS, no significant increases were detected for IL-1β, IL-6, IL-8, TNF-α, or IL-10 (Figure 1). Monocyte chemoattractant protein 1 (alternatively named CCL-2) is a chemokine demonstrated to possess a wide range of biological and immunomodulatory functions, the most well known of which is as a potent monocyte attractant.42,43 It is expressed by numerous tissues (including endothelial and epithelial cells) in response to cytokines (IL-1, IL-4, and TNF-α) and by exposure to bacterial agonist. Monocyte chemoattractant protein 1 is also involved in numerous biological processes including the promotion of tissue angiogenesis, bone remodelling, and has also been shown to act greatly to increase the permeability of the blood–brain barrier.42
Having first demonstrated that primary fetal ovine keratinocytes respond to UP in a manner substantially different from that induced by E coli LPS exposure, we sought to assess the nature of the response of the developing fetal skin to infection within the amniotic cavity with UP. In concordance with clinical observations, we have demonstrated previously that the ovine fetal skin responds vigorously to direct in utero exposure to E coli LPS, resulting in the epidermal migration of immunocytes and upregulation of key proinflammatory cytokines.25 In the present study, we demonstrate that exposure of the developing fetal skin to intra-amniotic UP infection leads to the development of a proinflammatory response involving upregulation of specific cytokines and chemokines, in conjunction with a vigorous and sustained basophilic migration to both the dermis and the epidermis (Figure 2). The inflammatory response to the 7-day UP exposure was characterized by marked increases in MCP-1, TNF-α, and IL-10. Cytokine/chemokine expression in response to a 69-day UP exposure was significantly reduced in comparison to the 7-day UP-exposure group, with none of the transcripts assessed remaining increased relative to control. We have demonstrated previously that exposure of fetal skin to E coli LPS induces a striking basophilic infiltration. Data presented here demonstrate a prolonged basophilic infiltration of the epidermis and dermis in fetal skin exposed to UP for either 7 or 69 days (Figure 2).
Our data suggest that significant differences exist in the mechanistic induction of inflammation in the fetal epidermis following exposure to UP or E coli. Inflammation resulting from E coli LPS exposure appears to involve the expression of proinflammatory cytokines by the epidermal keratinocytes themselves, whereas inflammation resulting from Ureaplasma exposure may involve invading immunocytes in response to elevated MCP-1 signaling from stimulated keratinocytes in the fetal skin.
We have demonstrated previously that prolonged or repeated exposure to E coli LPS induces endotoxin tolerance in fetal tissues and immunocytes.18,44 This tolerance conveys reduced responses to bacterial agonist and accordingly has significant implications for the preterm infant with respect to immunocompetence and ability to combat nosocomial infections.18,44 Intriguingly, skin from fetuses testing positive for UP after 69 days exposure exhibited significantly lower inflammation that are seen on the skin from 7-day exposures—despite our qPCR analysis demonstrating an equivalent UP gDNA load in each tissue group. These data suggest that the fetal skin develops an immunological tolerance for UP. Recent studies by Peebles and coworkers have suggested that in utero infections caused by multiple microorganisms may be significantly more common than previously thought.45 It will be of great interest to investigate the effect/effects of polymicrobial in utero infection on the ability of the skin and adaptive immune system to respond to the presence of bacterial agonist.
The findings of this study have a number of clinical implications. The fetal skin comprises a significant percentage of total fetal mass and possesses an ample vascular supply and thus has the potential to function as a large source of inflammatory mediators. Although we have demonstrated the fetal skin possess the ability to generate a localized inflammatory response to both E coli LPS and UP, the systemic effects of this inflammation on fetal well-being and development are presently unknown. Additionally, the immature fetal epidermis lacks an outer cornified envelope, potentially allowing inflammatory mediators generated within the fetal skin to cross into the amniotic fluid and interact with other fetal and maternal tissues.
A number of adult inflammatory disorders (ulcerative colitis, arthritis, and cardiovascular disease) are closely associated with the prolonged and inappropriate systemic elevation of inflammatory cytokines, including a number of those shown to be elevated in the present study (TNF-α and MCP-1). Ureaplasma spp are among a number of microorganisms identified in association with preterm birth and there is increasing evidence to suggest that polymicrobial infections of the amniotic fluid are more commonly associated with preterm birth than previously suspected. From a therapeutic perspective, understanding the mechanisms by which microorganisms commonly associated with preterm birth induce in utero inflammation allows for the identification of potential signalling cascade targets which may be able to be manipulated in an attempt to ameliorate in utero inflammation.
The present study has several limitations which need to be taken into consideration; the ability of the developing fetal ovine skin to respond to microorganisms at differing GAs is unknown and potential changes may occur in the viability of UP in utero during a chronic infection. Both factors may impact our analysis of fetal skin exposed to UP for 7 days or 69 days and suggest further investigations in this area are warranted.
Conclusions
In conclusion, this article presents the first experimental data (of which we are aware) to demonstrate the ability of the fetal skin to respond to in utero infection by UP with vigorous basophilic infiltration of the dermis and epidermis along with cytokine and chemokine production. Data from the present and previous studies demonstrate that the nature of the inflammatory response generated by the fetal skin changes depending on the initiating agonist and the length of exposure. On the basis of these data, we conclude (1) the fetal skin’s response to bacterial agonist is predicated upon both the nature and the length of exposure; and (2) primary fetal ovine keratinocytes respond to UP exposure by the production of MCP-1 but not inflammatory cytokines, suggesting that the UP-driven inflammatory response identified in the fetal skin derives from the subsequent production of proinflammatory cytokines and chemokines by invading immunocytes.
Acknowledgments
This work was supported by NHMRC grant 1010315 to JN, grants from the Ramaciotti Foundations (Australia), the Women and Infants Research Foundation (Western Australia), and the Financial Markets Foundation for Children (Australia) to MWK, by NIH grant HD 57869 to SK and by NIH grant HL 97064 to AJ. The authors wish to thank Rob Sprancis and the King Edward Memorial Hospital Histopathology Service for their expert technical assistance.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
References
- 1. Janeway CA, Travers P, Walport M, Shlomchik M. Basic Concepts in Immunobiology. New York: Garland; 2001 [Google Scholar]
- 2. Liggins GC. Cervical ripening as an inflammatory reaction. In: DA Ellwood, ABM Anderson. (Eds.), The Cervix in Pregnancy and Labour, Clinical and Biochemical Investigations. Edinburgh: Churchill Livingstone; 1981:1–9 [Google Scholar]
- 3. Sennstromm MB, Ekman G, Westergren-Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6(4):375–381 [DOI] [PubMed] [Google Scholar]
- 4. Kramer BW, Joshi SN, Moss TJ, et al. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L345–L353 [DOI] [PubMed] [Google Scholar]
- 5. Moss TJM, Knox CL, Kallapur SG, et al. Experimental amniotic fluid infection in sheep: Effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet. Gynecol. 2008;198(1):122.e121–122.e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newnham JP, Shub A, Jobe AH, et al. The effects of intra-amniotic injection of periodontopathic lipopolysaccharides in sheep. Am J Obstet Gynecol. 2005;193(2):313–321 [DOI] [PubMed] [Google Scholar]
- 7. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3-4):117–123 [DOI] [PubMed] [Google Scholar]
- 9. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195(6):1578–1589 [DOI] [PubMed] [Google Scholar]
- 10. Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179(1):194–202 [DOI] [PubMed] [Google Scholar]
- 11. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gomez R, Ghezzi F, Römern R, Yoon BH, Mazor M, Berry SM. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Acta Diabetologica Latina. 1997;176(1 part II). [Google Scholar]
- 14. Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197(5):518.e511–518.e518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171(6):1660–1667 [DOI] [PubMed] [Google Scholar]
- 16. Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17(7):619–628 [DOI] [PubMed] [Google Scholar]
- 17. Jobe AH, Newnham JP, Willet KE, et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162(5):1656–1661 [DOI] [PubMed] [Google Scholar]
- 18. Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171(1):73–77 [DOI] [PubMed] [Google Scholar]
- 19. Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192(4):1179–1186 [DOI] [PubMed] [Google Scholar]
- 20. Moss TJ, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189(6):1771–1776 [DOI] [PubMed] [Google Scholar]
- 21. Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2000;183(1):173–180 [DOI] [PubMed] [Google Scholar]
- 22. Grigsby PL, Novy MJ, Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17(1):85–94 [DOI] [PubMed] [Google Scholar]
- 23. Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194(5):1334–1340 [DOI] [PubMed] [Google Scholar]
- 24. Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol. 2002;186(3):523–530 [DOI] [PubMed] [Google Scholar]
- 25. Kemp MW, Saito M, Nitsos I, Jobe A, Kallapur S, Newnham J. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49(5):506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheah FC, Anderson TP, Darlow BA, Murdoch DR. Comparison of the mycoplasma duo test with PCR for detection of Ureaplasma species in endotracheal aspirates from premature infants. J Clin Microbiol. 2005;43(1):509–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang JS, Russell GC, Jann O, Glass EJ, Werling D, Haig DM. Molecular cloning and characterization of Toll-like receptors 1-10 in sheep. Vet Immunol Immunopathol. 2009;127(1-2):94–105 [DOI] [PubMed] [Google Scholar]
- 29. Sow FB, Gallup JM, Meyerholz DK, Ackermann MR. Gene profiling studies in the neonatal ovine lung show enhancing effects of VEGF on the immune response. Develop Comp Immunol. 2009;33(6):761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622 [DOI] [PubMed] [Google Scholar]
- 31. Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195(3):803–808 [DOI] [PubMed] [Google Scholar]
- 32. Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC. The Alabama Preterm Birth Study: intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am J Obstet Gynecol. 2006;195(6):1533–1537 [DOI] [PubMed] [Google Scholar]
- 33. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldenberg RL, Andrews WW, Goepfert AR, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43 e41–e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol. 2010;37(2):393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38(3):261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kasper DC, Mechtler TP, Reischer GH, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis. 2010;67(2):117–121 [DOI] [PubMed] [Google Scholar]
- 38. Oh KJ, Lee KA, Sohn YK, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203(3):211.e211–211.e218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70 [DOI] [PubMed] [Google Scholar]
- 40. Moss TJM, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol. 2005;192(4):1179–1186 [DOI] [PubMed] [Google Scholar]
- 41. Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29(1):15–26 [DOI] [PubMed] [Google Scholar]
- 42. Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clinica Chimica Acta. 2010;411(21-22):1570–1579 [DOI] [PubMed] [Google Scholar]
- 43. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kramer BW, Joshi SN, Moss TJM, et al. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol—Lung Cell Mol Physiol. 2007;293(2):L345–L353 [DOI] [PubMed] [Google Scholar]
- 45. Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4(12):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]