Abstract
Objectives: This study describes the distribution of dystroglycan (DG) in human placenta, membranes, and uterine decidua. Study design: Dystroglycan expression was characterized by Western blotting, immunohistochemistry, and immunofluorescence microscopy using human tissues and cultured cells. Results: Both α-DG and β-DG are expressed in the term syncytiotrophoblast, and α-DG is localized to the basement membrane. In first-trimester chorionic villi, α-DG and β-DG are localized to the periphery of the cytotrophoblast. Expression varies in term fetal membranes. α-Dystroglycan is not detectable in choriocarcinoma cells or HTR cells, but β-DG is present in both normal and cleaved forms. Conclusion: Dystroglycan is expressed at high levels in human trophoblasts, and localization of the α- and β-subunits varies with gestational age and trophoblast differentiation. Because DG expression inversely correlates with invasiveness in many cancers, its pattern of expression in trophoblasts suggests a possible function in inhibition of placental invasion.
Keywords: dystroglycan, placenta, trophoblast
Introduction
Abnormal placental implantation and development may result in a variety of complications, including preeclampsia, miscarriage, placenta previa, placenta accreta, or fetal growth restriction. Identifying cell adhesion molecules and signaling pathways involved in placental implantation and differentiation may reveal crucial steps necessary for normal development. Ultimately, better understanding of normal placental development could identify drug targets, treatment and prevention of implantation failure, recurrent miscarriage, preterm labor, preeclampsia, or intrauterine growth restriction.
Dystroglycan (DG) is an adhesion molecule that has been shown to connect the epithelial cell and the extracellular matrix (ECM) in many tissues. Dystroglycan is a highly conserved cell-membrane glycoprotein and is a core component of the dystrophin glycoprotein complex (DGC). Dystroglycan is a heterodimer1 of α and β subunits, encoded by the DAG1 gene, located on chromosome 3 band 21. A single messenger RNA (mRNA) encodes the precursor peptide which is then cleaved into the N-terminal α-DG and the C-terminal β-DG.2 The highly glycosylated extracellular α-DG subunit is a peripheral membrane protein, while the β-DG subunit is membrane spanning and connects the actin cytoskeleton to the α-DG subunit. The α-DG subunit binds ECM components such as laminin, perlecan, and agrin.1 α-Dystroglycan has a mucin domain between the N- and C-terminal regions which undergoes O-glycosylation as well as multiple sites for N-linked glycosylation.2 Binding of DG to the ECM is dependent on appropriate glycosylation of α-DG, and this extensive glycosylation of α-DG increases its molecular weight (MW)2 from the predicted 72 kDa to the observed 120 to 200 kDa.
Dystroglycan is localized to the basal lamina in most epithelial cells and has been implicated in proper maturation of postsynaptic neuronal elements.3 Dystroglycan influences cell stability and polarity and facilitates epithelial cell adhesion to the basement membrane.1 Dystroglycan ligand binding has also been proposed as an inhibitory signal for tumor invasion. Dystroglycan has been most extensively studied as it relates to the pathogenesis of muscular dystrophy. Abnormal forms and anomalous associations of DG with other proteins lead to varied types of muscular dystrophies.2 Specifically, atypical DG alters muscle contractility and exposes muscle fibers to injury.2 Secondary dystroglycanopathies are caused by abnormal α-DG glycosylation, which interferes with laminin binding.4
Dystroglycan is thought to be involved in embryogenesis, as DAG1 homozygous knockout is embryonically lethal in mice.5 Abnormal DG has also been implicated in the disruption of neuronal migration in the developing brain.2 Dystroglycan mRNA expression has been identified in mouse decidua 8.5 days postconception, with levels greater than 100-fold those of nonpregnant uterus and mature placenta.6 Mouse embryos that are homozygous for DG mutations dissolve prematurely secondary to lack of basement membrane formation between the maternal and fetal tissues.5,7,8 However, to our knowledge, expression of DG has yet to be investigated in human gestational tissue. We hypothesize that DG is expressed in human placenta and may be important for normal human placental development.
Materials and Methods
Tissue Collection
Placentas from 5 term (between 37 and 40 weeks of gestation) deliveries were obtained from patients at the University of Texas Medical Branch (UTMB) after repeat cesarean delivery as clinically indicated and approved by the Institutional Review Board (IRB). Tissues from elective abortions were obtained from Planned Parenthood Gulf Coast with maternal consent. Samples were obtained from patients who had requested and consented to the termination procedure prior to being approached for donation of tissue for research purposes. The protocol was approved by the IRB at UTMB, as well as by Planned Parenthood Federation of America.
Cell Culture
Both the JAR and JEG choriocarcinoma cell lines and first-trimester trophoblast cell line HTR-SV40Neo were grown and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and penicillin, streptomycin, and amphotericin B. All cells were incubated at 37°C and 5% CO2. Term cytotrophoblasts (CTBs) were isolated from fresh term placenta as previously described and >95% purity verified by immunofluorescent9 staining for cytokeratin 7.
Membrane Isolation
Cells were suspended in lysing buffer (20 mmol/L HEPES, 1.5 mmol/L MgCl2, 2.5 mmol/L EDTA, 2.5 mmol/L ethylene glycol tetraacetic acid (EGTA), and 1× protease inhibitor cocktail [PIC]) and Dounce homogenized. The mixture was sonicated and then centrifuged for 10 minutes at 1500g. The supernatant was centrifuged for 30 minutes at 12 000g and 4°C. The microsomal membrane pellet was suspended in 20 mmol/L HEPES, 1 mmol/L dithiothreitol, 1 mmol/L EGTA, and 1× PIC.
Antibodies
Mouse hybridoma MANDAG2 (clone 7D11), raised against the C-terminal 15 amino acid residues of the β-DG cytoplasmic domain, was obtained from the Iowa Developmental Studies Hybridoma Bank and grown in medium SFM4MAB (Thermo Scientific Hyclone, Logan, UT USA).10 Concentrated tissue culture supernatant was prepared using Amicon concentrators (Millipore, Billerca, MA USA). Anti-α-DG mouse monoclonal antibodies clone VIA4-1 and clone IIH6C4 were purchased from Millipore.10,11
Gel Electrophoresis and Western Blotting
Tissue for Western blotting was homogenized in radioimmunoprecipitation assay buffer (RIPA) buffer in the presence of PIC to extract proteins. Proteins present in the tissue homogenates, cell lysates, and microsomal membranes were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described.12 Comparable protein loading was verified using the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL USA) and by Ponceau stain after transfer to nitrocellulose. Horseradish peroxidase (HRP)-conjugated secondary antibodies were detected by chemiluminescence (Immobilon; Millipore). Peptide N-Glycosidase-F (New England Biolabs, Ipswich, MA USA) digestions were incubated at room temperature prior to gel electrophoresis, according to the manufacturer’s instructions.
Immunohistochemistry
Tissue was collected aseptically after dilation and curettage and placed in 10% neutral-buffered formalin. Immunohistochemistry (IHC) was performed on formalin-fixed term and first-trimester specimens using antibodies recognizing α- and β-DG. Sample preparation and IHC were performed in the Histopathology Special Procedures Laboratory of the UTMB Pathology Department. Sections were deparaffinized, rehydrated, and then underwent antigen retrieval in Target Retrieval Solution (Dako Corporation, Carpinteria, California; Cat #SP1699). Both avidin and biotin from the Avidin Biotin blocking kit (Vector Laboratories, Burlingame, California; Cat # SP2001) were used. Primary antibody was incubated in Labeled Streptavidin Biotin (LSAB) universal secondary antibody (Dako) and then LSAB2 label (Dako) was applied. Slides were counterstained with Harris Hematoxylin. A Nikon Eclipse E400 microscope equipped with Pan Fluor ×40 and Pan ×100 objectives, a DSQi1Mc cooled CCD camera with RGB filters, and NIS Elements BR image capture software was used to obtain images.
Immunoflourescence Microscopy
Cells were cultured on microscope coverslips and fixed with paraformaldehyde. Cells were permeabilized with 1% Triton X-100, and nonspecific interactions were blocked with gelatin. Monoclonal antibody IIH6 and rhodamine-conjugated MANDAG were applied to the cells and incubated. The coverslips were washed and then incubated with DyLight 488-conjugated goat anti-mouse immunoglobulin G (IgG) and 4′,6-diamidino-2-phenylindole (DAPI). Cells were fixed to slides and images were collected using a Zeiss LSM-510 META confocal microscope with a ×63, 1.4 numerical aperture oil immersion objective (Optical Microscopy Core at UTMB). The images were obtained using 2 different lines of excitation (488 and 543 nm) and 2 different channels of emission. After excitation with 488 nm, laser line emission was measured with a 505 to 530 nm filter; and after excitation with 543, laser line emission was measured with a 560 to 615 nm filter. All images were collected using 8-frame-Kallman, averaging with a pixel time of 2.51 μs, a pixel size of 39 nm, and frame size of 512 × 512 pixels and an optical slice of 1 μm.
Results
Dystroglycan is Expressed in Term Placenta and is Localized to the Syncytiotrophoblast
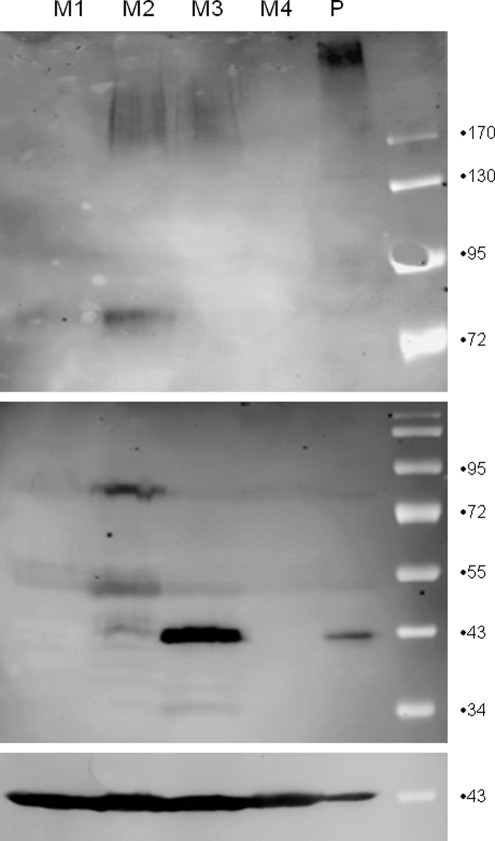
Western blots were performed comparing placenta with fetal membrane samples (Figure 1). α-Dystroglycan was found in all placenta samples. The α-DG in term placenta was highly glycosylated, weighing approximately 120 kDa and appearing as a diffuse band. The MW of β-DG in the untreated placenta and membranes was slightly larger than the expected 43 kDa, but the amount of the high MW and lower MW forms varies among individual participants tested. N-linked glycans were removed from β-DG by PNGase treatment. PNGase-treated placenta and membranes were compared with untreated placenta and membranes by Western blotting (Figure 2). Mobility shifts to 43 kDa in the PNGase-treated specimens, indicating that β-DG is N-glycosylated in these tissues. Immunohistochemistry strongly localizes maturely glycosylated α-DG to the basolateral syncytiotrophoblast (SCTB) but shows β-DG distributed throughout the SCTB in a vesicular pattern (Figure 3A), suggesting that the 2 subunits do not always colocalize. The predominance of DG staining in the trophoblast suggests that DG identified by Western blotting of whole tissue homogenates represents DG originating in the trophoblast.
Figure 1.
Dystroglycan (DG) expression varies in human fetal membranes. Whole tissue protein extracts from fetal membranes of 4 term pregnancies (M1-M4) delivered by repeat cesarean section and a representative term placenta (P) were analyzed by Western blotting with Ab IIH6C4 for expression of α-DG (top panel, 8% gel) and Ab MANDAG2 for detection of β-DG (middle panel, 12% gel), and β-actin (bottom panel, 12% gel). α-Dystroglycan is highly glycosylated, with a mobility greater than 170 kDa in term placenta but does not appear consistently in fetal membranes. β-Dystroglycan appears in varied molecular weight forms in placenta and fetal membranes.
Figure 2.
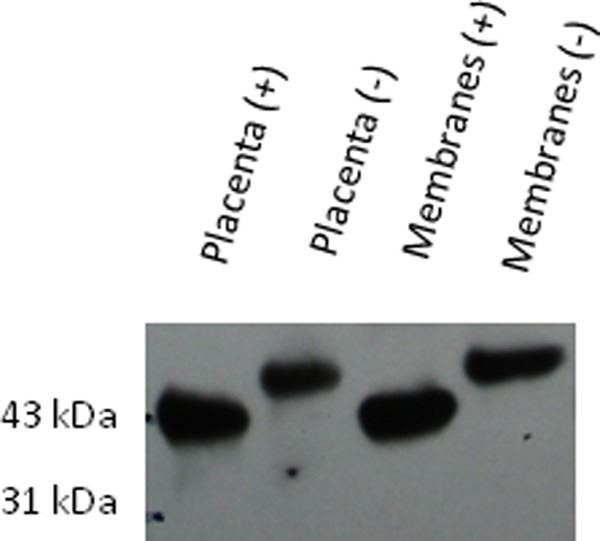
β-Dystroglycan (β-DG) is N-glycosylated in placenta and fetal membranes. Protein extract from human term placenta and fetal membranes was subjected to peptide N glycosidase F (PNGase F) digestion (+) or mock treatment (−) followed by Western blotting with Ab MANDAG2 to detect β-DG. A shift in molecular weight with PNGase treatment indicates the presence of N-glycosylation.
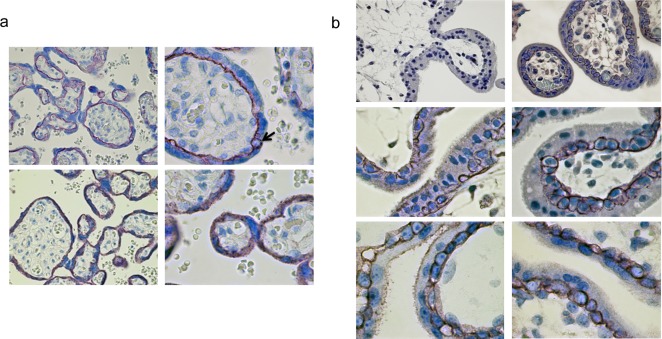
Figure 3.
A, Term placenta: top left, α-DG Ab VIA4-1, ×40 magnification (mag). Top right, α-DG ab VIA4-1, ×100 mag. Bottom left, β-DG Ab MANDAG2, ×40 mag. Bottom right, β-DG Ab MANDAG2, ×100 mag. α-DG is intensely localized to the basolateral SCTB, while β-DG is widely distributed throughout the SCTB. The arrow indicates a CTB. B, First trimester chorionic villi: top left, 12-week EGA normal mouse IgG, ×40 mag. Top right, 12-week EGA α-DG Ab VIA4-1, ×40 mag. Middle left, 12-week EGA α-DG Ab VIA4-1, ×100 mag. Middle right, 6-week EGA α-DG Ab VIA4-1, ×100 mag. Bottom left, 5-week EGA β-DG Ab MANDAG2, ×100 mag. Bottom right, 12-week EGA β-DG Ab MANDAG2, ×100 mag. α-DG and β-DG are localized to the CTB of terminal villi and not present in the SCTB in first-trimester specimens. DG indicates dystroglycan; SCTB, syncytiotrophoblast; CTB, cytotrophoblast; EGA, estimated gestational age; IgG, immunoglobulin.
Expression of α-DG in the Chorion and Amnion Varies From Moderate to Absent at Term
Western blot analysis comparing fetal membranes and placenta was performed to determine the presence of α-DG at term gestation (Figure 1). α-Dystroglycan was found in term placenta in all samples, but its expression was variable in fetal membranes from different participants. Immunohistochemistry of fetal membranes also shows variable expression of α- and β-DG in the chorion (Figure 4), with none detected in the amnion from any participant. No α- or β-DG was identified in isolated amnion samples by Western blotting (n = 3).
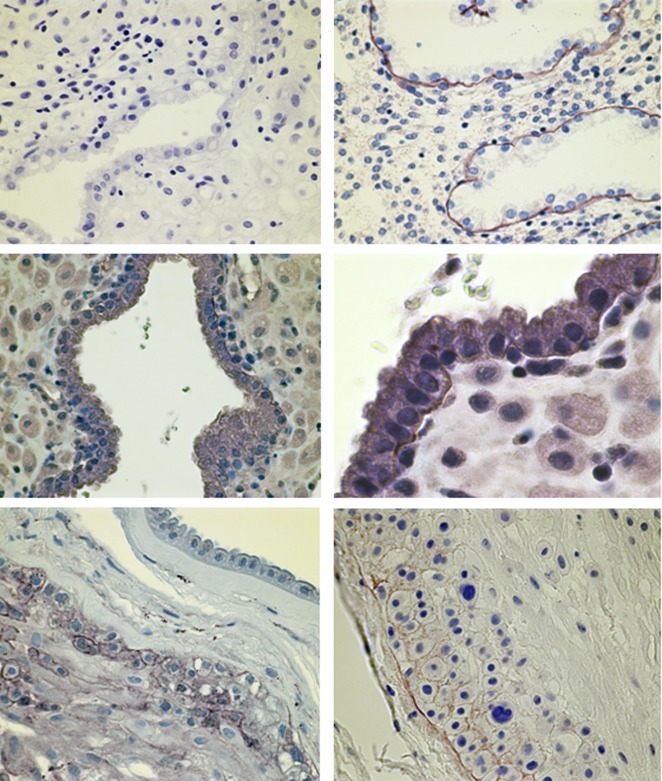
Figure 4.
DG localization in decidua and fetal membranes by immunohistochemistry. Top left, 6-week decidua, negative control mouse IgG, ×40 magnification. Top right, 6-week decidua α-DG Ab VIA4-1, ×40 mag. Middle left, 6-week decidua β-DG Ab MANDAG2, ×40 magnification. Middle right, 6-week decidua β-DG Ab MANDAG2, ×100 magnification. Bottom left, term fetal membranes β-DG Ab MANDAG2, ×40 magnification. Bottom right, term fetal membranes α-DG Ab VIA4-1, ×40 magnification. IHC detected varied expression of both subunits of DG in the chorion but neither subunit was detected in the amnion. DG indicates dystroglycan; IHC, immunohistochemistry.
Dystroglycan is Expressed in First-Trimester Chorionic Villi and Localizes to the CTB
Both subunits of DG are identified in the CTB of terminal villi at 6 weeks and 12 weeks of gestation by IHC (Figure 3B). Distribution is similar for both α- and β-DG, which surround the periphery of the CTB and do not appear to be present in the SCTB in the first trimester. First-trimester chorionic villi contain the 43-kDa form of β-DG but the mature α-DG are not detectable by Western blot analysis with these antibodies (not shown), suggesting that the overall DG protein expression may also be lower in first trimester than in term placenta.
α-Dystroglycan is found throughout gestational endometrial epithelial cells, whereas β-DG is localized to the basement membrane. Figure 4 depicts early first-trimester decidualized endometrium stained with antibodies to α- and β-DG. β-Dystroglycan is limited to the basement membrane (BM) underlying the endometrial epithelium, whereas intense staining with the α-DG antibody can be seen throughout the endometrial epithelium. This again suggests that the 2 subunits do not always colocalize.
Invasive Trophoblasts Do Not Express Cell Membrane-Localized DG
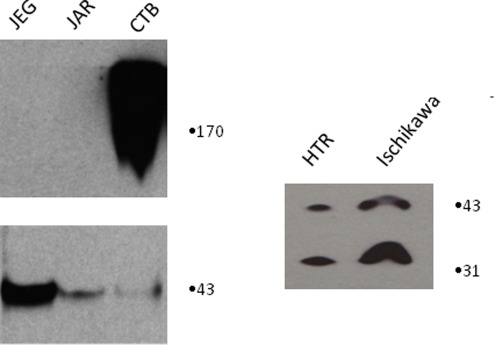
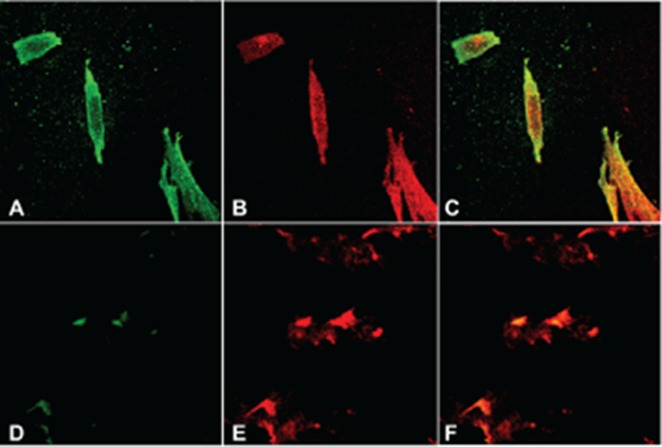
Western blotting of microsomal membranes prepared from choriocarcinoma cell lines JAR and JEG reveals absence of detectable α-DG (Figure 5). Cytotrophoblast, in contrast, contains large amounts α-DG and comparable amounts of β-DG. The invasive first-trimester trophoblast cell line HTRSV40 does not express detectable α-DG but does express both full-length and cleaved β-DG. To confirm the above findings, immunofluorescence (IF) confocal microscopy was used to evaluate the presence and location of α- and β-DG in JEG cells (Figure 6). Both subunits of DG are decreased in JEG as compared with MRC5 human fibroblast cells, and DG is not targeted to the cell membrane in JEG cells.
Figure 5.
Invasive trophoblast cell lines do not express α-DG. Cell lysates from choriocarcinoma cell lines JEG and JAR were compared with CTBs from term placenta, demonstrating the absence of normal α-DG in the invasive cells (top left) and presence of normal α-DG in the CTBs. All 3 contained the 43-kDa β-DG (bottom left). Microsomal membranes from the invasive trophoblast cell line HTR and the endometrial cancer cell line Ischikawa contained both the full length and the cleaved form of β-DG. Neither HTR nor Ischikawa had detectable α-DG (not shown). DG indicates dystroglycan; CTB, cytotrophoblast.
Figure 6.
Abnormal DG expression in choriocarcinoma cells by confocal immunoflourescence microscopy. JEG cells (bottom) were compared to MRC-5 human fibroblast cells (top) stained with VIA4-1 (left), MANDAG2 (center), and merged (right). MRC-5 cells showed normal membrane localized expression of α-DG, which was absent in JEG cells. Similar patterns were observed for HTR and JAR cells (not shown). DG indicates dystroglycan.
Comment
Dystroglycan is an adhesion molecule thought to be involved in murine embryogenesis and epithelial basement membrane formation. We propose that DG has a role in normal human trophoblast invasion and generation of the selectively permeable maternal–fetal barrier. Syncytiotrophoblast are responsible for gas and nutrient exchange as well as for the production of appropriate placental hormones and growth factors.13 The localization of DG to the basolateral aspect of the mature SCTB may be important for establishing a semipermeable basement membrane between the maternal and fetal circulation. By establishing and maintaining a BM underlying the mature SCTB, DG may help regulate maternal–fetal transport of nutrients, drugs, and infectious agents. α-Dystroglycan (α-DG) is also a known receptor for several infectious agents, including the congenital viral pathogen lymphocytic choriomeningitis virus, and its location at the maternal–fetal interface may mediate pathogen transport.14,15
In Figure 3, it appears that β-DG is located throughout the SCTB in term placenta, whereas the α-DG is identified only in the basement membrane. This finding was consistent across all specimens examined and mirrors the opposite finding in decidualized endometrial epithelium (Figure 4). Our data are inconsistent with the general belief that DG exists as a heterodimer localized to the cell membrane.2 Additionally, efforts to coimmunoprecipitate α-DG from term placenta using beads conjugated with mAb MANDAG2 recognizing β-DG were unsuccessful, despite detection of β-DG in the eluate. Possible explanations for this include failure of mAb VIA4-1 to recognize the predominant intracellular forms of α-DG because of immature carbohydrate motifs, causing incomplete overlap with MANDAG2-staining patterns. Alternatively, a portion of β-DG in SCTB may exist independent of α-DG, which would be a novel concept. Under some conditions, α-DG is known to undergo furin cleavage, which releases the glycosylated C-terminal domain for extracellular secretion.16 Should this occur, the retained membrane-bound β-DG may be recycled into endosomal compartments and could account for the intracellular vesicular-staining pattern observed uniquely in SCTB. Matrix metalloprotease (MMP) cleavage of the extracellular domain of β-DG could result in a similar scenario. This hypothesis requires additional investigation.
The β-DG localization also changes from first to third trimester, which could be explained by its role in the early invasive process of placental development. Villous invasion into the maternal myometrium can be too vigorous, leading to placental abnormalities such as accreta, increta, and percreta.16 We hypothesize that normally regulated DG expression inhibits trophoblast invasion, and the pattern of expression observed in chorionic villi and isolated trophoblasts supports this hypothesis. Abnormal DG expression may be involved in placental abnormalities.
We hypothesized that placental pathology deemed more invasive than normal would be associated with decreased amounts of α- and β-DG. Consistent with this, the α-subunit of DG was not found in the choriocarcinoma cell lines JAR and JEG on Western blot analysis. Lack of α-DG in studies of other types of invasive carcinoma cells has been attributed to increased enzymatic activity from MMPs and furin. Singh et al showed increased α-DG after treatment with MMP inhibitors in carcinoma cells.17 One explanation for our observations is that after proteolytic cleavage or incorrect enzymatic processing of α-DG, the monoclonal antibodies commercially available do not recognize their carbohydrate epitopes.16 Therefore, it is plausible that α-DG is present, but in abnormal forms that could not be recognized with the techniques utilized. Interestingly, increased proteolysis is a hallmark of malignant progression and placental invasion.18,19
Our evaluation of β-DG with Western blot showed both the expected 43 kDa band and an alternative 31 kDa band in the JAR choriocarcinoma and HTR invasive trophoblasts. Bozzi et al identified β-DG cleavage by MMP-9 at a specific site between amino acids His-715 and Leu-716, which results in a very stable protein weighing approximately 30 kDa.20 It has been postulated that the truncated version of β-DG is both physiological and pathological.20 Singh et al also found increased amounts of the 43 kDa MW β-DG in carcinoma cells after treatment with MMP inhibitors.17 Comparable decreases of the abnormal 31 kDa were found. We identified only the glycosylated form of β-DG in fetal membranes. Glycosylation is known to protect proteins from proteolytic cleavage and we hypothesize it protects β-DG from MMP processing. Matrix metalloprotease cleavage sites have been identified where the C-terminal domain of alpha DG and the N-terminal domain of β-DG interact.20 The degree of glycosylation may differ in human tissues, thus explaining the possibility of both physiological and pathological differences between forms of β-DG. More studies are needed to investigate the effect of N-glycosylation on susceptibility of β-DG to cleavage by MMP.
Our study is limited by several factors. We did not identify the smaller fragment of β-DG. The truncated version could be a splice variant or an abnormally processed fragment. Further studies are necessary to confirm that the fragment is a result of MMP cleavage. Also, we did not characterize the level of regulation of DG expression. Dystroglycan expression may be the result of differences in transcription, translation, processing, or degradation. The physiologic implication of variable DG expression in fetal membranes is not clear and we have yet to address the function of DG in trophoblast invasion.
Dystroglycan is expressed at high levels in human trophoblasts and localization of the α-DG and β-DG subunits varies with gestational age. We propose that spatial and temporal regulation of DG expression during trophoblast differentiation may be important for the prevention of overly aggressive placental invasion, and further research is planned to evaluate the role of DG in trophoblast invasion.
Acknowledgments
The tissue collection would not have been possible without the collaboration of Planned Parenthood Gulf Coast. Dr Adriana Paulucci-Holthauzen, Optical Microscopy Core at UTMB, was instrumental in obtaining images for our figures. The monoclonal antibody MANDAG2 developed by Glenn E. Morris was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA, USA.
Footnotes
Dr. Theiler is a member of the Bayer pharmaceuticals speakers' bureau. The other authors declared no conflicts of interest with regard to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: supported by National Institutes of Health K12 HD001269 Women’s Reproductive Health Research award.
References
- 1. Weir ML, Muschler J. Dystroglycan: emerging roles in mammary gland function. J Mammary Gland Biol Neoplasia. 2003;8(4):409–419 [DOI] [PubMed] [Google Scholar]
- 2. Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(pt 2):199–207 [DOI] [PubMed] [Google Scholar]
- 3. Haenggi T, Fritschy JM. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue. Cell Mol Life Sci. 2006;63(14):1614–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418(6896):417–422 [DOI] [PubMed] [Google Scholar]
- 5. Williamson RA, Henry MD, Daniels KJ, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6(6):831–841 [DOI] [PubMed] [Google Scholar]
- 6. Yotsumoto S, Fujiwara H, Horton JH, et al. Cloning and expression analyses of mouse dystroglycan gene: specific expression in maternal decidua at the peri-implantation stage. Hum Mol Genet. 1996;5(9):1259–1267 [DOI] [PubMed] [Google Scholar]
- 7. Anderson C, Winder SJ, Borycki AG. Dystroglycan protein distribution coincides with basement membranes and muscle differentiation during mouse embryogenesis. Dev Dyn. 2007;236(9):2627–2635 [DOI] [PubMed] [Google Scholar]
- 8. Henry MD, Williamson RA, Campbell KP. Analysis of the role of dystroglycan in early postimplantation mouse development. Ann N Y Acad Sci. Oct. 1998;857:256–259 [DOI] [PubMed] [Google Scholar]
- 9. Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol Med. 2006;121 (2):203–217 [DOI] [PubMed] [Google Scholar]
- 10. Pereboev AV, Ahmed N, thi Man N, Morris GE. Epitopes in the interacting regions of beta-dystroglycan (PPxY motif) and dystrophin (WW domain). Biochim Biophys Acta. 2001;1527(1-2):54–60 [DOI] [PubMed] [Google Scholar]
- 11. Zhou YW, Thomason DB, Gullberg D, Jarrett HW. Binding of laminin alpha1-chain LG4-5 domain to alpha-dystroglycan causes tyrosine phosphorylation of syntrophin to initiate Rac1 signaling. Biochemistry. 2006;45(7):2042–2052 [DOI] [PubMed] [Google Scholar]
- 12. Theiler RN, Compton T. Characterization of the signal peptide processing and membrane association of human cytomegalovirus glycoprotein O. J Biol Chem. 2001;276(42):39226–39231 [DOI] [PubMed] [Google Scholar]
- 13. van den Brule F, Berndt S, Simon N, et al. Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163–180 [DOI] [PubMed] [Google Scholar]
- 14. Cao W, Henry MD, Borrow P, et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081 [DOI] [PubMed] [Google Scholar]
- 15. Barton LL, Mets MB, Beauchamp CL. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am J Obstet Gynecol. 2002;187(6):1715–1716 [DOI] [PubMed] [Google Scholar]
- 16. Saito F, Saito-Arai Y, Nakamura A, Shimizu T, Matsumura K. Processing and secretion of the N-terminal domain of alpha-dystroglycan in cell culture media. FEBS Lett. 2008;582(3):439–444 [DOI] [PubMed] [Google Scholar]
- 17. Singh J, Itahana Y, Knight-Krajewski S, et al. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 2004;64(17):6152–6159 [DOI] [PubMed] [Google Scholar]
- 18. Losasso C, Di Tommaso F, Sgambato A, et al. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000;484(3):194–198 [DOI] [PubMed] [Google Scholar]
- 19. Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128(3):355–363 [DOI] [PubMed] [Google Scholar]
- 20. Bozzi M, Inzitari R, Sbardell D, et al. Enzymatic processing of beta-dystroglycan recombinant ectodomain by MMP-9: identification of the main cleavage site. IUBMB Life. 2009;61(12):1143–1152 [DOI] [PubMed] [Google Scholar]