Abstract
The majority of patients who present with hepatocellular carcinoma (HCC) are already at an advanced stage, and the tumors are unresectable. Radiotherapy (RT) technology can safely provide focused high-dose irradiation to these patients. A wide spectrum of RT technologiesis currently available, including internal RT consisting of Yttrium-90 (90Y), Iodine-131 (131I) anti-ferritin antibody and Homium-199 (199Ho) and external RT, such as three-dimensional conformal RT, intensity-modulated RT, helical tomotherapy, stereotactic body RT, and image-guided RT. However, it may be difficult for physicians to understand all of the available options and to select the optimal RT treatment. Physicians frequently query radiation oncologists on the practical indications of RT for managing patients with HCC. According to the Korean Liver Cancer Study Group practice guidelines, RT is considered appropriate for unresectable, locally advanced HCC without extrahepatic metastasis, a Child-Pugh class A or B, and tumors that occupy less than two-thirds of the liver with level II evidence. In this review, we discuss the application of various RT modalities based on disease status and the detailed indications for RT according to the Barcelona Clinic Liver Cancer staging system.
Keywords: Hepatocellular carcinoma, Radiotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) represents a critical issue in global health. Cancer statistics list this disease as the third most common cause of cancer-related deaths worldwide.1 According to guidelines of the American Association for the Study of Liver Diseases (AASLD), potentially curative therapies can treat the very early and early stages of the disease. However, less than 30% of HCC patients are detected with the disease in those stages.2 Another 20% of patients with terminal stage HCC receive recommendations for the best supportive treatment. Since HCC is unresectable in the majority of patients at the time of the first diagnosis, patients are often directed to nonsurgical treatments. Physicians have long overlooked radiotherapy (RT) for HCC as radiation might induce fatal hepatic toxicity at doses lower than the therapeutic doses.3,4 However, such limitation has been overcome by recent developments in RT technology involving precise delivery of focused high-dose on partial volume of the liver.5-10 According to the Korean Liver Cancer Study Group (KLCSG) practice guidelines, RT is considered appropriate for unresectable, locally advanced HCC without extrahepatic metastasis, Child-Pugh class A or B, and tumors occupying less than two-thirds of the liver. The evidence level is upgraded from level III in the 2003 version to level II in the 2009 KLCSG guidelines.11,12
In this review, we will first describe the application of various RT modalities by disease status. Then, we will discuss the indications for RT according to the HCC stage. Unlike other cancers, many staging systems are used for HCC, including the Barcelona Clinic Liver Cancer (BCLC) staging,13 tumor-node-metastasis (TNM),14 Okuda,15 Cancer of the Liver Italian Program (CLIP),16 and Japan Integrated Staging (JIS) scoring system.17 In our previous study comparing staging systems, The TNM staging system appears to be the best in predicting the prognosis for HCC patients treated with RT.18 However, our discussion will be based on the BCLC staging system as it currently serves as the basis for treatment decisions.
APPLICATION OF VARIOUS RT MODALITIES
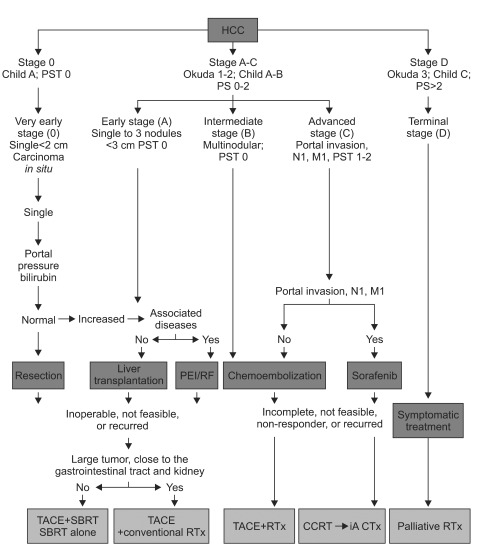
There are several strategies that may be used to deliver radiation to HCC. Currently, a variety of RT modes are available, which range from internal RT such as yttrium-90 (90Y) to external RT such as three-dimensional conformal RT (3D-CRT) and intensity modulated RT (IMRT). While some techniques are machine-dependent and involves protons, a modality which combines IMRT and image guided RT (IGRT) has been emerged for higher precision. However, modification of the fractionation scheme such as hypofractionated RT and stereotactic body RT (SBRT) may also be possible within a typical RT machine. 3D-CRT is the most commonly used platform of RT technology (Fig. 1).19,20
Fig. 1.
The application of various radiotherapy (RT) modalities. The type of RT should be determined according to the Barcelona Clinic Liver Cancer (BCLC) stage and liver function. The use of image-guided technology for the precise delivery of RT, such as intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT), is essential.
3D-CRT, three-dimensional conformal radiotherapy; IGRT, image guided radiotherapy.
INTERNAL RT
RT could be classified to internal and external RT. Internal RT using regional radionuclide therapeutic options are increasingly available for HCC patients and appears promising. Radiolabeled antibodies used by Pressman for radioimmunodetection of tumors showed that iodination with Iodine-131 (131I) did not destroy antibody activity and has resulted in tumor remissions in diverse cancers.21,22 Order et al.23 evaluated the efficacy of 131I anti-ferritin antibody and systemic CTx in treating HCC. Early attempts using monoclonal 131I anti-ferritin antibody were not successful, as outcomes were no better than with chemotherapy.24 Tumor-specificity and tumor-retention remain challenges in radioimmunotherapy.90Y, a pure β-emitter, decays to stable zirconiumi-90 with a physical half-life of 2.7 days. Radioembolization with 90Y represents a novel form of liver-directed brachytherapy.25,26 This approachcanbe applied to unresectable HCC. It also may be used for the treatment of unresectable HCC in patients with branch/partial portal vein thrombosis (PVT). A preliminary safety analysis in 15 patients with unresectable HCC and PVT without cavernous transformation has been reported.27 Kulik et al.28 evaluated phase II study about the safety and clinical benefit of radioembolization in a larger cohort of patients with unresectable HCC complicated by PVT. Homium-199 (199Ho), mostly beta and a little gamma emission with a half-life of 26.8 hours, has also been tried in chitosan complex form either intratumorally or transarterial approach. Percutaneous intratumoral Holmium injection showed excellent tumor control in HCCs with complete response rate 77.5% for tumors smaller than 3 cm and 91.7% for smaller than 2 cm.29 Intraarterial approach also showed promising result in tumors smaller than 5 cm.29,30
EXTERNAL RT
1. 3D-CRT
3D-CRT which involves shaping of the profile of each radiation beam to fit the profile of the target from the beam's eye view (BEV), uses a multi-leaf collimator (MLC) and a various number of beams. For the 3D-CRT, computed tomography (CT) scan images should be taken in the treatment position, and clinical target volume and target volume for RT needs to be delineated. The target is localized by establishing the positions of several optical markers relative to the target volume in a CT simulator.
Several factors should be considered when treating liver tumors with RT. First, the proximity of the liver to other radiosensitive organs should be considered, such as the duodenum, colon, small intestines, and kidneys according to Couinaud's segmentation. Park et al.10 reported that 26 of 47 patients (55.3%) who were irradiated on the right lobe only developed acute morbidity such as nausea and vomiting, and 11 of 12 patients (91.7%) who were irradiated on the left lobe developed acute morbidity. In our previous report on 50 HCC patients treated with 3D-CRT combined with transcatheter arterial chemoembolization (TACE), 7 patients developed gastro-duodenal side effects, 6 patients developed radiation-induced liver disease (RILD). One of them received the treatment for a tumor located in segment 5 of the liver and then developed subacute colitis.19
The second factor involves the liver and tumor movement along with respiration. When applying 3D-CRT without image-guided technology, cephalo-caudal movement of the target organ should be considered. Reducing respiratory motion can be attempted by abdominal compression which can decrease the target margins.
2. IMRT
IMRT, an advanced 3D-CRT, uses non-uniform beam intensity patterns with computer-aided optimization to achieve superior dose distribution.31 As it can change the intensity of individual rays within each beam, IMRT allows greater control of dose distribution and improves the ability to cover the treatment volume to concave tumor shapes. Cheng et al.32 compared dose-volume data between 3D-CRT and IMRT for patients with HCC. They found that IMRT achieved a large dose reduction in the spinal cord and spared the kidneys and stomach. IMRT exerted diverse dosimetric effects on the liver, significantly reducing the normal tissue complication probability (p=0.009), but significantly increasing the mean dose compared with 3D-CRT (p=0.009).
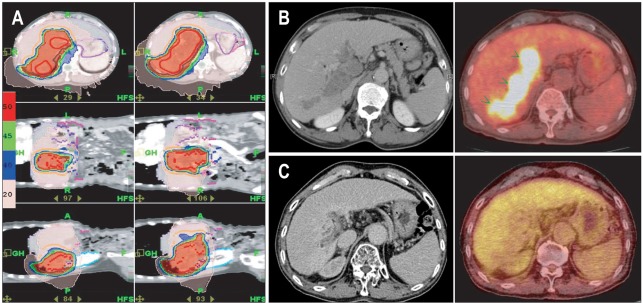
Helical tomotherapy (HT), another kind of fusion technology that combines IMRT and IGRT,33,34 provides better dose coverage for tumors, thanks to its 360° beam arrangement and helical delivery of radiation. Some studies have reported improved sparing of adjacent normal organs when using HT in various tumors, and HT offers increased dose conformity to the tumor and reduces doses delivered to sensitive structures.35,36 For liver tumors, HT could increase the dose conformity to the tumor with PVT and reduce the doses delivered to the non-cancerous parts of the liver. Fig. 2 is a case of 57-year-old man diagnosed HCC with PVT and an underlying chronic B viral hepatitis. He was treated with concurrent intra-arterial chemotherapy and HT (50 Gy/20 fractions) and received intra-arterial chemotherapy for 1 year. The main mass and PTV disappeared and he was followed with no evidence of disease until 30 months after completion of concurrent chemoradiotherapy (CCRT). Although he had recurred on the intrahepatic parenchyma after 30 months, he has been well with the disease after two times of TACE and radiofrequency ablation (RFA) once. In a dose-distribution comparison study of 3D-CRT, linac-based IMRT, and HT, we found that HT decreased high-dose radiation to certain critical structures like the stomach, whereas the mean hepatic dose increased.37
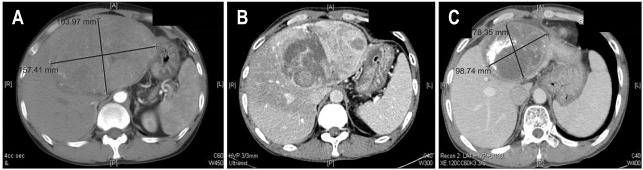
Fig. 2.
The case of a 51-year-old man diagnosed with a huge left hepatocellular carcinoma (15 cm) and portal vein thrombosis. He was treated with concurrent intra-arterial chemoradiotherapy and received 6 cycles of intra-arterial chemotherapy. The patient underwent a left lobectomy 15 months after treatment and showed complete pathologic remission. (A) The computed tomography images at the initial presentation, (B) after 1 month, and (C) showing a partial response after 15 months.
3. SBRT
SBRT offers a technique designed to very precisely deliver radiation to tumors anywhere in the body. The word "stereotactic" pertains to the precise positioning of a tumor in relationship to the body. The technology used in SBRT allows the delivery of external beam radiation with pinpoint accuracy. Such advancement in the accuracy of radiation treatments allows the delivery of higher doses of radiation, thus potentially improving the likelihood of killing cancer cells of a tumor. Another benefit to improved accuracy means that treatments require less time. Typically, SBRT consists of three to five treatments which are carried out over one to two weeks. The precision associated with SBRT simultaneously helps reduce the dose of radiation to normal tissue around a tumor, thus helping to reduce side effects for patients. Many studies, including the present one, have shown a dose-response relationship between conventional RT doses and responses in HCC.38,39 Seo et al.40 reported on the toxicity and efficacy of SBRT for the treatment of localized HCC in the absence of another standard treatment option. They administered SBRT dosages (33 to 57 Gy in three or four fractions) according to tumor volumes (median, 40.5 mL). They reported 2-year overall survival as 61.4%, local progression-free survival rates 66.4%, and a local response rate 63% at 3 months after SBRT. They found a high radiation dose independently related to survival. Furthermore, they reported a decline in liver function in six patients (16%) and Grade 3 musculoskeletal toxicity in one patient (2.7%). They suggested SBRT technique as a salvage treatment. Louis et al.41 evaluated 25 HCC patients who were not eligible for other modalities. A total dose of 45 Gy in three fractions of 15 Gy each was prescribed to the 80% isodose line (95% of the PTV received 45 Gy) and delivered to the target volume over 10 to 12 days. The actuarial 1- and 2-year local control rate was 95%. Overall 1- and 2-year actuarial survival rates were 79% and 52%, respectively.41 Several prospective studies have been conducted. Tse et al.42 reported outcomes of a phase I study of individualized SBRT for unresectable HCC and intrahepatic cholangiocarcinoma (IHC). The patients were treated with six-fraction SBRT during 2 weeks. Median survival of HCC and IHC patients was 11.7 months and 15.0 months, respectively.42 A Phase I dose escalation trial of SBRT for primary HCC at Indiana University showed one and 2-year overall survivals of 75% and 60%, respectively.43
4. IGRT
IGRT indicates the process of frequent two- and three-dimensional imaging during a course of radiation treatment to check physical uncertainties related to setup variation, organ movement, and tissue deformation. Target localization systems control such uncertainties, and the tools of images for IGRT include kilovoltage radiograph fluoroscopy, conebeam CT (CBCT), and megavoltage CT (MVCT). It is essential to use IGRT for precise RT such as IMRT and SBRT. SBRT entails the stereotactic delivery of ablative doses of radiation to a target/tumor volume, and it typically uses very tight margins to minimizes collateral damage to critical structures and organs. Therefore, a robust immobilization device is crucial to ensure a reproducibly accurate set-up, allowing a tighter margin expansion for planning treatment volume. Furthermore, tumors in the liver are subject to respiratory motion, which must be controlled and accounted for during CT simulation, treatment planning, and treatment delivery.44,45
PARTICLE BEAM THERAPY
1. Proton therapy
Proton therapy, a type of positively charged particle therapy, has a unique dose distribution that makes it suitable for treatment of deep tumors surrounded by normal structures, thanks to the unique physical characteristics of the depth-dose curve. Photon depth-dose curves show an exponentially decreasing energy deposition with increasing depth in tissue after a short build-up. By contrast, protons particles deposit their radiation energy as they slow down, and show a dose peak (Bragg peak) at a well-defined depth in tissue. Consequently, this results in no exit dose. This has advantages such as a lower dose in the entrance region than the dose delivered to the tumor regions even when using a single treatment angle. The second advantage, owing to the finite range and sharp distal fall-off, a radiation dose can be directed to a critical structure. Several authors reviewed clinical outcomes of HCC patients treated with proton therapy.46-48 The literature includes two prospective phase II studies on proton therapy for HCC. Bush et al.49 performed a study with 34 patients with locally unresectable HCC. The total dose included 63 cobalt Gy equivalents, administered in 15 fractions over 3 weeks. Three patients experienced duodenal or colonic bleeding. A 2-year actuarial local control rate of the treatment was 75%, and an overall survival rate was 55%.49 Kawashima et al.50 perfromed a phase II study of proton therapy for HCC patients, and reported a 2-year actuarial local progression-free rate of 96% and a 2-year actuarial overall survival rate of 66%. However, there are limited data regarding the efficacy of this treatment on HCC.
RT ACCORDING TO BCLC STAGE
The BCLC staging system, which is endorsed by the AASLD and European Association of Study of Liver (EASL), provides both tumor staging and treatment guidelines. For early stage HCCs, for example, resection, liver transplantation, or percutaneous ablation is recommended. Patients in intermediate or advanced stages are considered for palliation.
1. BCLC stage 0 or A
For small, solitary HCC lesions with BCLC stage 0 or A, the first treatment choice is local ablation such as RFA or percutaneous ethanol injection therapy (PEIT). However, the areas right below the hepatic dome and adjacent to the main portal vein are particularly susceptible to complications with other local ablation therapies. When these options are limited by technical difficulties in patients who are inoperable or refuse surgery, TACE has been widely used in Asian countries.51-54 Recently, there are several reports that SBRT also can be an appropriate alternative or adjuvant (Fig. 1).42,55,56 Andolino et al.56 evaluated the safety and efficacy of SBRT for the treatment of primary HCC. Sixty patients with liver-confined HCC were treated with SBRT and the median follow-up time was 27 months. The 2-year local control, progression-free survival, and overall survival were 90%, 48%, and 67%, respectively, with median time to progression of 47.8 months. They showed SBRT is a safe, effective, noninvasive option for patients with HCC ≤6 cm. Authors also suggested that SBRT should be considered when bridging to transplant or as definitive therapy for those ineligible for transplant. Takeda et al.57 recommended combination therapy of TACE plus SBRT for solitary tumors distant from the gastrointestinal tract and kidneys with a tumor volume <100 cm3. Large tumors or tumors close to adjacent radiosensitive organs should be treated with conventional or precise RT such as IMRT. Table 1 summarized the clinical results of RT according to BCLC stages.
Table 1.
A Summary of the Definition of BCLC Stages and the Results of Radiotherapy
BCLC, Barcelona Clinic Liver Cancer; PST, performance status; SBRT, stereotactic body radiotherapy; TACE, transcatheter arterial embolization; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; iA CTx, intra-arterial chemotherapy.
2. BCLC stage B
For BCLC B, TACE is recommended. The efficacy of TACE has been reported for enhancing survival of patients with BCLC in intermediate stage.52,58,59 However, the effect of TACE limited by vascular shunting, recanalization around the tumor capsule, as well as development of multiple feeding vessels.60,61 TACE was repeatedly performed to overcome the limitation. However, it frequently results in outgrowth of HCCs refractory to TACE. Instead, RT can provide a complementary effect.
The effect of RT in addition to TACE has been investigated by comparing TACE followed by RT vs TACE only or repeated TACE. The result showed a significant improvement in overall survival with TACE combined with RT. Shim et al.9 used RT following incomplete responses to TACE and reported response rates greater than 60%. Of 73 patients with HCC with an incomplete response to TACE, 38 patients received RT and 35 received repeated TACE alone. Patients treated with RT showed a significant improvement in 2-year survival rate (37% vs 14% for TACE plus RT vs TACE alone, p=0.001). The survival difference was greater in patients with large tumors; 2-year survival rates in TACE plus RT versus TACE alone were 63% vs 42% in 5 to 7 cm tumors, 50% vs 0% in 8 to 10 cm tumors, and 17% vs 0% in tumors larger than 10 cm, respectively. Other investigators have reported a similar range of response rates with TACE followed by RT.62-65
For multiple nodular lesions, repeated TACE is a common treatment option. However, with an increasing number of TACE procedures, incomplete TACE triggers tumor hypoxia, subsequently resulting in HCC either refractory to the treatment or facilitating intra- or extra hepatic metastasis. For focal HCC, delivery of concurrent RT can improve local control. In patients presenting with a large tumor and multiple small nodules, TACE can effectively control the small lesions, while RT could be used to target the largest lesion. Koom et al.66 questioned the usefulness of local RT in multifocal HCC. In their report, patients with viable intrahepatic tumors not targeted with RT had worse survival that those treated with targeted RT; in patients with intrahepatic tumors treated with TACE but without targeted RT, outcomes were comparable to patients with a single tumor.
3. BCLC stage C
BCLC stage C represents a variety of disease spectrum, including metastasis, portal vein invasion, and performance 0 to 2. In this stage, sorafenib is suggested as the standard of care. Sorafenib is an orally-active inhibitor of multiple tyrosine kinases including vascular endothelial growth factor receptor and Raf/mitogen-activated protein kinase. The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) and Asia-Pacific SHARP trial found that treatment of HCC patients with sorafenib resulted in improved overall survival. However, the gain in survival is modest and new treatment strategies are still needed.67,68 In a radiation oncologist's view, BCLC stage C with portal vein invasion or lymph node involvement could be treated with RT when the tumor does not respond to sorafenib or chemoembolization or shows progression (Fig. 3).69 The KLCSG practice guidelines suggest RT for locally advanced tumors with evidence of level II. Locally advanced HCC which is considered not amenable to surgical resection or immediate liver transplantation, should be locally advanced as defined by the BCLC intermediate stage (B) or the BCLC advanced stage (C) without extrahepatic spread, except regional lymph node involvement.70
Fig. 3.
Radiotherapy according to the Barcelona Clinic Liver Cancer stage. Inoperable, not feasible for curative treatment, or locally recurring hepatocellular carcinoma (HCC) can be managed using radiotherapy. Solitary tumors distant from the gastrointestinal tract and kidneys with a tumor volume <100 cm3 are eligible for stereotactic body radiotherapy (SBRT).
PST, performance status; PEI, percutaneous ethanol injection; RF, radiofrequency; TACE, transcatheter arterial embolization; CCRT, concurrent chemoradiotherapy; iA CTx, intraarterial chemotherapy.
For patients with PVT, several studies reported promising response level of the 3D-CRT.71,72 Objective response ranged from 37.5% to 50%.73,74 Kim et al.75 reported that RT induced 45.8% objective response with a median survival time of 10.7 months in responders and 5.3 months in non-responders for PVT in patients with HCC. They found a dose-response relationship between the RT dose and PVT response. RT for advanced HCC may be used in combination with systemic agents. Han et al.76 reported localized CCRT followed by hepatic arterial infusion chemotherapy (HAIC) in patients with locally advanced HCC, PVT, and good reserve liver function. They observed an objective response in 18 of 40 patients (45%) and an actuarial 3-year overall survival rate of 24.1%. The same group updated the treatment outcomes in 101 patients, leading to a median survival of 16.7 months. In selected patients, CCRT can convert unresectable HCCs to resectable ones. In our institute, among 156 unresectable HCC patients who received CCRT, 14 patients (9%) underwent hepatic resection (Fig. 4). RT directed to the PVT area has been reported an objective response rate of 37.5% to 71.4%, with a median survival time of 6.7 to 10.7 months.71,73,74,77 Lin et al.77 analyzed the recanalization rate of PVT and treatment toxicity after SBRT or 3D-CRT in 14 patients in a prospective study with a total of 43 patients. The crude response rate was 79%, and the median survival time was 6.0 for the SRT group and 6.7 months for 3D-CRT group.
Fig. 4.
The case of a 57-year-old man diagnosed with hepatocellular carcinoma and portal vein thrombosis. He was treated with concurrent intra-arterial chemotherapy and helical tomotherapy (HT) (50 Gy/20 fractions) and received intra-arterial chemotherapy for 1 year. (A) Axial, coronal, and sagittal isodose distribution of HT, (B) Positron emission tomography-computed tomography (PET-CT) images before HT, (C) PET-CT images 2 years after HT.
To determine the scope of the radiation field, our institute recommends that the field should cover the primary gross tumor, including PVT. Since this group of patients had already advanced disease, a high risk for failure can easily be expected. In our retrospective study of 161 HCC patients treated by CCRT through hepatic arterial infusion, several factors were identified for predicting treatment failure. The pretreatment AFP ≥500 ng/mL was a significant factor influencing intrahepatic-outfield and extrahepatic failure, and <55 years age at diagnosis increased the incidence of extrahepatic failure. The previous treatment before CCRT was associated with infield failure.78
4. BCLC stage D
Patients with HCC in terminal stage D need full symptomatic palliation for local disease or distant metastasis. Palliative RT is indicated for metastasis to lymph node, bone, brain, or other sites. For lymph node metastases from HCC, Yoon et al.79 suggested that RT doses of 45 Gy or higher to achieve a significant response. Seong et al.80 reported an overall response rate of 79.5% in 39 patients. The response rate was 87.5% in patients receiving ≥40 Gy10 (biologically effective dose, alpha/beta=10) and 42.9% in patients receiving <40 Gy10 (p=0.02). Responders had a median survival time of 10 months, and non-responders had a response rate of 6 months (p=0.01). For bone metastasis, RT showed complete pain relief in 50% of patients and partial pain relief in 80% to 90%. Nakamura et al.81 evaluated the therapeutic effects of RT on spinal metastases from HCC. They reported the ambulatory rate of 85% after 3 months and 63% after 6 months, and the local progression-free rate was 53% after 3 months and 47% after 6 months. Brain metastasis from HCC is extremely rare. Choi et al.82 carried out a retrospective review of 62 patients. Seventeen of them were treated with whole-brain radiation therapy (WBRT) alone, 10 others with gamma knife surgery alone, 6 patients surgical resection only, and 5 patients with surgical resection followed by WBRT. The median survival time was 6.8 weeks (95% confidence interval, 3.8 to 9.8 weeks) since diagnosis of brain metastasis. Treatment modality, number of brain lesions, and Child-Pugh classification represented significant prognostic factors for survival.
SUMMARY AND CONCLUSION
As discussed in the review, RT can be a useful therapy for tumors in various stages according to the BCLC system. It can serve as a nonsurgical curative therapy for stage 0 or A. It also can be combined with other treatments such as TACE for stage B. For stage C, RT in combination treatment can prolong the survival time in selected patients who present locally advanced HCC associated with portal vein invasion but not distant metastasis. For patients with stage D tumors, RT can provide effective palliation.
A variety of new RT machines are currently available, which could make it difficult for physicians when determining their choice of treatment. Although 3D-CRT has been the standard mode, it is highly recommended to use a precision RT technology involving intensity modulation as well as image-guided one. In particular, IGRT is an essential component of the advanced RT process.
However, the superiority of these sophisticated technologies has not been proven in terms of survival benefits yet.48,83 Further clinical study in the radiation treatment of HCC is necessary to confirm its role in multidisciplinary management of HCC.
ACKNOWLEDGEMENTS
This work was supported by a National R&D Program grant for cancer control, Ministry of Health and Welfare (0620390).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M Practice Guidelines Committee; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Cochrane AM, Murray-Lyon IM, Brinkley DM, Williams R. Quadruple chemotherapy versus radiotherapy in treatment of primary hepatocellular carcinoma. Cancer. 1977;40:609–614. doi: 10.1002/1097-0142(197708)40:2<609::aid-cncr2820400203>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 5.Lawrence TS, Tesser RJ, ten Haken RK. An application of dose volume histograms to the treatment of intrahepatic malignancies with radiation therapy. Int J Radiat Oncol Biol Phys. 1990;19:1041–1047. doi: 10.1016/0360-3016(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JM, McGinn CJ, Walker S, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39:1087–1092. doi: 10.1016/s0360-3016(97)00550-6. [DOI] [PubMed] [Google Scholar]
- 8.Seong J, Keum KC, Han KH, et al. Combined transcatheter arterial chemoembolization and local radiotherapy of unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:393–397. doi: 10.1016/s0360-3016(98)00415-5. [DOI] [PubMed] [Google Scholar]
- 9.Shim SJ, Seong J, Han KH, et al. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Park JW Korean Liver Cancer Study Group and National Cancer Center. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. [PubMed] [Google Scholar]
- 12.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 14.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 17.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 18.Seong J, Shim SJ, Lee IJ, et al. Evaluation of the prognostic value of Okuda, Cancer of the Liver Italian Program, and Japan Integrated Staging systems for hepatocellular carcinoma patients undergoing radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1037–1042. doi: 10.1016/j.ijrobp.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Seong J, Park HC, Han KH, et al. Clinical results of 3-dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma in the cirrhotic patients. Hepatol Res. 2003;27:30–35. doi: 10.1016/s1386-6346(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 20.Cheng SH, Lin YM, Chuang VP, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 21.Pressman D, Day ED, Blau M. The use of paired labeling in the determination of tumor-localizing antibodies. Cancer Res. 1957;17:845–850. [PubMed] [Google Scholar]
- 22.Tang ZY, Liu KD, Bao YM, et al. Radioimmunotherapy in the multimodality treatment of hepatocellular carcinoma with reference to second-look resection. Cancer. 1990;65:211–215. doi: 10.1002/1097-0142(19900115)65:2<211::aid-cncr2820650205>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Order SE, Stillwagon GB, Klein JL, et al. Iodine 131 antiferritin, a new treatment modality in hepatoma: a Radiation Therapy Oncology Group study. J Clin Oncol. 1985;3:1573–1582. doi: 10.1200/JCO.1985.3.12.1573. [DOI] [PubMed] [Google Scholar]
- 24.Order S, Pajak T, Leibel S, et al. A randomized prospective trial comparing full dose chemotherapy to 131I antiferritin: an RTOG study. Int J Radiat Oncol Biol Phys. 1991;20:953–963. doi: 10.1016/0360-3016(91)90191-6. [DOI] [PubMed] [Google Scholar]
- 25.Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10(2 Suppl 1):S107–S110. doi: 10.1002/lt.20036. [DOI] [PubMed] [Google Scholar]
- 26.Geschwind JF, Salem R, Carr BI, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S194–S205. doi: 10.1053/j.gastro.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15:335–345. doi: 10.1097/01.rvi.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- 28.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Han KH, Lee JT, et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin Cancer Res. 2006;12:543–548. doi: 10.1158/1078-0432.CCR-05-1730. [DOI] [PubMed] [Google Scholar]
- 30.Sohn JH, Choi HJ, Lee JT, et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology. 2009;76:1–9. doi: 10.1159/000173735. [DOI] [PubMed] [Google Scholar]
- 31.Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001;51:880–914. doi: 10.1016/s0360-3016(01)01749-7. [DOI] [PubMed] [Google Scholar]
- 32.Cheng JC, Wu JK, Huang CM, et al. Dosimetric analysis and comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy for patients with hepatocellular carcinoma and radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2003;56:229–234. doi: 10.1016/s0360-3016(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 33.Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 34.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 35.Cattaneo GM, Dell'oca I, Broggi S, et al. Treatment planning comparison between conformal radiotherapy and helical tomotherapy in the case of locally advanced-stage NSCLC. Radiother Oncol. 2008;88:310–318. doi: 10.1016/j.radonc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–596. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 37.Lee IJ, Seong J, Koom WS, et al. Selection of the optimal radiotherapy technique for locally advanced hepatocellular carcinoma. Jpn J Clin Oncol. 2011;41:882–889. doi: 10.1093/jjco/hyr053. [DOI] [PubMed] [Google Scholar]
- 38.Park HC, Seong J, Han KH, et al. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 39.Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 40.Seo YS, Kim MS, Yoo SY, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 41.Louis C, Dewas S, Mirabel X, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9:479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 42.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 43.Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 44.Cárdenes HR. Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clin Transl Oncol. 2009;11:276–283. doi: 10.1007/s12094-009-0355-5. [DOI] [PubMed] [Google Scholar]
- 45.Dawson LA, Eccles C, Bissonnette JP, Brock KK. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys. 2005;62:1247–1252. doi: 10.1016/j.ijrobp.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 46.Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799–3805. doi: 10.1158/1078-0432.CCR-04-1350. [DOI] [PubMed] [Google Scholar]
- 47.Hata M, Tokuuye K, Sugahara S, et al. Proton beam therapy for hepatocellular carcinoma with limited treatment options. Cancer. 2006;107:591–598. doi: 10.1002/cncr.22039. [DOI] [PubMed] [Google Scholar]
- 48.Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 49.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127(5 Suppl 1):S189–S193. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 50.Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 51.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 52.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 53.Matsui O, Kadoya M, Yoshikawa J, et al. Small hepatocellular carcinoma: treatment with subsegmental transcatheter arterial embolization. Radiology. 1993;188:79–83. doi: 10.1148/radiology.188.1.8390073. [DOI] [PubMed] [Google Scholar]
- 54.Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18:365–376. doi: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Son SH, Choi BO, Ryu MR, et al. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073–1080. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Takeda A, Takahashi M, Kunieda E, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: preliminary results for efficacy and toxicity. Hepatol Res. 2008;38:60–69. doi: 10.1111/j.1872-034X.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- 58.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 59.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 60.Sakurai M, Okamura J, Kuroda C. Transcatheter chemo-embolization effective for treating hepatocellular carcinoma. A histopathologic study. Cancer. 1984;54:387–392. doi: 10.1002/1097-0142(19840801)54:3<387::aid-cncr2820540303>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 61.Yu YQ, Xu DB, Zhou XD, et al. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62–65. doi: 10.1002/1097-0142(19930101)71:1<62::aid-cncr2820710111>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Yasuda S, Ito H, Yoshikawa M, et al. Radiotherapy for large hepatocellular carcinoma combined with transcatheter arterial embolization and percutaneous ethanol injection therapy. Int J Oncol. 1999;15:467–473. [PubMed] [Google Scholar]
- 63.Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73:1091–1097. doi: 10.1259/bjr.73.874.11271902. [DOI] [PubMed] [Google Scholar]
- 64.Chia-Hsien Cheng J, Chuang VP, Cheng SH, et al. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer. 2001;96:243–252. doi: 10.1002/ijc.1022. [DOI] [PubMed] [Google Scholar]
- 65.Zeng ZC, Tang ZY, Fan J, et al. A comparison of chemoembolization combination with and without radiotherapy for unresectable hepatocellular carcinoma. Cancer J. 2004;10:307–316. doi: 10.1097/00130404-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Koom WS, Seong J, Han KH, Lee do Y, Lee JT. Is local radiotherapy still valuable for patients with multiple intrahepatic hepatocellular carcinomas? Int J Radiat Oncol Biol Phys. 2010;77:1433–1440. doi: 10.1016/j.ijrobp.2009.07.1676. [DOI] [PubMed] [Google Scholar]
- 67.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 68.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 69.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 70.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 71.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 72.Zeng ZC, Fan J, Tang ZY, et al. A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. 2005;61:432–443. doi: 10.1016/j.ijrobp.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 73.Tazawa J, Maeda M, Sakai Y, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660–665. doi: 10.1046/j.1440-1746.2001.02496.x. [DOI] [PubMed] [Google Scholar]
- 74.Ishikura S, Ogino T, Furuse J, et al. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189–193. doi: 10.1097/00000421-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 75.Kim DY, Park W, Lim DH, et al. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419–2426. doi: 10.1002/cncr.21043. [DOI] [PubMed] [Google Scholar]
- 76.Han KH, Seong J, Kim JK, et al. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008;113:995–1003. doi: 10.1002/cncr.23684. [DOI] [PubMed] [Google Scholar]
- 77.Lin CS, Jen YM, Chiu SY, et al. Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three-dimensional conformal radiotherapy. Jpn J Clin Oncol. 2006;36:212–217. doi: 10.1093/jjco/hyl006. [DOI] [PubMed] [Google Scholar]
- 78.Cha HJ, Seong JS, Yoon HI, Pyun H, Koom WS. Clinical factors related to recurrence after hepatic arterial concurrent chemoradiotherapy for locally advanced hepatocellular carcinoma. Korean J Hepatol. 2011;17:S39. doi: 10.1093/jrr/rrt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoon SM, Kim JH, Choi EK, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004;36:79–84. doi: 10.4143/crt.2004.36.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 81.Nakamura N, Igaki H, Yamashita H, et al. A retrospective study of radiotherapy for spinal bone metastases from hepatocellular carcinoma (HCC) Jpn J Clin Oncol. 2007;37:38–43. doi: 10.1093/jjco/hyl128. [DOI] [PubMed] [Google Scholar]
- 82.Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome: brain metastasis from HCC. J Neurooncol. 2009;91:307–313. doi: 10.1007/s11060-008-9713-3. [DOI] [PubMed] [Google Scholar]
- 83.McIntosh A, Hagspiel KD, Al-Osaimi AM, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. 2009;115:5117–5125. doi: 10.1002/cncr.24552. [DOI] [PubMed] [Google Scholar]