Abstract
Diseases of the gallbladder are common and costly. The best epidemiological screening method to accurately determine point prevalence of gallstone disease is ultrasonography. Many risk factors for cholesterol gallstone formation are not modifiable such as ethnic background, increasing age, female gender and family history or genetics. Conversely, the modifiable risks for cholesterol gallstones are obesity, rapid weight loss and a sedentary lifestyle. The rising epidemic of obesity and the metabolic syndrome predicts an escalation of cholesterol gallstone frequency. Risk factors for biliary sludge include pregnancy, drugs like ceftiaxone, octreotide and thiazide diuretics, and total parenteral nutrition or fasting. Diseases like cirrhosis, chronic hemolysis and ileal Crohn's disease are risk factors for black pigment stones. Gallstone disease in childhood, once considered rare, has become increasingly recognized with similar risk factors as those in adults, particularly obesity. Gallbladder cancer is uncommon in developed countries. In the U.S., it accounts for only ~ 5,000 cases per year. Elsewhere, high incidence rates occur in North and South American Indians. Other than ethnicity and female gender, additional risk factors for gallbladder cancer include cholelithiasis, advancing age, chronic inflammatory conditions affecting the gallbladder, congenital biliary abnormalities, and diagnostic confusion over gallbladder polyps.
Keywords: Gallstones, Cholecystectomy, Gallbladder polyps, Gallbladder cancer
INTRODUCTION
Diseases of the gallbladder commonly manifest as gallstones and gallbladder cancer. To identify risk factors in a given population, epidemiological studies must first define the frequency of disease. Studies employing necropsy surveys or healthcare databases carry biases by their implicit nature: being postmortem or requiring biliary symptoms/complications, respectively.1-3 Another potential measure of disease burden, the frequency of cholecystectomy, is a limited marker for the prevalence of gallstones, as the perceived threshold for surgery and patient access to care differ greatly.4 Some epidemiological studies have been confounded by inadequate sample size or selection bias. Small sample size is open to a beta-II type error: a failure to accurately identify a true difference (i.e., a false negative result). Selection bias may lead to spurious differences (i.e., a false positive result). More reliable epidemiological studies now use transabdominal ultrasound to screen robust numbers in defined asymptomatic populations. Ultrasonography is an ideal means to quantitate the frequency of gallstone disease, being a noninvasive and safe imaging technique that accurately can detect the point prevalence of gallstones in a defined asymptomatic population.
GALLSTONE DISEASE
1. Burden of gallstone disease
Gallstones constitute a significant health problem in developed societies, affecting 10% to 15% of the adult population, meaning 20 to 25 million Americans have (or will have) gallstones.2,5-7 The resultant direct and indirect cost of gallbladder disease represents a consumption of ~$6.2 billion annually in the U.S., constituting a major health burden that has increased more than 20% over the last 3 decades.2,8,9 With an estimated 1.8 million ambulatory care visits each year, gallstone disease is a leading cause for hospital admissions related to gastrointestinal problems.10 These numbers are likely an underestimate because laparoscopic cholecystectomy is often performed as a day procedure and thus not captured by hospital statistics that require overnight admission. Although the mortality rate for gallstones disease is relatively low at 0.6%, the high burden of disease imposes troubling mortality figures, such as an estimated 1,092 gallstone-related deaths for 2004 in the U.S. Fortunately, case fatality rates have steadily diminished from over 5,000 deaths in 1950, falling >50% between the years 1979 and 2004. This decline represents the greatest decrease for any digestive disease.9
Gallstone disease per se also carries inherent risks. Prospective population-based surveys have revealed an increased overall mortality, particularly from cardiovascular disease and cancer, as seen in Americans and Pima Indians with cholelithiasis.11,12 Further, as the incidence of gallstone disease escalates, there is a concomitant increase in complications like gallstone-related pancreatitis.13
The number of surgical procedures for cholelithiasis has risen markedly in developed countries since 1950.14 The introduction of laparoscopic cholecystectomy in 1989 further increased the cholecystectomy rate.14-16 From 1990 to 1993, for example, there was a 28% escalation in the number of cholecystectomies performed.17 The change in practice emanated from the laparoscopic surgical approach, which represented a less invasive, more cosmetically acceptable operation while providing a lower surgical risk compared to the then conventional or "open" procedure. This likely resulted in more surgeries being done in patients previously thought to be too high a risk, or in those with minimal symptoms. Although there is undoubtedly an element of overuse, cholecystectomy is now the most common elective abdominal surgery performed in the U.S., with over 750,000 operations being performed annually.6,16,18 The cholecystectomy rate, though increased, fortunately appears to have stabilized in the late 1990s and may even be on the decline in the U.S.19
2. Clinical aspects of gallstone disease
1) Asymptomatic/Silent gallstones
Gallstones are common. 10% to 20% of Americans will develop stones at some time.20 The majority will not develop symptoms: up to 80% will never experience biliary pain or complications such as acute cholecystitis, cholangitis, or pancreatitis.21 Hence, most gallstones are clinically "silent," an incidental finding often uncovered during abdominal ultrasound being performed for another reason.22 People with such asymptomatic cholelithiasis, however, eventually may develop symptoms (biliary pain) that require treatment,23 but this risk is quite low averaging 2% to 3% per year,24 10% by 5 years.1,23 An even lower proportion, 1% to 2% per year, develop major gallstone complications.20,25 Therefore, expectant management is an appropriate choice for silent gallstones in the general population. The exception is patients at high risk for experiencing biliary complications:
(1) Large gallstones (>3 cm) or gallbladders crammed with stones that carry a higher risk of developing gallbladder cancer, perhaps an indication for prophylactic cholecystectomy.26,27
(2) Sickle cell disease is associated with the development of pigment gallstones, frequently necessitating cholecystectomy. Prophylactic cholecystectomy should be considered because stone complications is frequently difficult to distinguish from the clinical features of a sickle cell crisis or its complications such as infarction of the liver or abdominal viscera.28 When performed early, outside the emergency setting, cholecystectomy lessens the surgical risks, but still carries a high mortality rate at 1% and postoperative complications of >30%.29
(3) Solid organ transplantation (heart, lung, kidney, pancreas). Although stem cell (bone marrow) transplantation carries its own problems from cholelithiasis and biliary sludge developing, more problematic is the aftermath of solid organ transplantation in which gallstones that develop frequently progress to symptoms and complications like cholecystitis, principally during the first 2 years.30 Liver transplantation is exempt; the gallbladder is removed at the time of hepatectomy. Controversy exists in patients with asymptomatic gallstone disease who are undergoing solid organ transplantation: expectant management with routine screening ultrasonography vs prophylactic (pre-/posttransplantation) cholecystectomy.
(4) Abdominal surgery, performed for other reasons, may benefit from a simultaneous cholecystectomy in situations where the risk of gallstone formation and complications are high. Prophylactic cholecystectomy therefore should be considered in morbidly obese patients undergoing bariatric surgery.31
2) Symptomatic gallstone disease
Since most gallstones are asymptomatic, it is essential to define exactly which symptoms are caused by gallstones: true biliary pain and/or complications, versus nonspecific abdominal complaints including dyspepsia.32-34 Gallstone-associated pain seems to follow a certain pattern in most patients.35,36 Consensus groups have attempted to establish criteria for biliary pain relative to defined characteristics (e.g., episodic, steady, severe pain located in the upper abdomen and lasting more than 30 minutes) and some accompanying features (e.g., nocturnal onset; nausea and vomiting; radiating through to the back).11 The importance for clarifying what constitutes true biliary pain is to better predict relief following cholecystectomy. Currently, cholecystectomy does not relieve biliary pain in 10% to 33% of people with documented gallstones.37,38 Confusion with other functional gut disorders like irritable bowel syndrome (IBS) and dyspepsia will not provide a favorable outcome from cholecystectomy.39,40 The avoidance of an unnecessary cholecystectomy becomes critically germane in an era of escalating rates of surgery.
3) Functional (acalculous) gallbladder disease
Biliary pain seemingly results from increased intraluminal pressure as the gallbladder contracts against an obstructed outlet. In gallstone disease, the obstruction is obvious: a stone in the cystic duct. In functional gallbladder disease (also termed; acalculous gallbladder disease, gallbladder dyskinesia or biliary dyskinesia), the pain mechanism may be obstruction located at the gallbladder outlet, incoordination between gallbladder contraction and sphincter of Oddi relaxation, or visceral hypersensitivity. A clue to its existence is impaired gallbladder emptying, reliably quantitated by cholecystokinin-cholescintigraphy.41,42 Yet the frequency and management of acalculous gallbladder disease remains unclear. Eliminating the apparent problem, the gallbladder, via laparoscopic cholecystectomy is fraught with challenges, particularly in selecting those who would most benefit. Although the exact frequency of biliary dyskinesia is unknown, any increase in the employment of cholecystectomy for such cases most certainly would impact surgical rates. Thus, there is insufficient evidence to support a role for cholecystectomy in functional gallbladder disease at this time.43 Hence, patients with suspected functional biliary pain but whose intact gallbladder lacks ultrasonographic evidence of gallstones should be carefully evaluated to exclude other causes for their symptoms.
3. Risk factors for gallstone formation
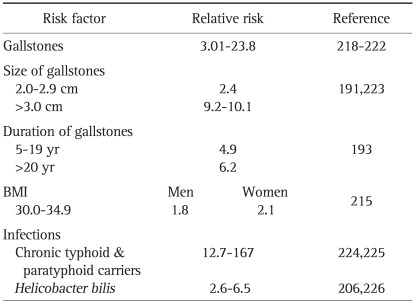
Important risk factors have been identified as being associated with gallstones (Table 1).2 Multiple case-control studies, comparing those with gallstones versus those without, have shown that gallstone formation is multifactorial. Some features, such as ethnicity, genetics, advancing age and female gender cannot be modified, whereas others (e.g., diet, physical activity, rapid weight loss and obesity) are modifiable.
Table 1.
Risk Factors for Gallstone Disease
TPN, total parental nutrition.
1) Ethnicity
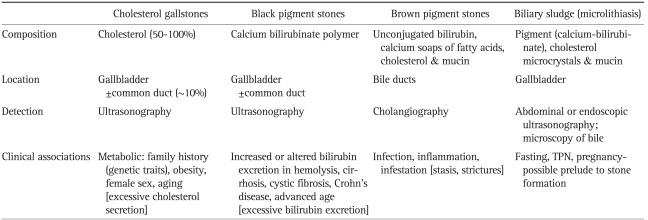
Geography and particularly ethnicity play an enormous role in the prevalence of gallstone disease and also the type of stone that forms: cholesterol gallstones predominate in the developed countries of the Western world; brown pigment stones in the bile ducts are more common in Asia (Table 2, Fig. 1).44
Table 2.
Types of Gallbladder and Biliary Tract Stones: Characteristics and Clinical Associations
TPN, total parental nutrition.
Fig. 1.
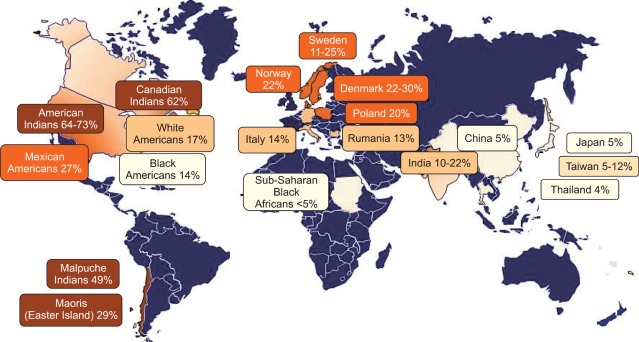
Worldwide prevalence of gallstones in females based on ultrasonographic surveys varies.45 Prevalence is inordinately high in American Indians and their admixtures, and also Northern Europeans; somewhat lower in European and American whites; intermediate in Asians and black Americans, and quite low in black Africans.
North American Indians have the highest reported rates of cholelithiasis, afflicting 64.1% of women and 29.5% of men.2,45 The aboriginal populations of South America also have an exceedingly high prevalence of gallstones: 49.4% of native Mapuche Indians of Chile women and 12.6% of men harbor gallstones.46 Mexican Americans are also at heightened risk when compared to White Americans; however, this risk is directly related to the degree of Amerindian admixture.47-50 White Americans have an overall prevalence of 16.6% in women and 8.6% in men.6,47 Intermediate prevalence rates occur in Asian populations51,52 and Black Americans (13.9% of women; 5.3% of men).6 The lowest frequencies occur in sub-Saharan Black Africans (<5%).53 The majority of gallstones in developed countries consist predominantly of cholesterol (>85%), whereas the remainder constitutes black pigment stones (i.e., composed of calcium bilirubinate) (Table 2).2,7
The situation differs in East Asia where brown pigment stones are located in bile ducts, predominately associated with parasitic infestation. In developed countries, however, these bile duct stones arise in association with the inflammation and infection that result from biliary strictures and malignancies. Brown pigment stones consist of some calcium bilirubinate (hence their dark color), fatty acid soaps (calcium palmitate and calcium stearate, hence their greasy feel), some cholesterol, and mucinous glycoproteins (a product of bacterial biofilms). They form de novo in the common bile duct (choledocholithiasis) or the intrahepatic bile ducts (hepatolithiasis). These primary ductal stones result from bacterial infection, biliary parasites (Clonorchis sinensis, Opisthorchis species, Fasciola hepatica) and stasis from partial biliary obstruction. Brown pigment stones are the predominant type in Asia where they can cause Oriental cholangiohepatitis: biliary obstruction with recurrent cholangitis, dilatation and stricturing of the biliary tree. In hepatolithiasis, stones are present in the intrahepatic bile ducts, regardless of any coexistent stones residing elsewhere in the biliary system (i.e., the extrahepatic ducts or the gallbladder). The brown pigment stones that arise in intrahepatic sites (hepatolithiasis) possess relatively more cholesterol and less bilirubin than those that form in extrahepatic sites, presumably due to a different mechanism for their formation. The frequency of hepatolithiasis, as a proportion of all bile duct stones, is as high as 20% in China and Taiwan, yet as low at 2% to 3% in Japan, Singapore, and Hong Kong.54 The stone type curiously has recently shifted in developing Asian countries from pigment to cholesterol stones. The basis for this change may reflect a decreased rate of chronic biliary infections and consumption of a more Westernized diet.2
2) Family history & genetics
Genetic susceptibility is a key factor in gallstone formation.55 Familial studies reveal an increased frequency: a nearly 5 times elevated risk in the relatives of gallstone patients. These rate are even higher in monozygotic twins at 12% and dizygotic twins at 6%.56,57 Yet spouses of affected patients do not have any increased risk, thereby eliminating a shared environment as the basis - i.e., similar dietary and other common habits among family members as the explanation for this apparent association.58 In a Swedish twin study, genetic effects accounted for 25%, shared environmental influences for 13% and unique environmental effects for 62% of the phenotypic variance.59
No mode of simple Mendelian pattern of inheritance can account for the majority of cases with gallstone disease. In fact, stone formation is a complex interaction of genes and environmental factors, particularly diet-gene interactions.55,60 Several genes have been associated with gallstone disease.61 Identified so far have been: the apolipoproteins E (APOE) and B (APOB),62 cholesterol ester transporting protein (CETP), cholesterol 7 α-hydroxylase,63 cholecystokinin receptor A (CCKAR),64 the LDL receptor (LDLR)65 and the CETP.66 Genome-wide association analysis has revealed that variants for the hepatic cholesterol secretion (ABCG8 19H and ABCB4) represent a susceptibility factor for human gallstones.67,68 Conferring an odds ratios of 2 to 3 for heterozygous and 7 for homozygous carriers, these variants account for 11% of the total gallstone risk. Such human susceptibility ("gallstone") genes therefore are not common and so embody a rather modest contribution. Cholelithiasis most likely is a polygenetic disease entity.
3) Age
The frequency of gallstones increases with age, escalating markedly after age 40 to become 4 to 10 times more likely in older individuals.2,69 The stone type also changes with age: initially being composed predominantly of cholesterol (corresponding to an increased cholesterol secretion into and saturation of bile) but in late life tending to be black pigment stones. Further, symptoms and complications increase with age, leading to more frequent cholecystectomies.70
4) Gender and female sex hormones
The female gender has a most compelling association with gallstone disease, especially during the fertile years. Women are almost twice as likely as men to form stones; the gap narrows following menopause after which men begin to catch up.2 The underlying mechanism is female sex hormones; parity, oral contraceptive use and estrogen replacement therapy are established risk factors for cholesterol gallstone formation.71-73 Female sex hormones adversely influence hepatic bile secretion and gallbladder function. Estrogens increase cholesterol secretion and diminish bile salt secretion, while progestins act by reducing bile salt secretion and impairing gallbladder emptying leading to stasis. A new 4th generation progestin, drospirenone, used in some oral contraceptives may further heighten the risk of gallstone disease and cholecystectomy; however, the increased risk is quite modest and not likely to be clinically meaningful.74
During pregnancy when female sex hormones are endogenously raised, biliary sludge (particulate material that is composed of cholesterol, calcium bilirubinate, and mucin) appears in 5% to 30% of women. Resolution frequently transpires during the post-partum period: sludge disappears in two-thirds; small (<1 cm) gallstones (microlithiasis) vanish in one-third, but definitive gallstones become established in ~5%.75,76 Additional risk factors for stone formation during pregnancy include obesity (prior to the pregnancy), reduced high density lipoprotein (HDL) cholesterol and the metabolic syndrome.76,77
5) Obesity
The exploding prevalence of obesity now reaches epidemic levels in both developed and developing nations like China.78,79 Obesity, particularly abdominal or centripetal obesity, is a well-established risk factor for gallstone disease.2,80-83 At least 25% of morbidly obese individuals have evidence of gallstone disease.84 Obesity in the late teenage years carries the greatest risk, whereas thinness protects against cholelithiasis.2,85 Females with obesity have an even increased risk of stones formation. Women with severe obesity (body mass index [BMI] >32 kg/m2) showed an age-adjusted relative risk of 6.0 for the development of gallstones compared with nonobese controls; their annual incidence of developing gallstones is 2%.85 Obesity is associated with an increased activity of the rate-limiting step in cholesterol synthesis, the hepatic enzyme, 3-hydroxyl-3-methyl-glutaryl co-enzyme A (HMG-CoA) reductase, leading to increased cholesterol synthesis in the liver and its heightened secretion into bile.80,86,87
6) Dyslipidemia, diabetes mellitus and the metabolic syndrome
Cholesterol gallstone disease is a metabolic problem, which correlates with lipid abnormalities, diabetes mellitus and adiposity. A low HDL cholesterol88 and hypertriglyceridemia89,90 carry an increased risk of developing stones. In contrast, there is no definite association with hypercholesterolemia.2,91 High homocysteine levels also may correlate with gallstone disease.92
The metabolic syndrome is defined by the presence of at least 3 features out of: abdominal obesity, high blood pressure, high fasting glucose, increased triglyceride levels and reduced HDL levels.93,94 Both the metabolic syndrome and diabetes mellitus are risk factors for gallstone disease.95 The metabolic syndrome has also been associated with stone complications.96 Insulin resistance predisposes to cholesterol gallstone formation,97,98 suggesting altered cholesterol and bile salt metabolism. Hepatic insulin resistance may act by enhancing hepatic cholesterol secretion, depressing bile salt synthesis and/or impairing gallbladder motility.99-101
7) Rapid weight loss
Low caloric diets and/or bariatric surgery with rapid weight loss are associated with gallstones developing in 30% to 71% of such individuals.102-108 Weight loss that exceeds 1.5 kg/wk following bariatric surgery increases the risk for stone formation; these stones are most likely to become apparent during the first 6 weeks after surgery when weight loss is most profound.109,110 Weight loss-associated gallstones are typically asymptomatic; only 7% to 16% develop symptoms, best predicted by a postoperative weight loss exceeding 25% of the body weight.84 Even less extreme weight fluctuations create a risk for stone formation,111 as is a history of dieting.112
8) Diet and total parental nutrition (TPN)
Other than a high caloric intake that leads to obesity, any importance of the dietary content is unclear and difficult to analyze.113-115 Diets specifically high in cholesterol,116 fatty acids, 117 carbohydrates118,119 or legumes120 seem to increase the risk of cholelithiasis. In contrast, unsaturated fats,121 coffee,122,123 fiber,124,125 ascorbic acid (vitamin C),126,127 calcium114 and moderate consumption of alcohol118,124,128 reduce the risk. Certainly, the shift to a more Western diet, high in refined carbohydrates and fat (triglycerides) and low in fiber, best explains the profound increase in cholesterol gallstones amongst American Indians (unmasking their presumed genetic burden) and in European countries following World War II. This dietary change also might account for the shift from pigment to cholesterol stones in Asian countries2,129,130 Genetic variations, especially in the genes that control cholesterol metabolism, might underscore why some respond to dietary change by developing cholesterol gallstones.16,60
TPN is a well-known risk factor for developing microlithiasis (biliary sludge) and gallstone disease, in addition to acute acalculous cholecystitis in critically ill patients.131-134 In an intensive care setting, biliary sludge appears after 5 to 10 days of fasting.17,49 After 4 weeks of TPN, half of those on TPN develop gallbladder sludge on ultrasonography; after 6 weeks all show evidence of sludge.135 Most are asymptomatic. Fortunately, sludge resolves within 4 weeks of discontinuing TPN and resuming an oral intake, a pattern similar to sludge appearing during pregnancy and rapid weight loss and then disappearing once the inciting event resolves.136 A possible explanation for this relates to loss of the enteric stimulation of the gallbladder in the absence of eating, leading to gallbladder stasis.134 Additionally, ileal disorders such as Crohn's disease or ileal resection, in which TPN is frequently required, can affect the enterohepatic cycling of bile acids and so augment bilirubin absorption and subsequent hepatic excretion.86
9) Lifestyle factors and socioeconomic status
The exact role of socioeconomic status and gallstones is controversial.2 A previous cross-sectional study of non-Hispanic Whites and Mexican Americans, found gallbladder disease inversely related to socioeconomic status.137 Socioeconomic status, however, may merely be an indirect marker for other risk factors like obesity and chronic medical conditions. The role of smoking in cholelithiasis is unclear.138
Reduced physical activity heightens the risk of gallstone disease whereas increased physical activity helps prevent cholelithiasis, independent of its role in weight loss.122,139 Increased endurance exercise (to 30 minutes 5 times a week) may avert symptomatic gallstones developing in men.140
10) Underlying chronic diseases
(1) Liver disease
Advanced cirrhosis is a well-established risk factor for gallstones, with an overall prevalence at 25% to 30%.141-143 Usually the stones consist of the black pigment type in patients with cirrhosis.144 This is likely related to altered pigment secretion, abnormal gallbladder motility and/or increased estrogen levels.2 Gallstone disease is also associated with chronic hepatitis C viral infection and nonalcoholic fatty liver disease;145-147 other factors for this are the metabolic syndrome and obesity.148
(2) Crohn's disease
There is a two-three fold increased risk of developing gallstones in patients with extensive ileal Crohn's disease.77,149 An obvious explanation for this is ileal disease or loss leading to bile acid malabsorption and depletion, reduced hepatic secretion of bile acids and bile that is supersaturated with cholesterol, leading to cholesterol stone formation. The cholesterol content in bile however can be rather normal or even low in these patients.150 Instead, there appears to be an increased frequency of pigment stones. Failure of terminal ileal transport in Crohn's disease allows excess bile acids to escape into the colon, where these biological detergents solubilize unconjugated bilirubin and so facilitate their absorption and return to the liver. The liver then secretes excessive pigment that subsequently precipitates as gallstones.151 Other explanations include fasting in patients with Crohn's disease, or altered bacterial colonic flora that enhance the deconjugation of bilirubin, which can then be passively absorbed; the result is an upregulated enterohepatic cycling of bile pigment.152
(3) Cystic fibrosis
Similar to ileal Crohn's disease, cystic fibrosis is associated with bile acid malabsorption due to its binding to undigested dietary nutrients. Gallstone prevalence in cystic fibrosis is increased 10% to 30%.153
(4) Other diseases
In sickle cell disease, chronic hemolysis leads to excessive bilirubin excretion with the formation of black pigment stones composed of calcium bilirubinate. These tend to be small in size, permitting some to travel into the common duct; the resultant obstruction is low-grade, not necessarily accompanied by duct dilation or cholangitis. Due to potential complications and the difficulty in distinguishing biliary-type pain from other complications of sickle cell disease, prophylactic cholecystectomy should be considered.29
Spinal cord injury is associated with a threefold increase in gallstone formation.154-156 Possible explanations for this include gallbladder stasis with sludge formation and intestinal hypomotility that alters bile acid metabolism.
IBS presents with abdominal pain in addition to other features, perhaps fostering cholecystectomy as a more common operation in patients with IBS. This may reflect inappropriate surgery caused by diagnostic confusion157 or post-surgical IBS symptoms as a consequence of the operation.158
11) Drugs
(1) Octreotide
Octreotide, a long-acting analogue of somatostatin that inhibits cholecystokinin release, results in decreased gallbladder motility and stasis.159 Inhibition of cholecystokinin also depresses small intestine motility; the resultant intestinal stasis enhances the formation of secondary bile acids like deoxycholic acid. Deoxycholic acid adversely influences bile formation (increasing cholesterol secretion) and augments the synthesis of gallbladder mucin (important for the precipitation of cholesterol microcrystals from bile and their subsequent growth into stones). Greater than 50% of patients receiving octreotide accordingly will develop cholelithiasis, although the majority are asymptomatic.160,161
(2) Ceftriaxone
Ceftriaxone, a third generation cephalosporin antibiotic, is secreted unmetabolized into bile, achieving high concentrations.86 This can result in biliary sludge and "pseudolithiasis" in those patients, particularly children, receiving ceftriaxone. Most remain asymptomatic. Meanwhile, the sludge resolves once the medication is discontinued.162,163
(3) Thiazide diuretics
Thiazide treatment may increase biliary cholesterol saturation leading to gallstones developing.164 Some case-controlled reports have suggested that thiazide use is associated with a heightened risk of acute cholecystitis.165,166 Others have not found any association.140 Most likely, thiazide use conveys a modest effect. In one prospective study concerning women taking thiazide diuretics, the relative risk of cholecystectomy rose 36% for past users and 57% for current users.167
(4) Statins
Drugs that inhibit HMG-CoA reductase seem to prevent cholesterol gallstone disease by diminishing cholesterol synthesis in the liver and decreasing its secretion into bile.168
12) Gallbladder disease in children
Cholelithiasis in the pediatric age group has been considered rare, historically thought to be black pigment stones related to prematurity and TPN use in infants or chronic hemolysis in adolescents. Cholesterol stones, in fact, are becoming increasingly more common in children.169-171 In unselected pediatric populations, the prevalence rates are reported between 0.1% to 1.0%.170,172 One explanation for this increase is greater access to and use of abdominal ultrasonography in children.173 A more prominent factor now is obesity, accounting for some 8% to 33% of gallstones observed in children.174,175 In obese children and adolescents, the prevalence may be as high as 2.0%.171 Other risk factors for gallstone disease in childhood include: female gender, pregnancy and oral contraceptive use; being of Mexican-American origin; drug exposure to cephalosporins, ceftriaxone or diuretics; a history of cardiac surgery or bowel resection, and having cystic fibrosis.149,172,173,176 In fact, the risk factors for pediatric gallbladder disease now more closely resemble those in adults.
40% to 51% of children with gallstones are asymptomatic.173,177 Those with no symptoms have a lower rate of complications, while gallstones may resolve in 17%. Therefore conservative management is recommended in these children. Pediatric patients with symptomatic cholelithiasis are at risk for complications (18% to 28%); the most common presentation is right upper quadrant abdominal pain.173,178 Complications include acute cholecystitis, choledocholithiasis and pancreatitis.
Laparoscopic cholecystectomy is safe and can be quite effective in children. Cholecystectomy for children with symptomatic gallstones therefore is the standard of care. Surgery is also being used more frequently for functional gallbladder disease (biliary dyskinesia), an entity that bears scrutiny given the absence of clear diagnostic criteria or effective management schemes.169
GALLBLADDER CANCER
Gallbladder cancer is a notoriously rare though lethal malignancy with marked ethnic and geographical variations. The presenting symptoms are typically vague so that its diagnosis commonly occurs at an advanced stage. This late diagnosis plus the anatomic feature that the gallbladder lacks a serosa culminates in a rather dismal prognosis.179-181 The overall mean survival rate for patients with advanced gallbladder cancer is 6 months, with a 5-year survival rate of 5%.182 Early gallbladder cancer (confined to the mucosa), though infrequent, offers the potential for a cure though cholecystectomy. Most (>80%) gallbladder cancers are adenocarcinomas that originate from the fundus (60%), body (30%), or neck (10%). The basis likely is genetic susceptibility, perhaps elicited by chronic gallbladder inflammation, often a product of cholelithiasis.181,183,184 One reasonable hypothesis focuses on chronic irritation of the mucosa (e.g., from the physical presence of the stones and/or superimposed chronic infection such as from Salmonella typhi) leading to dysplasia (perhaps abetted by mutagenic secondary bile acids) and terminating in malignant change.
The risk factors for developing gallbladder cancer therefore include ethnicity, genetic susceptibility, lifestyle factors and infections. Elucidating such risk factors (Table 3) not only provides insight into its pathogenesis accounting for its geographic and ethnic variances (Fig. 2), but more importantly should yield strategies to prevent and treat this unusual malignancy.185
Table 3.
Risk Factors for Gallbladder Cancer
Fig. 2.
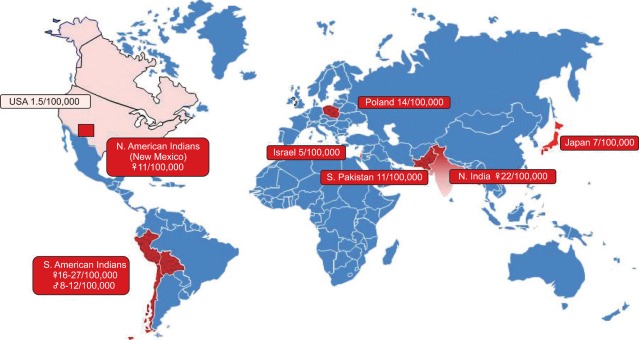
Incidence of gallbladder cancer worldwide (From National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program. Available from http://seer.cancer.gov/). Carcinoma of the gallbladder is more common in certain ethnic groups: native American Indians, white Hispanics from North and South America, and those from northern India and Eastern Europe.198 Elsewhere in the world, the incidence is low at <2/100,000.
1. Ethnicity, gender, and age
Gallbladder cancer is rare in developed countries. In the U.S., it only accounts for 0.5% of all gastrointestinal malignancies, accounting for less than 5,000 cases per year (1 to 2.5 per 100,000).183 Worldwide, gallbladder cancer has a low occurrence <2 per 100,000, but has a wide variance (Fig. 2). High annual incidence rates occur in North and South American Indians, generating an inordinate mortality, particularly amongst women: 15.5 per 100,000 in women (vs 7.5/100,000 in men) from La Paz, Bolivia, and 11.3 per 100,000 in women (vs 4/100,000 in men) from New Mexico. Hence, carcinoma of the gallbladder is the leading cause of cancer death in Chilean women, exceeding even breast, lung and cervical cancers.186,187 Other high-risk regions are scattered though Eastern Europe (14/100,000 in Poland), northern India (as high as 21.5/100,000 for women from Delhi) and south Pakistan (11.3/100,000).185 Intermediate incidences (3.7 to 9.1 per 100,000) occur elsewhere in South Americans of Indian descent, and in Israel (5/100,000) and Japan (7/100,000).184 The frequency is increasing in Shanghai, China and now accounts for the most frequent gastrointestinal malignancy and is a substantial cause of mortality.188 Although the majority of the world has decreasing mortality trends in gallbladder cancer, Iceland, Costa Rica, and Korea have an increase in mortality for men.189 There appears to be a modest decline in prevalence over the past two decades (National Cancer Institute. Surveillance, Epidemiology and End Results (SEER) Program [http://seer.cancer.gov/]).
Gender differences exist with geographic variances, generally being unfavorable for women. In those locals with the highest incidence, women have frequency rates greater than men. With age, gallbladder cancer increases.
2. Gallstones
A history of gallstones appears to carry the highest risk for gallbladder cancer, with a relative risk of 4.9.185 Most (69% to 100%) but not all people with gallbladder cancer have cholelithiasis. Further, these 2 entities frequently co-exist in the same populations, suggesting that stones may function as a co-factor for this carcinoma.190 American Indians, who have a quite high prevalence of cholesterol gallstone disease, also have a high incidence of carcinoma of the gallbladder; yet in other settings, there is a low incidence of gallbladder cancer despite an overall high frequency of cholelithiasis. Increasing stone size (>3 cm),191,192 number, volume, and weight, all are associated with an increased risk of cancer.190 Less important is the duration of cholelithiasis.193 Cholesterol stones seem to be more common than pigment stones in gallbladder cancer patients.194 Further attesting to gallstones being a risk factor for gallbladder carcinoma, the incidence of this cancer rises when the cholecystectomy rate declines.184,195 Nevertheless, consensus does not generally favor prophylactic cholecystectomy for asymptomatic stones196,197 as cholelithiasis is too common and gallbladder cancer too rare. Potential exceptions include large stones greater than 3 cm, which have a risk of 4% over 20 years,27,198 and elderly American Indian females with gallstones.199
3. Chronic inflammation
Chronic inflammation from any cause may lead to calcium being deposited in the gallbladder wall, termed the "porcelain gallbladder" because of its bluish color and fragile, brittle consistency.200 This entity is rare, being identified pathologically in less than 1% of gallbladder specimens. The calcium deposits can be detected on diagnostic imaging - plain abdominal radiographs, ultrasounds or computed tomography images. Controversy exists whether or not the porcelain gallbladder is truly associated with an increased risk of cancer. Some studies indicate that 25% (range, 12% to 61%) are associated with gallbladder cancer,199,201 whereas more recent reports negate any such association.202 Only gallbladders with partial calcification, stippled or multiple punctate calcifications in the glandular spaces of the mucosa, are premalignant and therefore should be removed prophylactically. Those with a broad continuous band of calcification in the muscularis appear not to be harbingers of gallbladder cancer.
Chronic bacterial infections also cause irritation and inflammation in the gallbladder. S. typhi carriers have an 8 to 12-fold increased risk with 6% developing gallbladder cancer.203-205 In contrast to typhoid carriers, however, a past history of typhoid fever is not associated with the development of gallbladder cancer.206 Helicobacter pilis is also implicated in gallbladder cancer with an odds ratio of 6.5 in Japanese patients and 5.86 in Thai patients.206
Primary sclerosing cholangitis (PSC) is typically associated with an increased risk of cholangiocarcinoma. As dysplasia occurs in 37% and adenocarcinoma in 14% of gallbladders from patients with PSC, their general predilection for biliary carcinoma as cholangiocarcinoma may place these individuals at heightened risk for developing gallbladder cancer.207
4. Congenital biliary abnormalities
An anomalous pancreaticobiliary junction is a rare congenital anomaly of the biliary tract in which the pancreatic and biliary ducts join outside the duodenal wall, forming an abnormally long channel that lies beyond the sphincter of Oddi.208 Such an anomaly defeats sphincter of Oddi gatekeeper function, potentially allowing pancreatic secretions to regurgitate into the biliary system and gallbladder, and so leading to malignant changes in the mucosa.209 The anomalous pancreaticobiliary junction is more prevalent in Asian (particularly Japanese) populations and carries an increased risk of gallbladder cancer at 3% to 18%.183,210 Hence, prophylactic cholecystectomy is recommended due to the high frequency of gallbladder carcinoma.
5. Genetic factors
There are undoubtedly genetic and environmental factors that coincide to become expressed as gallbladder cancer. A family history of gallbladder cancer is clearly a risk factor.211,212 The only responsible gene so far identified seems to be that for apolipoprotein B function (the APOB gene), which influences cholesterol handling yet is not associated with gallstones. In fact, the link between cholesterol gallstones and gallbladder cancer may relate to an interdependent disposal pathway that increases the export of both cholesterol and environmental toxins into bile. As gallbladder cancer is more common in women, such mutagenic toxins secreted reside longer in the gallbladder due to stasis from impaired contractility associated with the female hormone, progesterone. This protracted exposure allows environmental carcinogens to then cause malignant transformation, helping to reconcile the schism of seed versus soil and incorporate the predilection to the development of gallstones (also requiring some gallbladder stasis) and gallbladder cancer.213
6. Gallbladder polyps
Polypoidal masses of the gallbladder affect 5% of adults and may be confused with gallbladder cancer.214 Over two-thirds of polyps are composed of cholesterol esters; the other lesions are adenomas, leiomyomas or inflammatory polyps. Although occasionally associated with biliary colic, the vast majority of gallbladder polyps are asymptomatic, being found incidentally when abdominal imaging is performed for other purposes. Features that predict malignancy are: large polyps (>10 mm), a solitary or sessile mass, associated gallstones, patient age over 50 and most importantly, rapid polyp growth. Prophylactic cholecystectomy is warranted in patients with polyps that possess such malignant-appearing features.
7. Other lifestyle factors
The association of gallstones with gallbladder cancer likely explains why some of the traditional risk factors for gallstones are also risk factors for gallbladder cancer including obesity, female gender, and multiparity. In over 84,000 men and 97,000 women included in The Cancer Prevention Study II Nutrition Cohort, the relative risk of gallbladder cancer was 1.8 (95% confidence interval [CI], 1.1 to 2.9) in obese men with a BMI of 30.0 to 34.9 compared to men with a normal BMI (18.5 to 24.9). Obese women (BMI, 30.0 to 34.9) had a relative risk of 2.1 (95% CI, 1.6 to 2.9) compared to women with a normal BMI.215 Overall, obesity has a relative risk of 1.66 (95% CI, 1.47 to 1.88) for gallbladder cancer.216 Other lifestyle risks involve cigarette smoking, and alcohol consumption (in men only).217
CONCLUSION
The prevalence of gallbladder disease at any point in time (i.e., prevalence) has advanced with the use of ultrasonographic surveys as opposed to previous studies based on clinical or necropsy evidence.2,3 These population surveys have better defined important risk factors, both unchangeable and modifiable. The implications of changing environmental risk factors predict an increase in the numbers of individuals with gallstones. An aging population plus the rising epidemic of obesity and the metabolic syndrome are certain to aggravate the frequency and complications of gallstone disease. Identifying risk factors that can be altered (i.e., extreme obesity, rapid weight loss, sedentary lifestyle, and key dietary factors) should provide an opportunity to prevent cholelithiasis. Several risk factors for gallstones are also implicated in the pathogenesis of gallbladder cancer. Although the frequency of gallbladder cancer is relatively low in the U.S., if the incidence of gallstones rises, gallbladder cancer most likely will also increase.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med. 1982;307:798–800. doi: 10.1056/NEJM198209233071305. [DOI] [PubMed] [Google Scholar]
- 2.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 3.Kratzer W, Mason RA, Kächele V. Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound. 1999;27:1–7. doi: 10.1002/(sici)1097-0096(199901)27:1<1::aid-jcu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen G, Hoem D, Andrén-Sandberg A. Influence of laparoscopic cholecystectomy on the prevalence of operations for gallstones in Norway. Eur J Surg. 2002;168:464–469. doi: 10.1080/110241502321116460. [DOI] [PubMed] [Google Scholar]
- 5.Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants. 2005;15:329–338. doi: 10.1615/jlongtermeffmedimplants.v15.i3.90. [DOI] [PubMed] [Google Scholar]
- 6.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 7.Tazuma S. Gallstone disease: epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic) Best Pract Res Clin Gastroenterol. 2006;20:1075–1083. doi: 10.1016/j.bpg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 9.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–386. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140:508–516. doi: 10.1053/j.gastro.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimaldi CH, Nelson RG, Pettitt DJ, Sampliner RE, Bennett PH, Knowler WC. Increased mortality with gallstone disease: results of a 20-year population-based survey in Pima Indians. Ann Intern Med. 1993;118:185–190. doi: 10.7326/0003-4819-118-3-199302010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Lindkvist B, Appelros S, Manjer J, Borgström A. Trends in incidence of acute pancreatitis in a Swedish population: is there really an increase? Clin Gastroenterol Hepatol. 2004;2:831–837. doi: 10.1016/s1542-3565(04)00355-6. [DOI] [PubMed] [Google Scholar]
- 14.Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Zatz SL. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270:1429–1432. [PubMed] [Google Scholar]
- 15.Marshall D, Clark E, Hailey D. The impact of laparoscopic cholecystectomy in Canada and Australia. Health Policy. 1994;26:221–230. doi: 10.1016/0168-8510(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 16.Kang JY, Ellis C, Majeed A, et al. Gallstones: an increasing problem: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther. 2003;17:561–569. doi: 10.1046/j.1365-2036.2003.01439.x. [DOI] [PubMed] [Google Scholar]
- 17.Nenner RP, Imperato PJ, Rosenberg C, Ronberg E. Increased cholecystectomy rates among Medicare patients after the introduction of laparoscopic cholecystectomy. J Community Health. 1994;19:409–415. doi: 10.1007/BF02260323. [DOI] [PubMed] [Google Scholar]
- 18.Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004;126:1448–1453. doi: 10.1053/j.gastro.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Zacks SL, Sandler RS, Rutledge R, Brown RS., Jr A population-based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol. 2002;97:334–340. doi: 10.1111/j.1572-0241.2002.05466.x. [DOI] [PubMed] [Google Scholar]
- 20.Gibney EJ. Asymptomatic gallstones. Br J Surg. 1990;77:368–372. doi: 10.1002/bjs.1800770405. [DOI] [PubMed] [Google Scholar]
- 21.Sakorafas GH, Milingos D, Peros G. Asymptomatic cholelithiasis: is cholecystectomy really needed? A critical reappraisal 15 years after the introduction of laparoscopic cholecystectomy. Dig Dis Sci. 2007;52:1313–1325. doi: 10.1007/s10620-006-9107-3. [DOI] [PubMed] [Google Scholar]
- 22.Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. Br J Surg. 2004;91:734–738. doi: 10.1002/bjs.4547. [DOI] [PubMed] [Google Scholar]
- 23.Thistle JL, Cleary PA, Lachin JM, Tyor MP, Hersh T. The natural history of cholelithiasis: the National Cooperative Gallstone Study. Ann Intern Med. 1984;101:171–175. doi: 10.7326/0003-4819-101-2-171. [DOI] [PubMed] [Google Scholar]
- 24.Ransohoff DF, Gracie WA, Wolfenson LB, Neuhauser D. Prophylactic cholecystectomy or expectant management for silent gallstones. A decision analysis to assess survival. Ann Intern Med. 1983;99:199–204. doi: 10.7326/0003-4819-99-2-199. [DOI] [PubMed] [Google Scholar]
- 25.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165:399–404. doi: 10.1016/s0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 26.Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants. 2005;15:329–338. doi: 10.1615/jlongtermeffmedimplants.v15.i3.90. [DOI] [PubMed] [Google Scholar]
- 27.Kapoor VK. Cholecystectomy in patients with asymptomatic gallstones to prevent gall bladder cancer: the case against. Indian J Gastroenterol. 2006;25:152–154. [PubMed] [Google Scholar]
- 28.Bonatsos G, Birbas K, Toutouzas K, Durakis N. Laparoscopic cholecystectomy in adults with sickle cell disease. Surg Endosc. 2001;15:816–819. doi: 10.1007/s004640000383. [DOI] [PubMed] [Google Scholar]
- 29.Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol. 2010;8:483–489. doi: 10.1016/j.cgh.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Kao LS, Kuhr CS, Flum DR. Should cholecystectomy be performed for asymptomatic cholelithiasis in transplant patients? J Am Coll Surg. 2003;197:302–312. doi: 10.1016/S1072-7515(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 31.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–1005. [PubMed] [Google Scholar]
- 32.Jørgensen T. Abdominal symptoms and gallstone disease: an epidemiological investigation. Hepatology. 1989;9:856–860. doi: 10.1002/hep.1840090611. [DOI] [PubMed] [Google Scholar]
- 33.Traverso LW. Clinical manifestations and impact of gallstone disease. Am J Surg. 1993;165:405–409. doi: 10.1016/s0002-9610(05)80931-6. [DOI] [PubMed] [Google Scholar]
- 34.Fenster LF, Lonborg R, Thirlby RC, Traverso LW. What symptoms does cholecystectomy cure? Insights from an outcomes measurement project and review of the literature. Am J Surg. 1995;169:533–538. doi: 10.1016/S0002-9610(99)80212-8. [DOI] [PubMed] [Google Scholar]
- 35.Festi D, Sottili S, Colecchia A, et al. Clinical manifestations of gallstone disease: evidence from the multicenter Italian study on cholelithiasis (MICOL) Hepatology. 1999;30:839–846. doi: 10.1002/hep.510300401. [DOI] [PubMed] [Google Scholar]
- 36.Berhane T, Vetrhus M, Hausken T, Olafsson S, Søndenaa K. Pain attacks in non-complicated and complicated gallstone disease have a characteristic pattern and are accompanied by dyspepsia in most patients: the results of a prospective study. Scand J Gastroenterol. 2006;41:93–101. doi: 10.1080/00365520510023990. [DOI] [PubMed] [Google Scholar]
- 37.Weinert CR, Arnett D, Jacobs D, Jr, Kane RL. Relationship between persistence of abdominal symptoms and successful outcome after cholecystectomy. Arch Intern Med. 2000;160:989–995. doi: 10.1001/archinte.160.7.989. [DOI] [PubMed] [Google Scholar]
- 38.Vetrhus M, Berhane T, Søreide O, Søndenaa K. Pain persists in many patients five years after removal of the gallbladder: observations from two randomized controlled trials of symptomatic, noncomplicated gallstone disease and acute cholecystitis. J Gastrointest Surg. 2005;9:826–831. doi: 10.1016/j.gassur.2005.01.291. [DOI] [PubMed] [Google Scholar]
- 39.Mertens MC, Roukema JA, Scholtes VP, De Vries J. Risk assessment in cholelithiasis: is cholecystectomy always to be preferred? J Gastrointest Surg. 2010;14:1271–1279. doi: 10.1007/s11605-010-1219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thistle JL, Longstreth GF, Romero Y, et al. Factors that predict relief from upper abdominal pain after cholecystectomy. Clin Gastroenterol Hepatol. 2011;9:891–896. doi: 10.1016/j.cgh.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer E. Acalculous biliary pain: new concepts for an old entity. Dig Liver Dis. 2003;35(Suppl 3):S20–S25. doi: 10.1016/s1590-8658(03)00089-6. [DOI] [PubMed] [Google Scholar]
- 42.DiBaise JK, Richmond BK, Ziessman HH, et al. Cholecystokinin-cholescintigraphy in adults: consensus recommendations of an interdisciplinary panel. Clin Gastroenterol Hepatol. 2011;9:376–384. doi: 10.1016/j.cgh.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Gurusamy KS, Junnarkar S, Farouk M, Davidson BR. Cholecystectomy for suspected gallbladder dyskinesia. Cochrane Database Syst Rev. 2009;(1):CD007086. doi: 10.1002/14651858.CD007086.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Everhart JE, Yeh F, Lee ET, et al. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- 46.Miquel JF, Covarrubias C, Villaroel L, et al. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937–946. doi: 10.1016/s0016-5085(98)70266-5. [DOI] [PubMed] [Google Scholar]
- 47.Everhart JE. Gallstones and ethnicity in the Americas. J Assoc Acad Minor Phys. 2001;12:137–143. [PubMed] [Google Scholar]
- 48.Diehl AK, Stern MP. Special health problems of Mexican-Americans: obesity, gallbladder disease, diabetes mellitus, and cardiovascular disease. Adv Intern Med. 1989;34:73–96. [PubMed] [Google Scholar]
- 49.Maurer KR, Everhart JE, Ezzati TM, et al. Prevalence of gallstone disease in Hispanic populations in the United States. Gastroenterology. 1989;96(2 Pt 1):487–492. doi: 10.1016/0016-5085(89)91575-8. [DOI] [PubMed] [Google Scholar]
- 50.Hanis CL, Hewett-Emmett D, Kubrusly LF, et al. An ultrasound survey of gallbladder disease among Mexican Americans in Starr County, Texas: frequencies and risk factors. Ethn Dis. 1993;3:32–43. [PubMed] [Google Scholar]
- 51.Singh V, Trikha B, Nain C, Singh K, Bose S. Epidemiology of gallstone disease in Chandigarh: a community-based study. J Gastroenterol Hepatol. 2001;16:560–563. doi: 10.1046/j.1440-1746.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 52.Chen CY, Lu CL, Huang YS, et al. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing. 1998;27:437–441. doi: 10.1093/ageing/27.4.437. [DOI] [PubMed] [Google Scholar]
- 53.Bagi Abdel M, Arabi M, Abdel Rahim B, et al. Prevalence of gallbladder disease in Sudan: first sonographic field study in adult population. Gastroenterology. 1991;100:A307. [Google Scholar]
- 54.Shoda J, Tanaka N, Osuga T. Hepatolithiasis: epidemiology and pathogenesis update. Front Biosci. 2003;8:e398–e409. doi: 10.2741/1091. [DOI] [PubMed] [Google Scholar]
- 55.Lammert F, Matern S. The genetic background of cholesterol gallstone formation: an inventory of human lithogenic genes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:163–170. doi: 10.2174/1568008054064841. [DOI] [PubMed] [Google Scholar]
- 56.Sarin SK, Negi VS, Dewan R, Sasan S, Saraya A. High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology. 1995;22:138–141. [PubMed] [Google Scholar]
- 57.Gilat T, Feldman C, Halpern Z, Dan M, Bar-Meir S. An increased familial frequency of gallstones. Gastroenterology. 1983;84:242–246. [PubMed] [Google Scholar]
- 58.van der Linden W, Westlin N. The familial occurrence of gallstone disease. II. Occurrence in husbands and wives. Acta Genet Stat Med. 1966;16:377–382. doi: 10.1159/000151986. [DOI] [PubMed] [Google Scholar]
- 59.Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005;41:1138–1143. doi: 10.1002/hep.20654. [DOI] [PubMed] [Google Scholar]
- 60.Rudkowska I, Jones PJ. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr Rev. 2008;66:343–348. doi: 10.1111/j.1753-4887.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 61.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Mittal B, Mittal RD. Genetics of gallstone disease. J Postgrad Med. 2002;48:149–152. [PubMed] [Google Scholar]
- 63.Pullinger CR, Eng C, Salen G, et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider H, Sänger P, Hanisch E. In vitro effects of cholecystokinin fragments on human gallbladders. Evidence for an altered CCK-receptor structure in a subgroup of patients with gallstones. J Hepatol. 1997;26:1063–1068. doi: 10.1016/s0168-8278(97)80115-8. [DOI] [PubMed] [Google Scholar]
- 65.Feng D, Han T, Chen S. Polymorphism at the LDL receptor gene locus in patients with cholesterol gallstone disease. Zhonghua Yi Xue Za Zhi. 1998;78:63–65. [PubMed] [Google Scholar]
- 66.Juvonen T, Savolainen MJ, Kairaluoma MI, Lajunen LH, Humphries SE, Kesäniemi YA. Polymorphisms at the apoB, apoA-I, and cholesteryl ester transfer protein gene loci in patients with gallbladder disease. J Lipid Res. 1995;36:804–812. [PubMed] [Google Scholar]
- 67.Buch S, Schafmayer C, Völzke H, et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39:995–999. doi: 10.1038/ng2101. [DOI] [PubMed] [Google Scholar]
- 68.Grünhage F, Acalovschi M, Tirziu S, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- 69.Einarsson K, Nilsell K, Leijd B, Angelin B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N Engl J Med. 1985;313:277–282. doi: 10.1056/NEJM198508013130501. [DOI] [PubMed] [Google Scholar]
- 70.Völzke H, Baumeister SE, Alte D, et al. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 71.Cirillo DJ, Wallace RB, Rodabough RJ, et al. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- 72.Thijs C, Knipschild P. Oral contraceptives and the risk of gallbladder disease: a meta-analysis. Am J Public Health. 1993;83:1113–1120. doi: 10.2105/ajph.83.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 74.Etminan M, Delaney JA, Bressler B, Brophy JM. Oral contraceptives and the risk of gallbladder disease: a comparative safety study. CMAJ. 2011;183:899–904. doi: 10.1503/cmaj.110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maringhini A, Ciambra M, Baccelliere P, et al. Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med. 1993;119:116–120. doi: 10.7326/0003-4819-119-2-199307150-00004. [DOI] [PubMed] [Google Scholar]
- 76.Valdivieso V, Covarrubias C, Siegel F, Cruz F. Pregnancy and cholelithiasis: pathogenesis and natural course of gallstones diagnosed in early puerperium. Hepatology. 1993;17:1–4. [PubMed] [Google Scholar]
- 77.Ko CW, Beresford SA, Schulte SJ, Lee SP. Insulin resistance and incident gallbladder disease in pregnancy. Clin Gastroenterol Hepatol. 2008;6:76–81. doi: 10.1016/j.cgh.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 79.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 80.Erlinger S. Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol. 2000;12:1347–1352. doi: 10.1097/00042737-200012120-00015. [DOI] [PubMed] [Google Scholar]
- 81.Amaral JF, Thompson WR. Gallbladder disease in the morbidly obese. Am J Surg. 1985;149:551–557. doi: 10.1016/s0002-9610(85)80055-6. [DOI] [PubMed] [Google Scholar]
- 82.Johansen C, Chow WH, Jørgensen T, Mellemkjaer L, Engholm G, Olsen JH. Risk of colorectal cancer and other cancers in patients with gall stones. Gut. 1996;39:439–443. doi: 10.1136/gut.39.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80:38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 84.Li VK, Pulido N, Fajnwaks P, Szomstein S, Rosenthal R, Martinez-Duartez P. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc. 2009;23:1640–1644. doi: 10.1007/s00464-008-0204-6. [DOI] [PubMed] [Google Scholar]
- 85.Maclure KM, Hayes KC, Colditz GA, Stampfer MJ, Speizer FE, Willett WC. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med. 1989;321:563–569. doi: 10.1056/NEJM198908313210902. [DOI] [PubMed] [Google Scholar]
- 86.Lambou-Gianoukos S, Heller SJ. Lithogenesis and bile metabolism. Surg Clin North Am. 2008;88:1175–1194. doi: 10.1016/j.suc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 87.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest. 1977;59:828–840. doi: 10.1172/JCI108705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petitti DB, Friedman GD, Klatsky AL. Association of a history of gallbladder disease with a reduced concentration of high-density-lipoprotein cholesterol. N Engl J Med. 1981;304:1396–1398. doi: 10.1056/NEJM198106043042305. [DOI] [PubMed] [Google Scholar]
- 89.Ahlberg J. Serum lipid levels and hyperlipoproteinaemia in gallstone patients. Acta Chir Scand. 1979;145:373–377. [PubMed] [Google Scholar]
- 90.Barbara L, Sama C, Morselli Labate AM, et al. A population study on the prevalence of gallstone disease: the Sirmione Study. Hepatology. 1987;7:913–917. doi: 10.1002/hep.1840070520. [DOI] [PubMed] [Google Scholar]
- 91.Thijs C, Knipschild P, Brombacher P. Serum lipids and gallstones: a case-control study. Gastroenterology. 1990;99:843–849. doi: 10.1016/0016-5085(90)90978-a. [DOI] [PubMed] [Google Scholar]
- 92.Sakuta H, Suzuki T. Plasma total homocysteine and gallstone in middle-aged Japanese men. J Gastroenterol. 2005;40:1061–1064. doi: 10.1007/s00535-005-1691-z. [DOI] [PubMed] [Google Scholar]
- 93.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 94.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 95.Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–1657. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ata N, Kucukazman M, Yavuz B, et al. The metabolic syndrome is associated with complicated gallstone disease. Can J Gastroenterol. 2011;25:274–276. doi: 10.1155/2011/356761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology. 2000;31:299–303. doi: 10.1002/hep.510310206. [DOI] [PubMed] [Google Scholar]
- 98.Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol. 2006;45:299–305. doi: 10.1016/j.jhep.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 99.Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Twisk J, Hoekman MF, Lehmann EM, Meijer P, Mager WH, Princen HM. Insulin suppresses bile acid synthesis in cultured rat hepatocytes by down-regulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase gene transcription. Hepatology. 1995;21:501–510. [PubMed] [Google Scholar]
- 101.Nakeeb A, Comuzzie AG, Al-Azzawi H, Sonnenberg GE, Kissebah AH, Pitt HA. Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg. 2006;10:940–948. doi: 10.1016/j.gassur.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 102.Everhart JE. Contributions of obesity and weight loss to gallstone disease. Ann Intern Med. 1993;119:1029–1035. doi: 10.7326/0003-4819-119-10-199311150-00010. [DOI] [PubMed] [Google Scholar]
- 103.Yang H, Petersen GM, Roth MP, Schoenfield LJ, Marks JW. Risk factors for gallstone formation during rapid loss of weight. Dig Dis Sci. 1992;37:912–918. doi: 10.1007/BF01300390. [DOI] [PubMed] [Google Scholar]
- 104.Weinsier RL, Ullmann DO. Gallstone formation and weight loss. Obes Res. 1993;1:51–56. doi: 10.1002/j.1550-8528.1993.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 105.Liddle RA, Goldstein RB, Saxton J. Gallstone formation during weight-reduction dieting. Arch Intern Med. 1989;149:1750–1753. [PubMed] [Google Scholar]
- 106.Shiffman ML, Sugerman HJ, Kellum JM, Brewer WH, Moore EW. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86:1000–1005. [PubMed] [Google Scholar]
- 107.Broomfield PH, Chopra R, Sheinbaum RC, et al. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319:1567–1572. doi: 10.1056/NEJM198812153192403. [DOI] [PubMed] [Google Scholar]
- 108.Wudel LJ, Jr, Wright JK, Debelak JP, Allos TM, Shyr Y, Chapman WC. Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res. 2002;102:50–56. doi: 10.1006/jsre.2001.6322. [DOI] [PubMed] [Google Scholar]
- 109.Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98:115–117. doi: 10.1016/S0002-9343(99)80394-5. [DOI] [PubMed] [Google Scholar]
- 110.Al-Jiffry BO, Shaffer EA, Saccone GT, Downey P, Kow L, Toouli J. Changes in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obestity. Can J Gastroenterol. 2003;17:169–174. doi: 10.1155/2003/392719. [DOI] [PubMed] [Google Scholar]
- 111.Syngal S, Coakley EH, Willett WC, Byers T, Williamson DF, Colditz GA. Long-term weight patterns and risk for cholecystectomy in women. Ann Intern Med. 1999;130:471–477. doi: 10.7326/0003-4819-130-6-199903160-00003. [DOI] [PubMed] [Google Scholar]
- 112.Jørgensen T, Jørgensen LM. Gallstones and diet in a Danish population. Scand J Gastroenterol. 1989;24:821–826. doi: 10.3109/00365528909089221. [DOI] [PubMed] [Google Scholar]
- 113.Méndez-Sánchez N, Zamora-Valdés D, Chávez-Tapia NC, Uribe M. Role of diet in cholesterol gallstone formation. Clin Chim Acta. 2007;376:1–8. doi: 10.1016/j.cca.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 114.Cuevas A, Miquel JF, Reyes MS, Zanlungo S, Nervi F. Diet as a risk factor for cholesterol gallstone disease. J Am Coll Nutr. 2004;23:187–196. doi: 10.1080/07315724.2004.10719360. [DOI] [PubMed] [Google Scholar]
- 115.Tseng M, Everhart JE, Sandler RS. Dietary intake and gallbladder disease: a review. Public Health Nutr. 1999;2:161–172. doi: 10.1017/s136898009900021x. [DOI] [PubMed] [Google Scholar]
- 116.Lee DW, Gilmore CJ, Bonorris G, et al. Effect of dietary cholesterol on biliary lipids in patients with gallstones and normal subjects. Am J Clin Nutr. 1985;42:414–420. doi: 10.1093/ajcn/42.3.414. [DOI] [PubMed] [Google Scholar]
- 117.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-chain saturated fatty acids consumption and risk of gallstone disease among men. Ann Surg. 2008;247:95–103. doi: 10.1097/SLA.0b013e31815792c2. [DOI] [PubMed] [Google Scholar]
- 118.Scragg RK, McMichael AJ, Baghurst PA. Diet, alcohol, and relative weight in gall stone disease: a case-control study. Br Med J (Clin Res Ed) 1984;288:1113–1119. doi: 10.1136/bmj.288.6424.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut. 2005;54:823–828. doi: 10.1136/gut.2003.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nervi F, Covarrubias C, Bravo P, et al. Influence of legume intake on biliary lipids and cholesterol saturation in young Chilean men. Identification of a dietary risk factor for cholesterol gallstone formation in a highly prevalent area. Gastroenterology. 1989;96:825–830. [PubMed] [Google Scholar]
- 121.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med. 2004;141:514–522. doi: 10.7326/0003-4819-141-7-200410050-00007. [DOI] [PubMed] [Google Scholar]
- 122.Leitzmann MF, Willett WC, Rimm EB, et al. A prospective study of coffee consumption and the risk of symptomatic gallstone disease in men. JAMA. 1999;281:2106–2112. doi: 10.1001/jama.281.22.2106. [DOI] [PubMed] [Google Scholar]
- 123.Leitzmann MF, Stampfer MJ, Willett WC, Spiegelman D, Colditz GA, Giovannucci EL. Coffee intake is associated with lower risk of symptomatic gallstone disease in women. Gastroenterology. 2002;123:1823–1830. doi: 10.1053/gast.2002.37054. [DOI] [PubMed] [Google Scholar]
- 124.Leitzmann MF, Tsai CJ, Stampfer MJ, et al. Alcohol consumption in relation to risk of cholecystectomy in women. Am J Clin Nutr. 2003;78:339–347. doi: 10.1093/ajcn/78.2.339. [DOI] [PubMed] [Google Scholar]
- 125.Attili AF, Scafato E, Marchioli R, Marfisi RM, Festi D. Diet and gallstones in Italy: the cross-sectional MICOL results. Hepatology. 1998;27:1492–1498. doi: 10.1002/hep.510270605. [DOI] [PubMed] [Google Scholar]
- 126.Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III) Arch Intern Med. 2000;160:931–936. doi: 10.1001/archinte.160.7.931. [DOI] [PubMed] [Google Scholar]
- 127.Simon JA, Hudes ES. Serum ascorbic acid and other correlates of gallbladder disease among US adults. Am J Public Health. 1998;88:1208–1212. doi: 10.2105/ajph.88.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leitzmann MF, Giovannucci EL, Stampfer MJ, et al. Prospective study of alcohol consumption patterns in relation to symptomatic gallstone disease in men. Alcohol Clin Exp Res. 1999;23:835–841. [PubMed] [Google Scholar]
- 129.Su CH, Lui WY, P'eng FK. Relative prevalence of gallstone diseases in Taiwan. A nationwide cooperative study. Dig Dis Sci. 1992;37:764–768. doi: 10.1007/BF01296436. [DOI] [PubMed] [Google Scholar]
- 130.Kameda H, Ishihara F, Shibata K, Tsukie E. Clinical and nutritional study on gallstone disease in Japan. Jpn J Med. 1984;23:109–113. doi: 10.2169/internalmedicine1962.23.109. [DOI] [PubMed] [Google Scholar]
- 131.Guglielmi FW, Boggio-Bertinet D, Federico A, et al. Total parenteral nutrition-related gastroenterological complications. Dig Liver Dis. 2006;38:623–642. doi: 10.1016/j.dld.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 132.Roslyn JJ, Pitt HA, Mann LL, Ament ME, DenBesten L. Gallbladder disease in patients on long-term parenteral nutrition. Gastroenterology. 1983;84:148–154. [PubMed] [Google Scholar]
- 133.Baudet S, Medina C, Vilaseca J, et al. Effect of short-term octreotide therapy and total parenteral nutrition on the development of biliary sludge and lithiasis. Hepatogastroenterology. 2002;49:609–612. [PubMed] [Google Scholar]
- 134.Angelico M, Della Guardia P. Review article: hepatobiliary complications associated with total parenteral nutrition. Aliment Pharmacol Ther. 2000;14(Suppl 2):54–57. doi: 10.1046/j.1365-2036.2000.014s2054.x. [DOI] [PubMed] [Google Scholar]
- 135.Messing B, Bories C, Kunstlinger F, Bernier JJ. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983;84(5 Pt 1):1012–1019. [PubMed] [Google Scholar]
- 136.Shaffer EA. Gallbladder sludge: what is its clinical significance? Curr Gastroenterol Rep. 2001;3:166–173. doi: 10.1007/s11894-001-0015-6. [DOI] [PubMed] [Google Scholar]
- 137.Diehl AK, Rosenthal M, Hazuda HP, Comeaux PJ, Stern MP. Socioeconomic status and the prevalence of clinical gallbladder disease. J Chronic Dis. 1985;38:1019–1026. doi: 10.1016/0021-9681(85)90100-6. [DOI] [PubMed] [Google Scholar]
- 138.Sahi T, Paffenbarger RS, Jr, Hsieh CC, Lee IM. Body mass index, cigarette smoking, and other characteristics as predictors of self-reported, physician-diagnosed gallbladder disease in male college alumni. Am J Epidemiol. 1998;147:644–651. doi: 10.1093/oxfordjournals.aje.a009505. [DOI] [PubMed] [Google Scholar]
- 139.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med. 1999;341:777–784. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 140.Banim PJ, Luben RN, Wareham NJ, Sharp SJ, Khaw KT, Hart AR. Physical activity reduces the risk of symptomatic gallstones: a prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:983–988. doi: 10.1097/MEG.0b013e32833732c3. [DOI] [PubMed] [Google Scholar]
- 141.Acalovschi M, Badea R, Dumitraçcu D, Varga C. Prevalence of gallstones in liver cirrhosis: a sonographic survey. Am J Gastroenterol. 1988;83:954–956. [PubMed] [Google Scholar]
- 142.Conte D, Barisani D, Mandelli C, et al. Cholelithiasis in cirrhosis: analysis of 500 cases. Am J Gastroenterol. 1991;86:1629–1632. [PubMed] [Google Scholar]
- 143.Conte D, Fraquelli M, Fornari F, Lodi L, Bodini P, Buscarini L. Close relation between cirrhosis and gallstones: cross-sectional and longitudinal survey. Arch Intern Med. 1999;159:49–52. doi: 10.1001/archinte.159.1.49. [DOI] [PubMed] [Google Scholar]
- 144.Alvaro D, Angelico M, Gandin C, Ginanni Corradini S, Capocaccia L. Physico-chemical factors predisposing to pigment gallstone formation in liver cirrhosis. J Hepatol. 1990;10:228–234. doi: 10.1016/0168-8278(90)90057-x. [DOI] [PubMed] [Google Scholar]
- 145.Loria P, Lonardo A, Lombardini S, et al. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176–1184. doi: 10.1111/j.1440-1746.2005.03924.x. [DOI] [PubMed] [Google Scholar]
- 146.Méndez-Sánchez N, Bermejo-Martínez LB, Viñals Y, et al. Serum leptin levels and insulin resistance are associated with gallstone disease in overweight subjects. World J Gastroenterol. 2005;11:6182–6187. doi: 10.3748/wjg.v11.i39.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 148.Acalovschi M, Buzas C, Radu C, Grigorescu M. Hepatitis C virus infection is a risk factor for gallstone disease: a prospective hospital-based study of patients with chronic viral C hepatitis. J Viral Hepat. 2009;16:860–866. doi: 10.1111/j.1365-2893.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 149.Whorwell PJ, Hawkins R, Dewbury K, Wright R. Ultrasound survey of gallstones and other hepatobiliary disorders in patients with Crohn's disease. Dig Dis Sci. 1984;29:930–933. doi: 10.1007/BF01312482. [DOI] [PubMed] [Google Scholar]
- 150.Hutchinson R, Tyrrell PN, Kumar D, Dunn JA, Li JK, Allan RN. Pathogenesis of gall stones in Crohn's disease: an alternative explanation. Gut. 1994;35:94–97. doi: 10.1136/gut.35.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brink MA, Slors JF, Keulemans YC, et al. Enterohepatic cycling of bilirubin: a putative mechanism for pigment gallstone formation in ileal Crohn's disease. Gastroenterology. 1999;116:1420–1427. doi: 10.1016/s0016-5085(99)70507-x. [DOI] [PubMed] [Google Scholar]
- 152.Lapidus A, Akerlund JE, Einarsson C. Gallbladder bile composition in patients with Crohn's disease. World J Gastroenterol. 2006;12:70–74. doi: 10.3748/wjg.v12.i1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vítek L, Carey MC. Enterohepatic cycling of bilirubin as a cause of 'black' pigment gallstones in adult life. Eur J Clin Invest. 2003;33:799–810. doi: 10.1046/j.1365-2362.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 154.Apstein MD, Dalecki-Chipperfield K. Spinal cord injury is a risk factor for gallstone disease. Gastroenterology. 1987;92:966–968. doi: 10.1016/0016-5085(87)90971-1. [DOI] [PubMed] [Google Scholar]
- 155.Rotter KP, Larraín CG. Gallstones in spinal cord injury (SCI): a late medical complication? Spinal Cord. 2003;41:105–108. doi: 10.1038/sj.sc.3101408. [DOI] [PubMed] [Google Scholar]
- 156.Xia CS, Han YQ, Yang XY, Hong GX. Spinal cord injury and cholelithiasis. Hepatobiliary Pancreat Dis Int. 2004;3:595–598. [PubMed] [Google Scholar]
- 157.Kennedy TM, Jones RH. Epidemiology of cholecystectomy and irritable bowel syndrome in a UK population. Br J Surg. 2000;87:1658–1663. doi: 10.1046/j.1365-2168.2000.01596.x. [DOI] [PubMed] [Google Scholar]
- 158.Ros E, Zambon D. Postcholecystectomy symptoms. A prospective study of gall stone patients before and two years after surgery. Gut. 1987;28:1500–1504. doi: 10.1136/gut.28.11.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Creutzfeldt W, Lembcke B, Fölsch UR, Schleser S, Koop I. Effect of somatostatin analogue (SMS 201-995, Sandostatin) on pancreatic secretion in humans. Am J Med. 1987;82(5B):49–54. doi: 10.1016/0002-9343(87)90426-8. [DOI] [PubMed] [Google Scholar]
- 160.Trendle MC, Moertel CG, Kvols LK. Incidence and morbidity of cholelithiasis in patients receiving chronic octreotide for metastatic carcinoid and malignant islet cell tumors. Cancer. 1997;79:830–834. doi: 10.1002/(sici)1097-0142(19970215)79:4<830::aid-cncr20>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 161.Roti E, Minelli R, Gardini E, et al. Chronic treatment with a long-acting somatostatin analogue in a patient with intestinal carcinoid tumor: occurrence of cholelithiasis. J Endocrinol Invest. 1990;13:69–72. doi: 10.1007/BF03348589. [DOI] [PubMed] [Google Scholar]
- 162.Lopez AJ, O'Keefe P, Morrissey M, Pickleman J. Ceftriaxone-induced cholelithiasis. Ann Intern Med. 1991;115:712–714. doi: 10.7326/0003-4819-115-9-712. [DOI] [PubMed] [Google Scholar]
- 163.Bickford CL, Spencer AP. Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature. Pharmacotherapy. 2005;25:1389–1395. doi: 10.1592/phco.2005.25.10.1389. [DOI] [PubMed] [Google Scholar]
- 164.Angelin B. Effect of thiazide treatment on biliary lipid composition in healthy volunteers. Eur J Clin Pharmacol. 1989;37:95–96. doi: 10.1007/BF00609433. [DOI] [PubMed] [Google Scholar]
- 165.Rosenberg L, Shapiro S, Slone D, Kaufman DW, Miettinen OS, Stolley PD. Thiazides and acute cholecystitis. N Engl J Med. 1980;303:546–548. doi: 10.1056/NEJM198009043031002. [DOI] [PubMed] [Google Scholar]
- 166.Van der Linden W, Ritter B, Edlund G. Acute cholecystitis and thiazides. Br Med J (Clin Res Ed) 1984;289:654–655. doi: 10.1136/bmj.289.6446.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Leitzmann MF, Tsai CJ, Stampfer MJ, Willett WC, Giovannucci E. Thiazide diuretics and the risk of gallbladder disease requiring surgery in women. Arch Intern Med. 2005;165:567–573. doi: 10.1001/archinte.165.5.567. [DOI] [PubMed] [Google Scholar]
- 168.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Statin use and the risk of cholecystectomy in women. Gastroenterology. 2009;136:1593–1600. doi: 10.1053/j.gastro.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.St Peter SD, Keckler SJ, Nair A, et al. Laparoscopic cholecystectomy in the pediatric population. J Laparoendosc Adv Surg Tech A. 2008;18:127–130. doi: 10.1089/lap.2007.0150. [DOI] [PubMed] [Google Scholar]
- 170.Palasciano G, Portincasa P, Vinciguerra V, et al. Gallstone prevalence and gallbladder volume in children and adolescents: an epidemiological ultrasonographic survey and relationship to body mass index. Am J Gastroenterol. 1989;84:1378–1382. [PubMed] [Google Scholar]
- 171.Kaechele V, Wabitsch M, Thiere D, et al. Prevalence of gallbladder stone disease in obese children and adolescents: influence of the degree of obesity, sex, and pubertal development. J Pediatr Gastroenterol Nutr. 2006;42:66–70. doi: 10.1097/01.mpg.0000187816.31213.06. [DOI] [PubMed] [Google Scholar]
- 172.Kratzer W, Walcher T, Arnold F, et al. Gallstone prevalence and risk factors for gallstone disease in an urban population of children and adolescents. Z Gastroenterol. 2010;48:683–687. doi: 10.1055/s-0028-1109957. [DOI] [PubMed] [Google Scholar]
- 173.Bogue CO, Murphy AJ, Gerstle JT, Moineddin R, Daneman A. Risk factors, complications, and outcomes of gallstones in children: a single-center review. J Pediatr Gastroenterol Nutr. 2010;50:303–308. doi: 10.1097/MPG.0b013e3181b99c72. [DOI] [PubMed] [Google Scholar]
- 174.Holcomb GW, Jr, Holcomb GW., 3rd Cholelithiasis in infants, children, and adolescents. Pediatr Rev. 1990;11:268–274. doi: 10.1542/pir.11-9-268. [DOI] [PubMed] [Google Scholar]
- 175.Friesen CA, Roberts CC. Cholelithiasis. Clinical characteristics in children. Case analysis and literature review. Clin Pediatr (Phila) 1989;28:294–298. doi: 10.1177/000992288902800701. [DOI] [PubMed] [Google Scholar]
- 176.Waldhausen JH, Benjamin DR. Cholecystectomy is becoming an increasingly common operation in children. Am J Surg. 1999;177:364–367. doi: 10.1016/s0002-9610(99)00063-x. [DOI] [PubMed] [Google Scholar]
- 177.Wesdorp I, Bosman D, de Graaff A, Aronson D, van der Blij F, Taminiau J. Clinical presentations and predisposing factors of cholelithiasis and sludge in children. J Pediatr Gastroenterol Nutr. 2000;31:411–417. doi: 10.1097/00005176-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 178.Kumar R, Nguyen K, Shun A. Gallstones and common bile duct calculi in infancy and childhood. Aust N Z J Surg. 2000;70:188–191. doi: 10.1046/j.1440-1622.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 179.Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493–1497. doi: 10.1002/1097-0142(19920915)70:6<1493::aid-cncr2820700608>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 180.Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 181.Lai CH, Lau WY. Gallbladder cancer: a comprehensive review. Surgeon. 2008;6:101–110. doi: 10.1016/s1479-666x(08)80073-x. [DOI] [PubMed] [Google Scholar]
- 182.Levy AD, Murakata LA, Rohrmann CA., Jr Gallbladder carcinoma: radiologic-pathologic correlation. Radiographics. 2001;21:295–314. doi: 10.1148/radiographics.21.2.g01mr16295. [DOI] [PubMed] [Google Scholar]
- 183.Pandey M. Risk factors for gallbladder cancer: a reappraisal. Eur J Cancer Prev. 2003;12:15–24. doi: 10.1097/00008469-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 184.Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 185.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 186.Andia ME, Hsing AW, Andreotti G, Ferreccio C. Geographic variation of gallbladder cancer mortality and risk factors in Chile: a population-based ecologic study. Int J Cancer. 2008;123:1411–1416. doi: 10.1002/ijc.23662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Serra I, Calvo A, Csendes A, Sharp A. Gastric and gallbladder carcinoma in Chile: epidemiological changes and control programs. Rev Med Chil. 1989;117:834–836. [PubMed] [Google Scholar]
- 188.Hsing AW, Bai Y, Andreotti G, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121:832–838. doi: 10.1002/ijc.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for gallbladder cancer across the world. HPB (Oxford) 2008;10:327–331. doi: 10.1080/13651820802007464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shrikhande SV, Barreto SG, Singh S, Udwadia TE, Agarwal AK. Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause. Eur J Surg Oncol. 2010;36:514–519. doi: 10.1016/j.ejso.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 191.Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250:2323–2326. [PubMed] [Google Scholar]
- 192.Csendes A, Becerra M, Rojas J, Medina E. Number and size of stones in patients with asymptomatic and symptomatic gallstones and gallbladder carcinoma: a prospective study of 592 cases. J Gastrointest Surg. 2000;4:481–485. doi: 10.1016/s1091-255x(00)80090-6. [DOI] [PubMed] [Google Scholar]
- 193.Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132–1138. doi: 10.1093/jnci/89.15.1132. [DOI] [PubMed] [Google Scholar]
- 194.Kimura W, Shimada H, Kuroda A, Morioka Y. Carcinoma of the gallbladder and extrahepatic bile duct in autopsy cases of the aged, with special reference to its relationship to gallstones. Am J Gastroenterol. 1989;84:386–390. [PubMed] [Google Scholar]
- 195.Diehl AK, Beral V. Cholecystectomy and changing mortality from gallbladder cancer. Lancet. 1981;2:187–189. doi: 10.1016/s0140-6736(81)90366-4. [DOI] [PubMed] [Google Scholar]
- 196.Tewari M. Contribution of silent gallstones in gallbladder cancer. J Surg Oncol. 2006;93:629–632. doi: 10.1002/jso.20529. [DOI] [PubMed] [Google Scholar]
- 197.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 198.Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95:1402–1410. doi: 10.1111/j.1572-0241.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 199.Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699–703. doi: 10.1067/msy.2001.113888. [DOI] [PubMed] [Google Scholar]
- 200.Berk RN, Armbuster TG, Saltzstein SL. Carcinoma in the porcelain gallbladder. Radiology. 1973;106:29–31. doi: 10.1148/106.1.29. [DOI] [PubMed] [Google Scholar]
- 201.Cunningham SC, Alexander HR. Porcelain gallbladder and cancer: ethnicity explains a discrepant literature? Am J Med. 2007;120:e17–e18. doi: 10.1016/j.amjmed.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 202.Kim JH, Kim WH, Yoo BM, Kim JH, Kim MW. Should we perform surgical management in all patients with suspected porcelain gallbladder? . Hepatogastroenterology. 2009;56:943–945. [PubMed] [Google Scholar]
- 203.Kumar S, Kumar S, Kumar S. Infection as a risk factor for gallbladder cancer. J Surg Oncol. 2006;93:633–639. doi: 10.1002/jso.20530. [DOI] [PubMed] [Google Scholar]
- 204.Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784–787. doi: 10.1111/j.1572-0241.2000.01860.x. [DOI] [PubMed] [Google Scholar]
- 205.Tewari M, Mishra RR, Shukla HS. Salmonella typhi and gallbladder cancer: report from an endemic region. Hepatobiliary Pancreat Dis Int. 2010;9:524–530. [PubMed] [Google Scholar]
- 206.Matsukura N, Yokomuro S, Yamada S, et al. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Cancer Res. 2002;93:842–847. doi: 10.1111/j.1349-7006.2002.tb01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31:907–913. doi: 10.1097/01.pas.0000213435.99492.8a. [DOI] [PubMed] [Google Scholar]
- 208.Nomura T, Shirai Y, Sandoh N, Nagakura S, Hatakeyama K. Cholangiographic criteria for anomalous union of the pancreatic and biliary ducts. Gastrointest Endosc. 2002;55:204–208. doi: 10.1067/mge.2002.121341. [DOI] [PubMed] [Google Scholar]
- 209.Babbitt DP, Starshak RJ, Clemett AR. Choledochal cyst: a concept of etiology. Am J Roentgenol Radium Ther Nucl Med. 1973;119:57–62. doi: 10.2214/ajr.119.1.57. [DOI] [PubMed] [Google Scholar]
- 210.Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Gallbladder carcinoma associated with anomalous pancreaticobiliary duct junction. Can J Gastroenterol. 2007;21:383–387. doi: 10.1155/2007/383949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 212.Hemminki K, Li X. Familial liver and gall bladder cancer: a nationwide epidemiological study from Sweden. Gut. 2003;52:592–596. doi: 10.1136/gut.52.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Venniyoor A. Cholesterol gallstones and cancer of gallbladder (CAGB): molecular links. Med Hypotheses. 2008;70:646–653. doi: 10.1016/j.mehy.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 214.Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol. 2002;16:187–194. doi: 10.1155/2002/787598. [DOI] [PubMed] [Google Scholar]
- 215.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94:2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 216.Larsson SC, Wolk A. Obesity and the risk of gallbladder cancer: a meta-analysis. Br J Cancer. 2007;96:1457–1461. doi: 10.1038/sj.bjc.6603703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yagyu K, Kikuchi S, Obata Y, et al. Cigarette smoking, alcohol drinking and the risk of gallbladder cancer death: a prospective cohort study in Japan. Int J Cancer. 2008;122:924–929. doi: 10.1002/ijc.23159. [DOI] [PubMed] [Google Scholar]
- 218.Chow WH, Johansen C, Gridley G, Mellemkjaer L, Olsen JH, Fraumeni JF., Jr Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer. 1999;79:640–644. doi: 10.1038/sj.bjc.6690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ishiguro S, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Risk factors of biliary tract cancer in a large-scale population-based cohort study in Japan (JPHC study); with special focus on cholelithiasis, body mass index, and their effect modification. Cancer Causes Control. 2008;19:33–41. doi: 10.1007/s10552-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 220.Zatonski WA, La Vecchia C, Przewozniak K, Maisonneuve P, Lowenfels AB, Boyle P. Risk factors for gallbladder cancer: a Polish case-control study. Int J Cancer. 1992;51:707–711. doi: 10.1002/ijc.2910510508. [DOI] [PubMed] [Google Scholar]
- 221.Khan ZR, Neugut AI, Ahsan H, Chabot JA. Risk factors for biliary tract cancers. Am J Gastroenterol. 1999;94:149–152. doi: 10.1111/j.1572-0241.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 222.Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer. 2007;97:1577–1582. doi: 10.1038/sj.bjc.6604047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lowenfels AB, Walker AM, Althaus DP, Townsend G, Domellöf L. Gallstone growth, size, and risk of gallbladder cancer: an interracial study. Int J Epidemiol. 1989;18:50–54. doi: 10.1093/ije/18.1.50. [DOI] [PubMed] [Google Scholar]
- 224.Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet. 1994;343:83–84. doi: 10.1016/s0140-6736(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 225.Strom BL, Soloway RD, Rios-Dalenz JL, et al. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer. 1995;76:1747–1756. doi: 10.1002/1097-0142(19951115)76:10<1747::aid-cncr2820761011>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 226.Murata H, Tsuji S, Tsujii M, et al. Helicobacter bilis infection in biliary tract cancer. Aliment Pharmacol Ther. 2004;20(Suppl 1):90–94. doi: 10.1111/j.1365-2036.2004.01972.x. [DOI] [PubMed] [Google Scholar]