Abstract
Background/Aims
Epigallocatechin-3-gallate (EGCG), the primary catechin in green tea, has anti-inflammatory and anti-oxidative properties. The aim of the current study was to characterize the impact of EGCG on lipopolysaccharide (LPS)-induced innate signaling in bone marrow-derived macrophages (BMMs) isolated from ICR mice.
Methods
The effect of EGCG on LPS-induced pro-inflammatory gene expression and nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling was examined using reverse transcription-polymerase chain reaction, Western blotting, immunofluorescence, and the electrophoretic mobility shift assay.
Results
EGCG inhibited accumulation of LPS-induced IL-12p40, IL-6, MCP-1, ICAM-1, and VCAM-1 mRNA in BMMs. EGCG blocked LPS-induced IκBα degradation and RelA nuclear translocation. EGCG blocked the DNA-binding activity of NF-κB. LPS-induced phosphorylation of ERK1/2, JNK, and p38 was inhibited by EGCG. U0126 (an inhibitor of MEK-1/2) suppressed the LPS-induced IL-12p40, IL-6, MCP-1, ICAM-1, and VCAM-1 mRNA accumulation in BMMs.
Conclusions
These results indicate that EGCG may prevent LPS-induced pro-inflammatory gene expression through blocking NF-κB and MAPK signaling pathways in BMMs.
Keywords: Epigallocatechin-3-gallate, Nuclear factor-κB, Mitogen-activated protein kinase, Macrophage
INTRODUCTION
Complementary and alternative medicine such as herbal/dietary therapy is becoming an increasingly attractive approach to the treatment and prevention of various inflammatory disorders, including inflammatory bowel disease (IBD).1,2 However, despite their clear popularity, absence of empirical data showing efficacy and mechanisms of action in vivo prevents their incorporation into mainstream medicine.
Green tea is one of the widely consumed beverages in the world. Many epidemiologic studies showed that green tea consumption has beneficial effects in preventing the development of atherosclerosis and prostatic cancer. It is generally agreed that these beneficial effects of green tea are mediated by its polyphenols. Green tea contains four polyphenolic compounds known as catechin; (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin gallate and (-)-epigallocatechin-3-gallate (EGCG). EGCG is the most abundant polyphenol in green tea, and has a variety of modulatory actions on physiological functions, such as anti-inflammatory, anti-oxidative, anti-mutagenic, and anticarcinogenic effects.3-7
IBD, such as Crohn's disease and ulcerative colitis, is a chronic and relapsing intestinal inflammation of unknown etiology. It has been proposed that IBD is caused by from aberrant mucosal immune responses to nonpathogenic bacteria and bacterial products in the intestine.8-11 In IBD, the intestinal epithelial cell damage results in an increased uptake of luminal antigens, including bacteria and bacterial products, and thus leads to the activation of immune cells in lamina propria and the mounting of inflammatory responses. Among the immune cells, macrophages and monocytes play an important role in the initiation, development and outcome of immune response and are also found in the inflamed gut mucosa.12-15 However, until now, the direct effects of EGCG on bone marrow-derived macrophages (BMMs) in intestinal inflammation have not been fully investigated.
Lipopolysaccharide (LPS), a gram negative bacteria-derived cell wall product, stimulates over-production of nitric oxide, release of inflammatory cytokines and recruitment of immune cells.16,17 Inflammatory cytokines are important pro-inflammatory mediators and may be responsible for the induction of chemokines, enzymes and adhesion molecules in intestinal inflammation. Most inflammatory cytokines are induced by the activation of transcription factors and protein kinases such as the transcriptional nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK).18-22 Mucosal inflammation in patients with IBD and in experimental models of intestinal inflammation is accompanied by elevated levels of activated NF-κB.23-26 It has been shown previously that MAPK plays a critical role in the transduction of inflammatory response in variable cell types. MAPK differentially regulates the production of pro- and anti-inflammatory cytokines in immune cells including dendritic cells.27-30
In the present study, we investigated the impact of EGCG on LPS-induced innate signaling and proinflammatory gene expression in BMMs.
MATERIALS AND METHODS
1. Isolation and culture of BMMs
Bone marrow cells were isolated from 5- to 8-week-old ICR mice (Samtako Science, Daejeon, Korea) as previously described.31 Mice were sacrificed by cervical dislocation. Femora and tibiae were aseptically removed and dissected free of adherent soft tissue. The bone ends were cut, and the marrow cavity was flushed out into a petri dish by slowly injecting MEM-α medium (Hyclone, Logan, UT, USA) at one end of the bone using a sterile 21-gauge needle. The bone marrow suspension was carefully agitated with a plastic Pasteur pipette to obtain a single cell suspension. Bone marrow cells were washed and depleted of red blood cell (RBC) by hypotonic lysis using RBC lysing buffer (Sigma-Aldrich, St. Louis, MO, USA). After washing twice with phosphate-buffered saline (PBS), the cells were suspended in MEM-α medium supplemented with 10% FBS and 50 units/mL penicillin, 50 µg/mL streptomycin (Gibco, Grand Island, NY, USA). The number of viable cells was determined with trypan blue (Gibco) and bone marrow cells were cultured on 10 cm2 tissue culture dishes in total amount of 2×106 cells/dish. 10 ng/mL of mouse macrophage colony stimulating factor (M-CSF; BioSource, Camarillo, CA, USA) was added to every 10 cm dish to differentiate BMMs. On day 3, non-adherent cells were discarded and adherent cells (immature BMMs) were suspended in fresh MEM-α with M-CSF and used in subsequent experiment. All of the cells were cultured at 37℃ under a humidified atmosphere containing 5% CO2. EGCG was obtained from Sigma-Aldrich and stored as 50 mM stock solutions in 4℃. LPS from Escherichia coli (serotype 0111:B4) was also purchased from Sigma-Aldrich and dissolved in sterile, pyrogen free PBS. U0126 (a specific inhibitor of MEK-1/2, an upstream effector of ERK1/2) was obtained from Calbiochem (San Diego, CA, USA). Cells were pretreated with various concentrations of EGCG (0-100 µM) or U0126 (20 µM) after which they were stimulated with LPS (0.5-1 µg/mL) for times indicated (0 to 1 hour). All methods used in this study was approved by the Animal Care and Use Committee at the Chonnam National University Medical School Research Institution and conformed to US National Institutes of Health (NIH publication No. 86-23 revised 1985) guidelines.
2. Cell viability
BMMs were plated to a 96-well plate at a density of 1×104 cells/well and incubated in medium with various concentrations of EGCG and LPS. After incubation for 24 hours, cell viability was determined by EZ-CyTox (tetrazolium salts, WST-1) cell viability Assay kit (Daeil Lab Inc., Seoul, Korea). After WST-1 reagent was added for 1 to 2 hours at 37℃, the absorbance was determined using a microplate reader (Infinite M200; Tecan Austria GmbH, Grödig, Austria) with Magellan V6 data analysis software (Tecan, Austria GmbH, Austria). Triplicate wells were used for each experimental condition and all experiments were repeated at least three times.
3. Western blotting
The cells were exposed to LPS (1 µg/mL) in the absence or presence of 100 µM EGCG-pretreatment. Following 10 or 30 minutes of incubation at 37℃, cells were washed twice with cold PBS and lysed with RIPA buffer (1 M Tris-HCl, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA) with 1 mM PMSF, Halt™ Phosphatase inhibitor and Halt™ Protease inhibitor cocktail (Thermo) for 15 minutes at 4℃. Lysates were cleared by centrifugation at 14,000 g for 20 minutes at 4℃. The protein concentrations of cell lysates were determined using BCA™ protein assay (Thermo, Rockford, IL, USA). Equivalent amounts of proteins were separated by 12% SDS-PAGE and electrophoretically transferred to PVDF membrane (Millipore, Billerica, MA, USA). The analysis was used primary antibodies as described by the manufacturer; polyclonal anti-IκBα, phospho-IκBα, ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, p38, phospho-p38 (Cell signaling, Danvers, MA, USA), polyclonal anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing three times with TBST, membranes were incubated with secondary HRP-conjugated anti-mouse IgG for 1 hour. After washing, the blots were detected with chemiluminescence (ECL) HRP substrate (Millipore) by image reader (Ras-4000; Fujifilm, Tokyo, Japan).
4. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the instructions provided by the manufacturer. The quantity and purity of total RNA were determined by measuring the optical density using Nanodrop (Nanodrop Technologies, Wilmington, DE, USA). RT was carried out with 1 µg total RNA using MMLV reverse transcriptase (Invitrogen) and RNAsin (Takara, Otsu, Shiga, Japan) for first strand cDNA synthesis. PCR amplification of cDNA was performed using gene-specific primers (Table 1). The quantitative real-time PCR reactions were performed using SYBR Green (SensiMixplus SYBR; Quantace, London, UK) on a Rotor-GeneTm 6000 real-time rotary analyzer (Corbett, San Francisco, CA, USA). The regular PCR was done by using Go taq. Polymerase (Promega, Madison, WI, USA).
Table 1.
Primers for Reverse Transcription-Polymerase Chain Reaction
5. Immunofluoroscence
The cells were plated on 8-chamber slide (Nunc, Rochester, NY, USA). The cells were exposed to LPS (1 µg/mL) for 10 minutes in the absence or presence of 100 µM EGCG-pretreatment. Then cells were washed with PBS and fixed with 4% formaldehyde in room temperature. Next, cells were permeabilized using PBS containing 0.25% Triton X-100 and blocked with PBS containing 1% BSA and 10% FBS. Cells were subjected to staining with polyclonal anti-p65 antibody (Santa Cruz Biotechnology) overnight at 4℃. RelA (p65) in cells was visualized with Alexa 488 (green) conjugated secondary antibody (Invitrogen). Coverslips were mounted in ProLong Gold antifade reagent containing DAPI (Invitrogen) and analyzed using fluorescent microscopy.
6. Electrophoretic mobility shift assay (EMSA)
Nuclear proteins were prepared following the previously described.32 The treated cells were washed twice with PBS and resuspended in lysis buffer (10 mM HEPES [pH7.9], 0.5 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2 mM PMSF, 0.1% NP40). Cells were left on ice for 5 minutes and centrifuged at 12,000×g for 5 minutes. Resulting pellet was resuspended in high salt buffer (20 mM HEPES [pH 7.9], 25% Glycerol, 1.5 mM MgCl2, 0.8 M KCl, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF) and centrifuged at 12,000×g for 20 minutes. The supernatant was collected as the nuclear extract and protein concentration was estimated using BCA™ protein assay (Thermo). The nuclear extracts were stored at -70℃. EMSA was performed using the LightShift™ Chemiluminescent EMSA kit (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. The reaction mixtures (10 µL) containing about 5 µg nuclear extracts were incubated with 10 fmol of the biotin-labeled double-stranded oligonucleotide probes (NF-κB/p65 oligonucleotides; 5'-CAT CGG AAA TTT CCG GAA ATT TCC GGA AAT TTC CGG C-3'/5'-GCC GGA AAT TTC TGG AAA TTT CCG GAA ATT TCC AT G-3') in reaction buffer for 20 minutes at room temperature. Samples were then run into a 5% nondenaturing polyacrylamide gel and transferred to Biodyne™ B Nylon membrane (Thermo, Rockford, IL, USA) in 0.5×TBE buffer. The biotin-labeled NF-κB/p65 probe was detected with a using the streptavidin-horseradish peroxidase conjugate and the chemiluminescent substrate.
7. Statistical analysis
For in vitro experiments, data were analyzed using a paired Student's t-test and differences were considered significant if 2-tailed p-values were <0.05.
RESULTS
1. Impact of EGCG on the viability of BMMs
In order to study the effect of EGCG on cell viability, cells were exposed to different concentrations of EGCG (0 to 200 µM) or LPS (0.5 to 1 µg/mL) for 24 hours. EGCG treatment or LPS stimulation did not affect cell viability in BMMs (data not shown).
2. EGCG inhibits LPS-induced proinflammatory gene expressions in BMMs
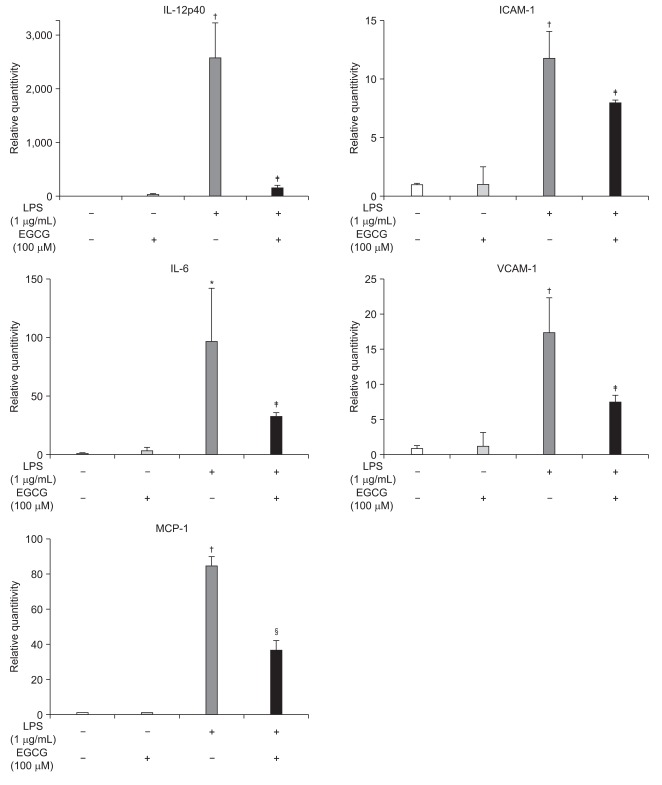
To examine whether EGCG could inhibit the LPS-induced inflammatory cytokines, chemokines and adhesion molecules expression in BMMs, mRNA levels of LPS-induced inflammatory cytokines, chemokines, and adhesion molecules was measured by RT-PCR. LPS-induced IL-12p40, IL-6, MCP-1, ICAM-1 and VCAM-1 mRNA accumulations in BMMs were inhibited by EGCG treatment (Fig. 1).
Fig. 1.
Epigallocatechin-3-gallate (EGCG) inhibited the lipopolysaccharide (LPS)-induced pro-inflammatory gene expression in bone marrow-derived macrophages (BMMs). The BMMs were pretreated with EGCG (100 µM) for 1 hour, stimulated with LPS (1 µg/mL) and harvested at 1 hour. RNA was isolated using the TRIzol procedure, and 1 µg of total RNA was reverse transcribed and amplified using primers for IL-12p40, IL-6, MCP-1, ICAM-1, VCAM-1, and GAPDH. *p<0.05, †p<0.005 compared to unstimulated cells; ‡p<0.05, §p<0.005 compared to LPS stimulation.
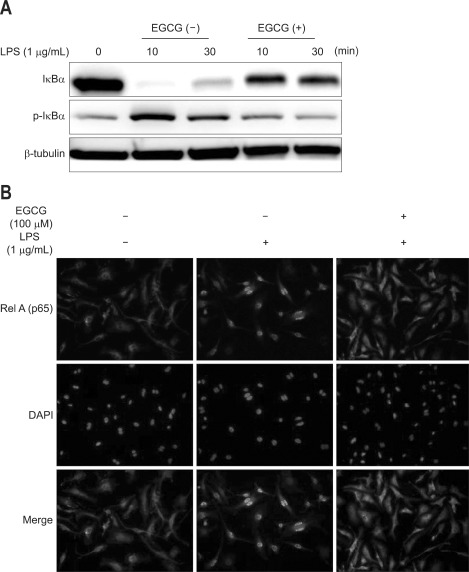
3. EGCG inhibits LPS-induced IκBα phosphorylation/degradation and RelA nuclear translocation in BMMs
To determine whether inhibition of LPS-induced NF-κB activation was due to reduced IκBα phosphorylation/degradation, cells were pretreated with EGCG for 1 hour, before exposing them to 1 µg/mL LPS for 30 minutes. In Western blotting, EGCG treatment inhibited LPS-induced IκBα phosphorylation/degradation (Fig. 2A). The nuclear translocation of NF-κB followed IκBα degradation. RelA (p65) nuclear translocation was evaluated by immunfluorescence. RelA nuclear translocation was blocked by EGCG treatment (Fig. 2B).
Fig. 2.
Epigallocatechin-3-gallate (EGCG) inhibited lipopolysaccharide (LPS)-induced IκBα phosphorylation/degradation and RelA nuclear translocation in bone marrow-derived macrophages (BMMs). (A) The BMMs were pretreated for 1 hour with EGCG (100 µM) and stimulated with LPS (1 µg/mL) for 10 and 30 minutes. Total protein was extracted, and 20 µg of protein was subjected to SDS-PAGE followed by IκBα and β-tubulin immunoblotting using the ECL technique. (B) The BMMs were pretreated with EGCG (100 µM) for 1 hour and stimulated with LPS (1 µg/mL) for 30 minutes. RelA localization was visualized using an anti-RelA primary antibody followed by a rhodamine-conjugated detection antibody.
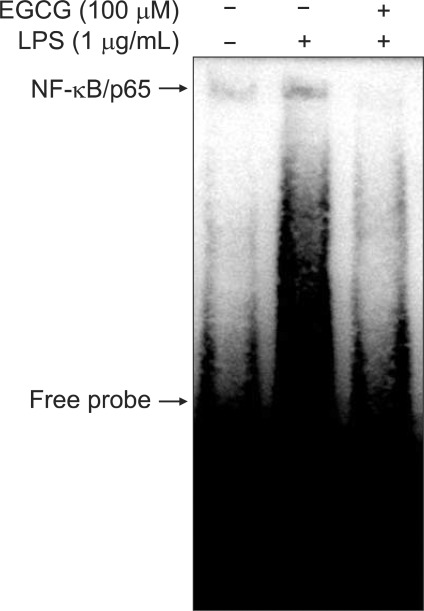
4. EGCG inhibits NF-κB-DNA binding activity in BMM
The DNA binding activity of NF-κB was evaluated by EMSA. BMMs were pretreated with EGCG (100 µM) for 1 hour and then stimulated with LPS (1 µg/mL) for 30 minutes. EGCG markedly suppressed the binding of nuclear extracts to NF-κB consensus nucleotide in BMMs (Fig. 3).
Fig. 3.
Epigallocatechin-3-gallate (EGCG) inhibited nuclear factor-κB (NF-κB)-DNA binding activity in bone marrow-derived macrophages (BMMs). The DNA-binding activity of NF-κB was evaluated by EMSA. The BMMs were pretreated with EGCG (100 µM) for 1 hour, stimulated with LPS (1 µg/mL) for 30 minutes, and nuclear extracts were prepared.
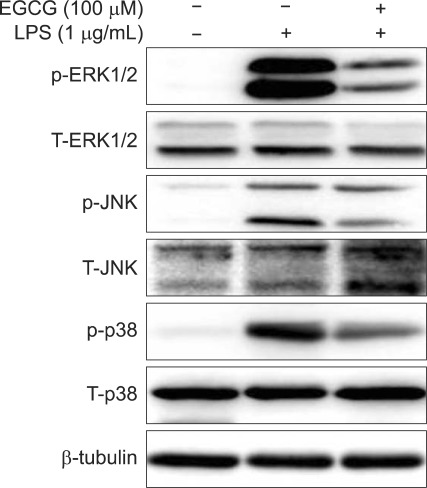
5. EGCG inhibits LPS-induced phosphorylation of MAPK signal proteins in BMMs
MAPK is a critical factor mediating inflammatory responses to external stimuli such as LPS. We next examined whether EGCG suppress MAPK signaling pathway in the presence of LPS by studying the phosphorylation level of MAPK signaling proteins in BMMs. Western blot analysis showed that EGCG inhibited LPS-induced phosphorylations of ERK1/2, JNK, and p38 in BMMs (Fig. 4).
Fig. 4.
Epigallocatechin-3-gallate (EGCG) inhibited the lipopolysaccharide (LPS)-induced phosphorylation of MAPK signaling proteins in bone marrow-derived macrophages (BMMs). The BMMs were pretreated for 1 hour with EGCG (100 µM) and stimulated with LPS (1 µg/mL) for 30 minutes. Total protein was extracted, and 20 µg of protein was subjected to SDS-PAGE followed by phospho-ERK1/2, total ERK1/2, phospho-JNK, total JNK, phospho-p38, total p38, and β-tubulin immunoblotting using the chemiluminescence (ECL) technique. The results are representative of three independent experiments.
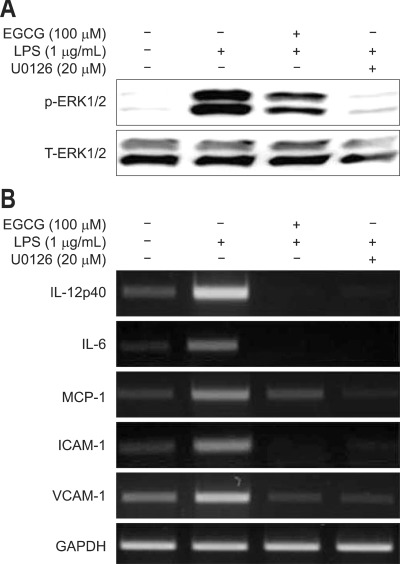
6. Effect of MAPK inhibitor on LPS-induced proinflammatory gene expression in BMMs
U0126 (a specific inhibitor of MEK-1/2, an upstream effector of ERK1/2) and EGCG suppressed the LPS-induced ERK1/2 phosphorylation and IL-12p40, IL-6, MCP-1, ICAM-1, and VCAM-1 mRNA accumulations in BMMs (Fig. 5).
Fig. 5.
The effect of a MAPK inhibitor on the lipopolysaccharide (LPS)-induced gene expression in bone marrow-derived macrophages (BMMs). The BMMs were pretreated with 20 µM U0126 and 100 µM epigallocatechin-3-gallate (EGCG) for 1 hour and stimulated with 1 µg/mL LPS for 1 hour. U0126 and EGCG suppressed LPS-induced ERK1/2 phosphorylation (A), and IL-12p40, IL-6, MCP-1, ICAM-1 and VCAM-1 accumulation of mRNA (B) in BMMs.
DISCUSSION
IBD appears to result from dysregulated immune response to various external stimuli.8-11 Current therapies for patients with IBD are largely based on immunosuppressants such as prednisone, azathioprine and 6-mercaptopurine or biologics such as infliximab.8-11 Although these are relatively effective, a number of patients develop significant side effects and/or become unresponsive to them. Recent data demonstrate that many plant-derived compounds show anti-inflammatory activities and most of their actions are related to cytokines, chemokines or adhesion molecules. Many inflammatory disorders are initially associated with an imbalance of cytokine networks. Therefore, complementary and alternative medicine including plant derived agents would be useful for the treatment of inflammatory disorders including IBD.33 Practically, IBD patients commonly use complementary and alternative medicine in addition to traditional therapy.
Green tea is one of the most popular beverages worldwide and believed to elicit anti-oxidant, anti-carcinogenic, anti-inflammatory and immunomodulatory properties. EGCG is the most abundant polyphenol present in green tea and is believed to provide the majority of these beneficial effects observed with green tea consumption.3-7 Although these beneficial effects of EGCG on inflammation and cancer have been studied extensively in various cell types, the scientific basis for these beneficial effects of EGCG is still lacking.
A prominent feature of IBD is the presence of inflammatory cells in the gut mucosa, and neutrophil migration depends on cytokines released by macrophages and mast cells. Especially, mucosal macrophages derived from circulating monocytes play a major role in chronic mucosal inflammation.12-15 Therefore, the aim of current study was to characterize the impact of EGCG on LPS-induced innate signaling in BMMs.
Cell signaling pathways that are responsible for maintaining a bal ance between cell proliferation and apoptosis, have emerged as rational targets for the management of inflammation and cancer.7 IBD is known to have alterations in multiple cellular signaling pathways and because of the complexities in the interaction among multiple signaling networks, definite treatment and cure for IBD are still elusive.
NF-κB is a mammalian transcription factor that regulates the expression of genes encoding proinflammatory cytokines, chemokines, immune receptors and adhesion molecules that play a key part in inflammation related injury such as IBD.18-20 In unstimulated cells, NF-κB, a predominant heterodimer consisting of p65 (RelA) and p50 subunits, is present as a latent, inactive, IκB bound complex in the cytoplasm but upon activation by various external stimuli, NF-κB rapidly translocates to the nucleus, binds to specific sequences in the promoter region and induces proinflammatory gene expression.18-20 Activated NF-κB and increased NF-κB DNA binding activity has been demonstrated in the inflammed colonic mucosa of patients with IBD.23-26 These findings have suggested that NF-κB may be an effective therapy target in treating intestinal inflammation. Previously, EGCG blocked NF-κB activation by inhibiting IκB kinase activity in intestinal epithelial cell line.34 And EGCG modulated the production of inflammatory cytokines and chemokines in human colon cancer cell lines, macrophage cell lines and bone marrow-derived dendritic cells.35-40 Moreover, EGCG ameliorated mucosal inflammation in mouse experimental colitis models.41,42 Our study showed that EGCG inhibited LPS-induced IκBα degradation, RelA nuclear translocation and NF-κB DNA binding activity in BMMs. EGCG inhibited proinflammatory gene expressions including cytokines, chemokines, and adhesion molecules in BMMs. These results suggest that EGCG exhibits potent anti-inflammatory effects by modulating proinflammatory gene productions via blockade of NF-κB activation.
MAPKs have been implicated in many physiologic processes, including cell proliferation, differentiation, and apoptosis. Three major types of MAPKs in mammarian cells are the ERK1/2, JNK, and p38 MAPKs.21,22 Activated MAPKs modulate a number of different steps in the inflammatory cascade. These include production of pro-inflammatory cytokines, degranulation of neutrophils, as well as the expression of important determining parameters of colon injury including cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS).27-30 Previously, EGCG has been shown to inhibit angiogenesis and induce arrest of cell cycle and apoptosis in variable cancer cell lines and protected oxidative stress-mediated damage which is partly mediated by suppression of MAPKs pathway.43-46 Our study showed that EGCG inhibits LPS-induced phosphorylation of ERK1/2, JNK, and p38 in BMMs. In addition, an inhibitor of MEK-1/2, U0126 suppressed the LPS-induced ERK1/2 phosphorylation and proinflammatory gene expressions including cytokines, chemokines and adhesion molecules in BMMs. These findings are similar with those of EGCG treatment. Therefore, these results indicate that inhibition of proinflammatory gene expressions by EGCG may be caused by inhibition of MAPK signaling pathway in BMMs.
Taken together, EGCG has been shown to modulate NF-κB and MAPK signaling pathways in a fashion that controls proinflammatory gene expression, thereby imparting strong anti-inflammatory effect. Therefore, modulation of these cell signaling pathways by EGCG could contribute to be beneficial in the treatment of IBD.
ACKNOWLEDGEMENTS
This work was supported by a grant (0720570) from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea, and partly by a grant from the Korea Science & Engineering Foundation through the Medical Research Center for Gene Regulation (R13-2002-013-04001-0) at Chonnam National University, Republic of Korea.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;(343):1–19. [PubMed] [Google Scholar]
- 2.Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the Asian adult population: United States, 2004-2006. Adv Data. 2008;(394):1–22. [PubMed] [Google Scholar]
- 3.Chen D, Milacic V, Chen MS, et al. Tea polyphenols, their biological effects and potential molecular targets. Histol Histopathol. 2008;23:487–496. doi: 10.14670/hh-23.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol. 2008;1:195–212. doi: 10.2174/1874467210801030195. [DOI] [PubMed] [Google Scholar]
- 9.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 11.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 17.Magness ST, Jijon H, Van Houten Fisher N, et al. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 18.Karrasch T, Jobin C. NF-kappaB and the intestine: friend or foe? Inflamm Bowel Dis. 2008;14:114–124. doi: 10.1002/ibd.20243. [DOI] [PubMed] [Google Scholar]
- 19.Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 20.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 21.Kong AN, Yu R, Chen C, Mandlekar S, Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res. 2000;23:1–16. doi: 10.1007/BF02976458. [DOI] [PubMed] [Google Scholar]
- 22.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 23.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 24.Davé SH, Tilstra JS, Matsuoka K, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–7859. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997;158:226–234. [PubMed] [Google Scholar]
- 26.Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–38178. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 27.Kong AN, Owuor E, Yu R, et al. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Hong S, Feng Z, et al. Regulation of lipopolysaccharide-induced inflammatory response by heat shock protein 27 in THP-1 cells. Cell Immunol. 2010;264:127–134. doi: 10.1016/j.cellimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez-Fidalgo S. Cpolysaccharide-induced inflammatory response by heat shock protein 27 in THP-1 cells. Cell Immunol 2010;264:127-134. lemmmation in mice. Eur J Pharmacol. 2010;633:78–84. doi: 10.1016/j.cellimm.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Sheng KC, Pietersz GA, Tang CK, Ramsland PA, Apostolopoulos V. Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J Immunol. 2010;184:2863–2872. doi: 10.4049/jimmunol.0903458. [DOI] [PubMed] [Google Scholar]
- 31.Chambers TJ, Owens JM, Hattersley G, Jat PS, Noble MD. Generation of osteoclast-inductive and osteoclastogenic cell lines from the H-2KbtsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1993;90:5578–5582. doi: 10.1073/pnas.90.12.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin Part II modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70:93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Oz HS, Barve S, et al. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol. 2001;60:528–533. [PubMed] [Google Scholar]
- 35.Porath D, Riegger C, Drewe J, Schwager J. Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J Pharmacol Exp Ther. 2005;315:1172–1180. doi: 10.1124/jpet.105.090167. [DOI] [PubMed] [Google Scholar]
- 36.Navarro-Perán E, Cabezas-Herrera J, Sánchez-Del-Campo L, García-Cánovas F, Rodríguez-López JN. The anti-inflammatory and anti-cancer properties of epigallocatechin-3-gallate are mediated by folate cycle disruption, adenosine release and NF-kappaB suppression. Inflamm Res. 2008;57:472–478. doi: 10.1007/s00011-008-8013-x. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa D, Matsui A, Imai M, Sonoda Y, Kasahara T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774.1. Biol Pharm Bull. 2004;27:1353–1358. doi: 10.1248/bpb.27.1353. [DOI] [PubMed] [Google Scholar]
- 38.Melgarejo E, Medina MA, Sánchez-Jiménez F, Urdiales JL. Epigallocatechin gallate reduces human monocyte mobility and adhesion in vitro. Br J Pharmacol. 2009;158:1705–1712. doi: 10.1111/j.1476-5381.2009.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers J, Perkins I, van Olphen A, et al. Epigallocatechin gallate modulates cytokine production by bone marrow-derived dendritic cells stimulated with lipopolysaccharide or muramyldipeptide, or infected with Legionella pneumophila. Exp Biol Med (Maywood) 2005;230:645–651. doi: 10.1177/153537020523000906. [DOI] [PubMed] [Google Scholar]
- 40.Ahn SC, Kim GY, Kim JH, et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-kappaB. Biochem Biophys Res Commun. 2004;313:148–155. doi: 10.1016/j.bbrc.2003.11.108. [DOI] [PubMed] [Google Scholar]
- 41.Ran ZH, Chen C, Xiao SD. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother. 2008;62:189–196. doi: 10.1016/j.biopha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Abboud PA, Hake PW, Burroughs TJ, et al. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579:411–417. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–1886. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 45.Kundu JK, Surh YJ. Epigallocatechin gallate inhibits phorbol ester-induced activation of NF-kappa B and CREB in mouse skin: role of p38 MAPK. Ann N Y Acad Sci. 2007;1095:504–512. doi: 10.1196/annals.1397.054. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Zhang HY. Cancer preventive mechanisms of the green tea polyphenol (-)-epigallocatechin-3-gallate. Molecules. 2007;12:946–957. doi: 10.3390/12050946. [DOI] [PMC free article] [PubMed] [Google Scholar]