Abstract
Background/Aims
Aging gastric mucosa is known to have decreased mucosal defenses and increased susceptibility to injury by nonsteroidal anti-inflammatory drugs. Depending on the type of nonsteroidal anti-inflammatory drug (NSAID), the underlying mechanisms and the extent of damage to the stomach or intestine may differ. This study was performed to evaluate the acute gastric damage caused by different doses of indomethacin, diclofenac and aspirin in rats of various ages.
Methods
For the acute models, indomethacin (10, 20 or 40 mg/kg), diclofenac (40 or 80 mg/kg) or aspirin (100 mg/kg) was given to 7- and 25-week-old and 1-year-old Sprague-Dawley rats by intragastric gavage. The gross ulcer index, damage area as assessed by imaging, histological index, myeloperoxidase (MPO) activity, and cytosolic phospholipase A2 (cPLA2) levels were measured after 24 hours.
Results
The gross ulcer index and damage area increased with age in the presence of three NSAIDs (p<0.05). The increases in MPO levels induced by diclofenac and aspirin were significantly higher in 1-year-old than 7-week-old rats (p<0.05). cPLA2 expression induced by indomethacin (10 and 40 mg/kg) was greater in the 1-year-old rats, compared with 7-week-old rats (p<0.05).
Conclusions
NSAID-induced acute gastric damage increased in a dose- and age-dependent manner.
Keywords: Nonsteroidal anti-inflammatory drugs, Aging, Gastric damage, Aspirin
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin are widely used as anti-inflammatory and analgesic agents, and are commonly prescribed by physicians. However, gastrointestinal toxicity associated with NSAIDs is an important medical problem. Gastric mucosal injury is thought to result when aggressive luminal factors (such as acid, NSAIDs, or Helicobacter pylori) overwhelm mucosal protective mechanism.1,2 Most previous studies examining gastric mucosal injury have investigated the mechanisms of NSAID-induced gastric mucosal lesions development and progression.3,4 Although the inhibition of cyclooxygenase (COX) induced by NSAIDs leading to the depletion of endogenous prostaglandins is known to be a major pathogenic element in the development of these lesions, there are other factors including neutrophil activation,5,6 decrease of gastric mucus production,7,8 hypermotility,9 and oxygen free radicals.10 However, the exact pathogenic mechanism remains to be elucidated. Mucosal proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-8 are considered to be key factors of gastric injury induced by NSAIDs like indomethacin.11 In addition to proinflammatory cytokines, the accumulation and activation of neutrophils, which may lead to microcirculation disturbances and the production of free radicals in gastric mucosa, are also important events in gastric injury due to NSAIDs.12,13
While increased exposure to NSAIDs among the elderly is an obvious risk factor the development of NSAID gastropathy and its complications, epidemiologic analyses indicate that aging is an independent risk factor.14 However, there are a few experimental data on age-related changes in gastric mucosal functions that may predispose the elderly to NSAID gastropathy.15-17 Experimental and limited clinical studies indicate that aging gastric mucosa has impaired mucosal defenses such as decreased mucus and bicarbonate secretion,17-19 decreased prostaglandin generation,15,20,21 reduced nitric oxide synthase activity,17,22 and reduced blood flow.23,24 Furthermore, aging increases the susceptibility to injury by a variety of damaging agents such ethanol7,23 as well as NSAIDs,25,26 and also impairs the healing of acute injury and chronic gastric ulcers.15,27
It has been reported that gastric toxicity is strongly influenced by the amount of drug dissolved under the pH conditions rather than the potency of the drug as an inhibitor of prostaglandin synthesis.28 For example, aspirin exerts its gastrotoxic effects predominantly by localized action during the gastric absorption of the drug; aspirin is much more gastrotoxic than other NSAIDs despite its relatively low potency as a COX inhibitor.28 In contrast, indomethacin is associated with greater intestinal toxicity compared to other NSAIDs, suggesting that it could be related to enterohepatic circulation and the continuous inhibition of prostaglandins.28 These results suggest that the extent of ulcer damage and the related mechanisms might be different depending on the type or dose of NSAID.
Based on these previous findings, we investigated the acute gastric damage caused by different dosages of three NSAIDs, indomethacin, diclofenac, and aspirin, in rats of various ages to provide information for understanding the mechanism underlying acute NSAIDs-induced gastric damage.
MATERIALS AND METHODS
1. Animals
Six-, 24-, and 51-week-old male Sprague-Dawley (SD) rats were purchased (Orient Co., Ltd., Seoul, Korea) and housed in wire-bottom cages maintained at 20℃ to 26℃, 35% to 75% humidity and in a 12/12-hour light/dark cycle (lights on, 8:00 to 20:00) under pathogen-free conditions. After 1 week of adaptation, 7-week-old (weighing 260 to 290 g), 25-week-old (weighting 600 to 800 g) and 1-year-old (weighting 600 to 850 g) rats were used for the experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital.
2. Experimental design
The rats were starved but given water for 24 hours prior to the experiments. NSAIDs (indomethacin, Sigma-Aldrich Co., St. Louis, MO, USA, 10, 20 or 40 mg/kg body weight; diclofenac, Sigma-Aldrich Co., 40 or 80 mg/kg body weight; or aspirin, Sigma-Aldrich Co., 100 mg/kg body weight) or 0.5% carboxymethylcellulose (CMC; 5 mL/kg body weight) as a control were administered orally by gavage through a metal tube attached to a 5- or 10-mL syringe. Each group consisted of seven rats. The indomethacin solution was dissolved in 0.5% (wt/vol) CMC with 10% ethanol, pH ranging from 1.3 to 1.5. The aspirin solution was prepared in the same way but with 20% ethanol which maintained aspirin in its readily-absorbed non-ionized form. The diclofenac solution was dissolved in 0.5% (wt/vol) CMC. As we used 10% and 20% ethanol to dissolve indomethacin and aspirin, respectively, we tested these percentages on control rat to confirm that it was not a potential confounder. There was no specific lesion occurred with 20% ethanol similar to 0.5% CMC, and 0.5% CMC was administered into control group as a vehicle. Twenty-four hours after NSAID or vehicle administration, the animals were humanely sacrificed and the gastric lesions were scored.
3. Gross damage (gross ulcer index and damage area) and histological index
After sacrifice, the isolated stomachs were cut open along the greater curvature and washed in ice-cold saline. To investigate the degree of gross mucosal damage, the mucosal sides of the stomachs were photographed using a digital camera, and part of the mucosa was immediately fixed with a 10% formalin solution. The mucosal surface was macroscopically observed and ulcer scores were determined. The gross damage of the gastric mucosa was assessed by two experienced gastroenterologists, who were blinded to the experiment, using a previously described gross ulcer index7,29 defined as (number of type I lesions)+(number of type II lesions)×2+(number of type II lesions)×3. The lesion type was classified as follows: type I indicated the presence of edema, hyperemia, or a single submucosal punctiform hemorrhage; type II was defined as the presence of submucosal hemorrhagic lesions with small erosions; and type III indicated the presence of a deep ulcer with erosions and invasive lesions. A total injury score for each stomach was calculated by summing the gross ulcer index of all lesions in that stomach. In addition, the damage area was also measured by the image program. The areas of gross damage (erosion or ulceration) were measured by using a computerized video analysis system (MetaMorph 7.0; Molecular Devices, Downington, PA, USA). The area of mucosal damage was expressed as a percentage of the total mucosal area.
The stomach was cut longitudinally to a 5 mm width from the cardia to the pylorus in the anterior aspect after opening stomach along greater curvature regardless of the damaged area or inflammation, and fixed in the formalin. The tissue specimens were dehydrated and embedded in paraffin. The longest part of the specimen was sectioned into 6 µm fragments, and the sections were stained with hematoxylin and eosin (H&E). An experienced pathologist who was blinded to the experiment examined all tissues. The mucosal damage was graded by assigning a previous described index of histological injury30 defined as (% type I damage)×1+(% type II damage)×2+(% type III damage)×3. The types of damage were defined as follows: type 0 damage meant all gastric mucosal cells appeared intact and had a normal shape, location, appearance, and density; type I damage indicated that surface epithelial cells and the uppermost 2 or 3 cells lining the glands were damaged; type II damage described damage greater than type I but involving <50% of the thickness of the gastric mucosa; and type III damage indicated damage involving >50% of the gastric mucosa depth.
4. Measurement of mucosal myeloperoxidase (MPO)
An assay of gastric mucosal MPO concentration was used to quantify the degree of neutrophil infiltration.31,32 Three hundred milligrams of scraped mucosa were homogenized for 30 seconds with a polytron homogenizer in 1.0 mL of ice-cold 0.5% hexadecyltrimethylammonium bromide in 50 mM phosphate buffer (pH 6.0). Hexadecyltrimethylammonium bromide was used to negate the pseudoperoxidase activity of hemoglobin and to solubalize membrane-bound MPO. The homogenate was sonicated for 10 seconds, freeze-thawed three times and centrifuged for 20 minutes at 18,000 ×g. The supernatant was isolated and the MPO concentration was determined using an ELISA kit (Immundiagnostik AG, Bensheim, Germany).
5. Western blotting for cytosolic phospholipase A2 (cPLA2)
Gastric expression of cPLA2 was used to quantify the degree of inflammation. The gastric mucosa was homogenized with lysis buffer containing 25 mM Tris-HCL (pH 7.4), EGTA (1 mM), DTT (1 mM), leupeptin (10 µg/mL), aprotinin (10 µg/mL), PMSF (1 mM), and Triton X-100 (0.1%). The proteins (30 µg for each sample) were separated by SDS-PAGE (7.5% gel) and transferred to nitrocellulose membranes. All procedures were done in Tris buffer (40 mM, pH 7.55) containing 0.3 M NaCl and 0.3% Tween 20. The membranes were then blocked with 6% (wt/vol) milk and subsequently incubated with an anti-cPLA2 antibody (mouse monoclonal IgG2b antibody, 1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4℃. The blots were then incubated with a secondary antibody (goat polyclonal antibody, 1:5,000 dilution; Santa Cruz Biotechnology) and an imaging analyzer was used to measure the band densities.
6. Statistical analysis
All statistical calculations were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). The results were compared using the Mann-Whitney U test and Kruskall-Wallis test. All values are reported as the mean±standard error (SEM). Statistical significance was set at p<0.05.
RESULTS
1. Gross findings of indomethacin-, diclofenac- and aspirininduced damage
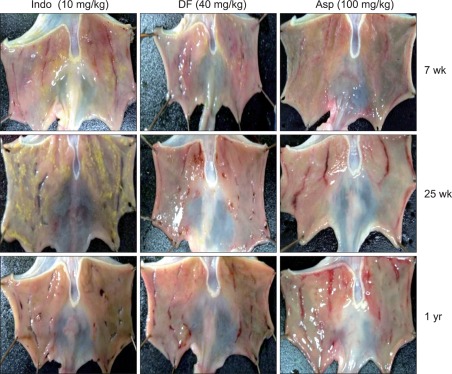
The gross appearance of gastric damage patterns was different depending on the type of NSAIDs, and became more apparent when the damage was severe (Fig. 1). Indomethacin caused a punctuate type clean ulcer, and was accompanied by hemorrhage when the damage was severe. The gastric damage induced by dicolfenac was similar to that caused by indomethacin, but the depth of ulcers was shallow and the damage extent was less severe than that caused by indomethacin (Fig. 1). In contrast, the ulcers caused by aspirin were usually shallow and had a linear shape, but were associated with hemorrhage more often than those caused by indomethacin or diclofenac (Fig. 1).
Fig. 1.
Gross findings of the gastric damage caused by indomethacin (10 mg/kg), diclofenac (40 mg/kg), and aspirin (100 mg/kg) in rats.
Indo, indomethacin; DF, diclofenac; Asp, aspirin.
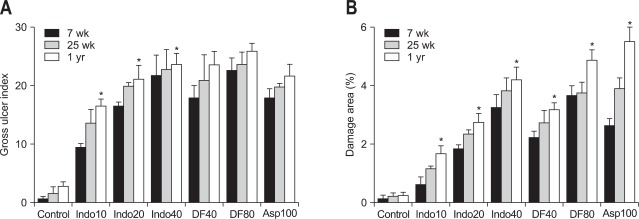
2. Gross ulcer index and damage area depending on age and dose of NSAIDs
All of the NSAID groups showed significantly higher gross ulcer index values than the control group (Fig. 2A). The gross ulcer index increased with aging in rat treated with all three kinds of NSAIDs (Fig. 2A). These were proportionally increased in animals treated with indomethacin (10, 20, or 40 mg/kg). That is, the gross ulcer index in a 1-year-old rat treated with indomethacin was significantly higher than that in a 7-week-old rat (p<0.05). However, the gross ulcer index of rats treated with diclofenac (40 and 80 mg/kg) as well as aspirin (100 mg/kg) were not significantly associated with age (Fig. 2A). The damage area measured by the image program was distinctly increased with aging in rats that received all three NSAID treatments (Fig. 2B). All three kinds of NSAIDs significantly induced more severe damage in 1-year-old rats than in 7-week-old rats.
Fig. 2.
(A) Gross ulcer index and (B) damaged area in the rat stomachs (7- and 25-week-old and 1-year-old rats) assessed by the image program. Mean±standard error with seven rats per group.
Indo10, indomethacin 10 mg/kg; Indo20, indomethacin 20 mg/kg; Indo40, indomethacin 40 mg/kg; DF40, diclofenac 40 mg/kg; DF80, diclofenac 80 mg/kg; Asp 100, aspirin 100 mg/kg.
*p<0.05 compared with the 7-week-old rats in the same nonsteroidal anti-inflammatory drug-treatment group.
When the extent of the damage was assessed according to the dose of indomethacin, there was a statistically significant increase in the gross ulcer index as well as the area of damage (Fig. 2) in the animals treated with 40 mg/kg indomethacin compared to ones that received 10 mg/kg regardless of age (7- and 25-week-old, or 1-year-old rats). With diclofenac, this dosedependent increase in the gross ulcer index was not observed but there was a significant difference in the area of damage (Fig. 2). That is, the damage area was significantly increased by 80 mg/kg diclofenac compared to 40 mg/kg diclofenac in 7-week- and 1-year-old rats.
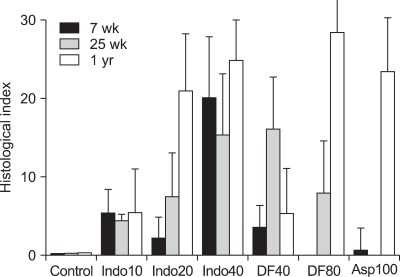
3. Histological index depending on age
The histological index tended to increase with age in the rats treated with NSAIDs (Fig. 3). Gastric damage caused by indomethacin, diclofenac, or aspirin were more severe in 1-year-old rats than 7-week-old rats, but there was no statistical significance (Fig. 3). In case of 7-week group of diclofenac 80 mg/kg and 25-week group in aspirin 100 mg/kg the histological index was near zero similar to control group.
Fig. 3.
Histological index in the rat stomachs (7- and 25-week-old and 1-year-old rats). Mean±standard error with seven rats per group. Indo10, indomethacin 10 mg/kg; Indo20, indomethacin 20 mg/kg; Indo40, indomethacin 40 mg/kg; DF40, diclofenac 40 mg/kg; DF80, diclofenac 80 mg/kg; Asp 100, aspirin 100 mg/kg.
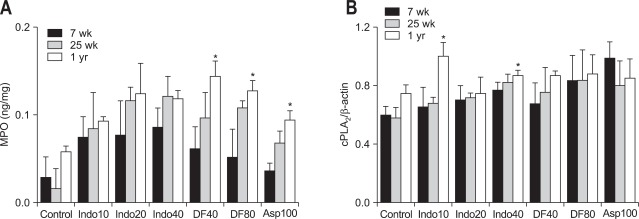
4. MPO levels and expression of cPLA2 depending on age
MPO levels were increased in the all of the NSAID-treated groups compared to the control group (Fig. 4A). When these levels were compared in each NSAID group according to age, the MPO levels in diclofenac or aspirin group were significantly higher in the 1-year-old rats than 7-week-old rats. In animals treated with indomethacin, the MPO levels were increased with age but there was no significant difference (Fig. 4A). cPLA2 expression in the rats treated with 10 and 40 mg/kg indomethacin was higher in 1-year-old rats than 7-week-old rats (Fig. 4B). However, there was no significant difference in groups treated with the other two NSAIDs (Fig. 4B).
Fig. 4.
Mucosal concentrations of (A) myeloperoxidase (MPO) and (B) cPLA2 expression in the rat stomachs (7- and 25-week-old and 1-year-old rats). Mean±standard error with seven rats per group.
Indo10, indomethacin 10 mg/kg; Indo20, indomethacin 20 mg/kg; Indo40, indomethacin 40 mg/kg; DF40, diclofenac 40 mg/kg; DF80, diclofenac 80 mg/kg; Asp100, aspirin 100 mg/kg.
*p<0.05 compared with the 7-week-old rats in the same nonsteroidal anti-inflammatory drug-treatment group.
DISCUSSION
Although the mechanisms of NSAID-induced gastric injury are not well understood, it is widely accepted that both COX-dependent and -independent mechanisms are involved. Furthermore, various factors such as the stimulation of gastric acid production, inflammatory cells infiltration, cytokines, mucosal blood flow, and free radicals are known to contribute to the development of NSAID-induced gastric mucosal damage. We previously suggested that the impairment of apoptosis, angiogenesis, and sensory neuron activity via the activation of early gross response-1 (Egr-1), phosphatase and tension homologue deleted on chromosome 10 (PTEN) might increase the susceptibility of the gastric mucosa to injury with aging using 6-, 30-, and 74-week-old and 2-year-old Fischer 344 rats.17 However, since the Fischer 344 rat, the well-known rat strain for the aging or the carcinogenicity, are not appropriate for the acute or chronic NSAID-induced inflammation experiment (not published), we decided to use SD rat in our present study, which was the commonly used rat strain in the previously published papers regarding NSAID-induced enteropathy. When we determined the gross damage caused by the different types or doses of NSAIDs using the gross ulcer index, the damage was found to be more severe in 1-year-old rats than 7-week-old rats. Furthermore, this age-related tendency became clearer when the damage area was calculated by the image program instead of simply counting the number of ulcer or erosion. When we measured the area of NSAID-induced damage, there was a statistically significant difference between 7-week-old and 1-year-old rats treated with either diclofenac (40, 80 mg/kg) or aspirin (100 mg/kg). However, when the damage was measured by the gross ulcer index, there was no statistical difference.
Both the gross ulcer index and the damage area increased in a dose-dependent manner by indomethacin. However, when the area of diclofenac-induced damage was compared between rats that received 40 or 80 mg/kg of the drug, there was a significant difference only in the damage area but not in the gross ulcer index. These results may be explained by the fact that the gross ulcer index could not measure the severity of damage in the long- or wide-shaped lesions which were frequently found in the diclofenac- and aspirin-treated groups. Similar to our results, there was a report that both indomethacin and diclofenac administration increases gastric damage in a dose-dependent manner.33
MPO concentration, a biochemical indicator of the degree of mucosal neutrophil infiltration, increases with the development of gastric mucosal injury. MPO levels have been shown to increase concomitantly with gastric injury in indomethacin-treated rats.34 With age, the capacity of the stomach lining to resist damage decreases which in turn increases the risk of peptic ulcer disease, especially in people who use aspirin and other NSAIDs.25 Several studies have reported an increased susceptibility of the gastric mucosa to various injuries in older animals.15,24,35,36 In the present study, MPO levels of the 1-year-old rats were significantly higher than those in the 7-week-old rats treated with diclofenac (40 and 80 mg/kg) or aspirin (100 mg/kg). However, this trend was not found in the indomethacin group, suggesting that the impact of aging could be different depending on the type of NSAID.
There are two pathways to metabolize the arachidonic acid released from membrane phospholipids to the chemical signal transmitter in the inflammation process. One of them is the prostagladins synthesis through COX and the other one is the leukotriene (leukotriene A4, B4, C4, D4, E4) synthesis through 5-LOX. LTB4, a by-product of 5-LOX and cPLA2, is the major chemotactic factor for leukocytes and has been reported to play an important pathophysiological role in the development of gastroduodenal ulcers.37 Recently, we also reported a high increase of cPLA2 and 5-LOX expression, and MPO mucosal levels after ethanol administration.38 In contrast to this ethanol model, NSAID-treated rats did not show much difference in cPLA2 expression although it was higher in 1-year-old rats compared to the 7-week-old rats treated with indomethacin (10 and 40 mg/kg). Furthermore, histological index, which was higher in the ethanol model,38 showed a tendency to increase with age in the NSAID-treated animals in the present study, but there was no statistical significance. These results altogether suggest that the damage after exposure of NSAIDs for 24 hours might not be sustained through neutrophil infiltration. Neutrophil infiltration into the injured mucosa has been shown to occur as early as 15 to 30 minutes after indomethacin administration in rats.39 In addition, when mucosal damage and repair observed at 3 and 24 hours after aspirin administration were compared, less damage and higher regeneration was found at 24 hours than at 3 hours.16 Thus, mucosal damage at the 24 hour-incubation in the present study might have been sustained after direct damage by NSAIDs instead of cPLA2-neutrophil pathway. Furthermore, NSAIDs promote neutrophil-endothelial adhesive interactions, but not neutrophil migration into the extravascular space.40,41 This may explain why the gastric inflammation induced by NSAIDs is less extensive than that caused by either H. pylori42 or ethanol43 in which gastric damage occurs through neutrophil migration. In addition, there was no statistical significance in the aspect of histological index in spite of statistical difference in the gross damage in the aged rat. This discrepancy could be explained by the different collection of data for gross and histological damage index. That is, the gross damage was counted from the entire gastric mucosa before separation of stomach for histology and biochemistry. However, the tissue for histology was collected from certain area of stomach regardless of the damaged area or inflammation. Furthermore, the histological damage counting system is based on neutrophil infiltration in the mucosa that NSAID-induced mucosal damage is milder than ethanol-induced model similar to cPLA2 results.
Until now, there have been few studies which compared various types of NSAID-induced gastric damage in animals. Zhang et al.34 reported that intragastric gavaged indomethacin (48 mg/kg) induced linear hemorrhagic lesions in the glandular mucosa. Takeuchi et al.44 also reported that indomethacin administration led to either non-hemorrhagic or hemorrhagic gastric lesions which were linear or dotted. Diclofenac administered subcutaneously at dose of 40 mg/kg45 or by intragastric gavage at 50 mg/kg46 caused hemorrhagic lesions. Intragastric gavage of aspirin (200 mg/kg) also produced linear and dotted erosion in the gastric mucosa.6 When the three NSAIDS were compared in the present study, gross NSAID-induced gastric damage was different depending upon the type of NSAIDs. Both indomethacin and diclofenac caused linear-shaped ulcers with dented spots, but the damage caused by indomethacin-induced ulcers was more severe. In contrast, the ulcers caused by aspirin were relatively shallow and linear-shaped, and were accompanied with hemorrhage more frequently than those caused by the other two NSAIDs.
The present study has a limitation. That is, the age-dependent effect on NSAIDs-induced gastric damage and difference of gastric damage depending on the type or dose of NSAID has been investigated in SD rat model. However, the underlying mechanisms (such as COX/prostaglandin pathway, mucus production, microcirculation etc.) and difference in susceptibility among NSAIDs have not been evaluated in the present study. Further studies regarding the underlying detailed mechanisms and susceptibility among NSAIDs are planned in the future.
In summary, the gross gastric damage induced by indomethacin, diclofenac, or aspirin increased according to dosage and age in rats. However, the differences in gastric damage were greater when the damaged area was measured by the image program than by the gross ulcer index. Increases in MPO levels induced by diclofenac and aspirin were significantly higher in 1-year-old rats compared to 7-week-old rats, and cPLA2 expression was higher in 1-year-old rats compared to 7-week-old rats among the animals treated with indomethacin and diclofenac. We also found that gross NSAID-induced gastric damage occurred in different patterns according to the type of NSAID. In conclusion, NSAID-induced acute gastric damage increased in a dose- and age-dependent manner.
ACKNOWLEDGEMENTS
This work was supported by a grant (no. 06-2009-032) from the Seoul National University Bundang Hospital Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Allen A, Flemström G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 2.Flemstrom G, Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G183–G193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- 3.Beck PL, Xavier R, Lu N, et al. Mechanisms of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterology. 2000;119:699–705. doi: 10.1053/gast.2000.16497. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Naito Y, Kishi A, et al. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida N, Yoshikawa T, Nakamura Y, et al. Role of neutrophil-mediated inflammation in aspirin-induced gastric mucosal injury. Dig Dis Sci. 1995;40:2300–2304. doi: 10.1007/BF02063228. [DOI] [PubMed] [Google Scholar]
- 6.Odashima M, Otaka M, Ohba R, et al. Attenuation of gastric mucosal inflammation induced by aspirin through inhibition of selective type III phospshodiesterase in rats. Dig Dis Sci. 2007;52:1355–1359. doi: 10.1007/s10620-006-9553-y. [DOI] [PubMed] [Google Scholar]
- 7.Nam SY, Kim N, Lee CS, et al. Gastric mucosal protection via enhancement of MUC5AC and MUC6 by geranylgeranylacetone. Dig Dis Sci. 2005;50:2110–2120. doi: 10.1007/s10620-005-3016-8. [DOI] [PubMed] [Google Scholar]
- 8.Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi K, Tanaka A, Hayashi Y, Kubo Y. Functional mechanism underlying COX-2 expression following administration of indomethacin in rat stomachs: importance of gastric hypermotility. Dig Dis Sci. 2004;49:180–187. doi: 10.1023/b:ddas.0000017436.05273.fd. [DOI] [PubMed] [Google Scholar]
- 10.Del Soldato P, Foschi D, Benoni G, Scarpignato C. Oxygen free radicals interact with indomethacin to cause gastrointestinal injury. Agents Actions. 1986;17:484–488. doi: 10.1007/BF01965518. [DOI] [PubMed] [Google Scholar]
- 11.Okada A, Kinoshita Y, Waki S, et al. Rat gastric mucosal cells express ICAM-1 and proinflammatory cytokines during indomethacin-induced mucosal injury. J Lab Clin Med. 1998;131:538–547. doi: 10.1016/s0022-2143(98)90062-2. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 14.Lee M. Prevention and treatment of nonsteroidal anti-inflammatory drug-induced gastropathy. South Med J. 1995;88:507–513. doi: 10.1097/00007611-199505000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, Feldman M. Age-related reductions in gastric mucosal prostaglandin levels increase susceptibility to aspirin-induced injury in rats. Gastroenterology. 1994;107:1746–1750. doi: 10.1016/0016-5085(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Hardman WE, Cameron I. Age-related changes in gastric mucosal repair and proliferative activities in rats exposed acutely to aspirin. Gerontology. 1998;44:198–203. doi: 10.1159/000022010. [DOI] [PubMed] [Google Scholar]
- 17.Kang JM, Kim N, Kim JH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147–G1153. doi: 10.1152/ajpgi.00218.2010. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Parekh D, Townsend CM, Jr, Thompson JC. Effects of aging on duodenal bicarbonate secretion. Ann Surg. 1990;212:332–337. doi: 10.1097/00000658-199009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinsull SM. Effect of colloidal bismuth subcitrate on age related gastric lesions in the rat. Gut. 1991;32:355–360. doi: 10.1136/gut.32.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogiagis D, Glare EM, Misajon A, Brown W, O'Brien PE. Cyclooxygenase-1 and an alternatively spliced mRNA in the rat stomach: effects of aging and ulcers. Am J Physiol Gastrointest Liver Physiol. 2000;278:G820–G827. doi: 10.1152/ajpgi.2000.278.5.G820. [DOI] [PubMed] [Google Scholar]
- 21.Cryer B, Redfern JS, Goldschmiedt M, Lee E, Feldman M. Effect of aging on gastric and duodenal mucosal prostaglandin concentrations in humans. Gastroenterology. 1992;102(4 Pt 1):1118–1123. [PubMed] [Google Scholar]
- 22.Grønbech JE, Lacy ER. Role of gastric blood flow in impaired defense and repair of aged rat stomachs. Am J Physiol. 1995;269(5 Pt 1):G737–G744. doi: 10.1152/ajpgi.1995.269.5.G737. [DOI] [PubMed] [Google Scholar]
- 23.Tarnawski A, Pai R, Deng X, et al. Aging gastropathy-novel mechanisms: hypoxia, up-regulation of multifunctional phosphatase PTEN, and proapoptotic factors. Gastroenterology. 2007;133:1938–1947. doi: 10.1053/j.gastro.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Goto H, Tachi K, Hisanaga Y, et al. Exacerbatory mechanism responsible for water immersion stress-induced gastric lesions in aged rats compared with young rats. Clin Exp Pharmacol Physiol. 2001;28:659–662. doi: 10.1046/j.1440-1681.2001.03497.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee M, Feldman M. The aging stomach: implications for NSAID gastropathy. Gut. 1997;41:425–426. doi: 10.1136/gut.41.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998;105(1B):31S–38S. doi: 10.1016/s0002-9343(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 27.Tsukimi Y, Okabe S. Changes in gastric function and healing of chronic gastric ulcers in aged rats. Jpn J Pharmacol. 1995;68:103–110. doi: 10.1254/jjp.68.103. [DOI] [PubMed] [Google Scholar]
- 28.Beck WS, Schneider HT, Dietzel K, Nuernberg B, Brune K. Gastrointestinal ulcerations induced by anti-inflammatory drugs in rats. Physicochemical and biochemical factors involved. Arch Toxicol. 1990;64:210–217. doi: 10.1007/BF02010727. [DOI] [PubMed] [Google Scholar]
- 29.Pandit S, Sur TK, Jana U, Bhattacharyya D, Debnath PK. Anti-ulcer effect of Shankha bhasma in rats: a preliminary study. Indian J Pharmacol. 2000;32:378–380. [Google Scholar]
- 30.Lacy ER, Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982;83:619–625. [PubMed] [Google Scholar]
- 31.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 32.Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Rhee HI, Jung IH, et al. SKI306X, an oriental herbal mixture, suppresses gastric leukotriene B4 synthesis without causing mucosal injury and the diclofenac-induced gastric lesions. Life Sci. 2005;77:1181–1193. doi: 10.1016/j.lfs.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Tajima K, Kageyama K, Kyoi T. Irsogladine maleate suppresses indomethacin-induced elevation of proinflammatory cytokines and gastric injury in rats. World J Gastroenterol. 2008;14:4784–4790. doi: 10.3748/wjg.14.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida M, Kawano O, Misaki N, Saitoh K, Irino O. Healing of acetic acid-induced gastric ulcer and gastric mucosal PGI2 level in rats. Dig Dis Sci. 1990;35:80–85. doi: 10.1007/BF01537227. [DOI] [PubMed] [Google Scholar]
- 36.Atchison CR, Balakumaran A, West AB, Hoffmann WE, Treinen-Moslen M. Aging enhances susceptibility of diclofenac-treated rats to gastric ulceration, while attenuating enteropathy. Dig Dis Sci. 2000;45:614–620. doi: 10.1023/a:1005422029918. [DOI] [PubMed] [Google Scholar]
- 37.Gambero A, Maróstica M, Becker TL, Pedrazzoli J., Jr Effect of different cyclooxygenase inhibitors on gastric adaptive cytoprotection induced by 20% ethanol. Dig Dis Sci. 2007;52:425–433. doi: 10.1007/s10620-006-9487-4. [DOI] [PubMed] [Google Scholar]
- 38.Kang JM, Kim N, Kim B, et al. Gastroprotective action of Cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA(2)/5-LOX pathway. Dig Dis Sci. 2009;54:2549–2560. doi: 10.1007/s10620-008-0671-6. [DOI] [PubMed] [Google Scholar]
- 39.Anthony A, Sim R, Dhillon AP, Pounder RE, Wakefield AJ. Gastric mucosal contraction and vascular injury induced by indomethacin precede neutrophil infiltration in the rat. Gut. 1996;39:363–368. doi: 10.1136/gut.39.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida N, Yoshikawa T. Effect of Helicobacter pylori-mediated inflammation on nonsteroidal anti-inflammatory drugs-induced gastric mucosal injury. Keio J Med. 2002;51(Suppl 2):45–50. doi: 10.2302/kjm.51.supplement2_45. [DOI] [PubMed] [Google Scholar]
- 41.Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol. 1992;262(5 Pt 1):G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida N, Granger DN, Evans DJ, Jr, et al. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 43.Kvietys PR, Twohig B, Danzell J, Specian RD. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990;98:909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi K, Ueki S, Okabe S. Importance of gastric motility in the pathogenesis of indomethacin-induced gastric lesions in rats. Dig Dis Sci. 1986;31:1114–1122. doi: 10.1007/BF01300266. [DOI] [PubMed] [Google Scholar]
- 45.Komoike Y, Takeeda M, Tanaka A, Kato S, Takeuchi K. Prevention by parenteral aspirin of indomethacin-induced gastric lesions in rats: mediation by salicylic acid. Dig Dis Sci. 2002;47:1538–1545. doi: 10.1023/a:1015867119014. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez S, Martín MJ, Ortiz P, Motilva V, Alarcón de la Lastra C. Effects of dipyrone on inflammatory infiltration and oxidative metabolism in gastric mucosa: comparison with acetaminophen and diclofenac. Dig Dis Sci. 2002;47:1389–1398. doi: 10.1023/a:1015395103160. [DOI] [PubMed] [Google Scholar]