Abstract
Background/Aims
The aim of this study was to evaluate the prevalence and risk factors for hepatitis B surface antigen (HBsAg) positivity in pregnant Ghanaian women.
Methods
We surveyed 1,500 pregnant women in Eastern region of Ghana. Direct interviews were performed by trained nurses using standardized questionnaires. Pregnant women were screened for human immunodeficiency virus (HIV) and hepatitis B infections, hemoglobin levels and sickle cell anemia as part of the antenatal check-up.
Results
The overall HBsAg positive rate was 10.6%, which varied among districts (13.8% for Kwahu West, 12.4% for Upper Manya, and 2.2% for Yilo Krobo). HBsAg positivity was significantly higher in women with depression (odds ratio [OR], 3.74; 95% confidence interval [CI], 2.13 to 6.57) and HIV (OR, 2.03; 95% CI, 1.06 to 3.89). Age, education, and gravidity were not related to HBsAg positivity. Anti-hepatitis B immunoglobulin for newborns of HBsAg-positive mothers is not provided at birth in public health facilities in Ghana. However, hepatitis B vaccination is provided as part of a routine vaccination schedule starting at 6 weeks of age.
Conclusions
To prevent mother-to-child transmission of hepatitis B, screening tests for HBsAg in pregnant women and hepatitis B vaccination of newborns immediately after birth need to be performed in this region.
Keywords: Hepatitis B, Ghana, Pregnant women, Risk factors, Prevalence
INTRODUCTION
It has been reported that general health state in Eastern Ghana is lower than that of other areas in the same country. In 2009, the life expectancy at birth of Ghanaian men and women are 57 and 64 years respectively, with under-five mortality rate (probability of dying by age 5 per 1,000 live births) reaching 69,1 a per capita total expenditure on health (PPP int. $) was $122 and total expenditure on health as a percentage of gross domestic product was 8.1% in 2009 according to World Health Organization (WHO) statistics.2 However, it was $100 in Eastern Ghana. The number of physicians per 10,000 is 0.85 in Ghana,2 and Eastern region is lower than this in 2009.3 Sub-Saharan Africa including Ghana is a highly endemic area of hepatitis B, reaching up to approximately 90% carrier rate in some rural areas.4 The hepatitis B prevention strategy of WHO states that routine infant immunization is required for all countries and additional vaccination is needed in accordance with every country's epidemiological characteristic. Hepatitis B virus (HBV) birth dose (vaccination in 24 hours) is recommended for countries with hepatitis B surface antigen (HBsAg) prevalence higher than 8%.5 Currently, an average Ghanaian receives vaccination routinely as part of the pentavalent vaccine including diphtheria, pertussis, tetanus, and haemophilus influenza type b at 6, 10, and 14 weeks after birth, respectively, but not immediately after birth. It is established that the main HBV transmission path of Sub-Saharan Africa is horizontal,6,7 but attention also needs to be given to the vertical transmission considering previous reports that most of the HBV infected children of this area was infected before the age of five.6,7 Under these circumstances, maternal and child health services is vital, yet little information is available on HBsAg prevalence of pregnant Ghanaian women. The more pregnant women are exposed to HBV, the higher the risk of vertical transmission which in turn keeps hepatitis B endemic. This study sought to investigate the HBsAg prevalence, as well as risk factors for its transmission amongst pregnant women in Eastern region of Ghana. Finally, the study aimed at suggesting practical alternatives of HBV prevention.
MATERIALS AND METHODS
Between December 2008 and March 2009, data was collected from 1,500 pregnant women who visited three hospitals in Eastern region of Ghana. The Eastern region occupies a land area of 19,323 km and constitutes 8.1% of the total land area of Ghana. The population of Eastern region was 2.3 million in 2008 which was 9.8% of Ghanaian population and three study districts occupied 10.8% (0.25 million) of the Eastern region (Fig. 1).
Fig. 1.
Map of Ghana. The three study districts were located in the Eastern region.
In three study districts of Eastern region, there were three hospitals; Holy Family Hospital (HFH) in Kwahu West district, Asesewa Government Hospital (AGH) in Upper Manya Krobo district, and Somanya Polyclinic (SPC) in Yilo Krobo district. HFH was an old general hospital established in 1945, has 3 doctors and assisted by a number of professional doctors who visit the hospital regularly. While AGH was new district hospital, there was only one physician. In Yilo Krobo district including SPC, there were no physicians.3 Aside from these hospitals there are also rural health centers; Reproductive & Child Health (RCH) or Community-based Health and Planning Services (CHPS). Public health workers are assigned in RCH or CHPS. Pregnant women visit RCH or CHPS to antenatal care or urgent delivery.
We have trained nurses of three hospitals and they directly interviewed the pregnant women. After signing a written informed consent, all the subjects responded to a questionnaire, which composed of six sections; general information, current pregnancy, pregnancy history, concerns, health care utilizations, and health examinations. They were screened for blood pressure, blood sugar, anemia, HBsAg, human immunodeficiency virus (HIV), and sickle cell anemia. Hemoglobin levels less than 10 g/dL was determined to be anemic. HIV was identified by HIV rapid antibody test performed using OraQuick Rapid HIV-1/2 and confirmed with First Response manufactured by OraSure Technology Inc. (Bethlehem, PA, USA) and Premier Medical Co., Ltd. (Daman, India), respectively. HBsAg was done using serum with Accu-Tell® HBsAg Strip manufactured by Accu Bio Tech Co., Ltd. (Beijing, China).
The Institutional Review Board of the Eulji University Hospital approved the study protocol (IRB 08-38). Permission was sought from the Eastern Regional Health Directorate of the Ghana Health Service and written informed consent for use of questionnaires and medical records for research purposes was obtained from every study participant.
We analyzed correlation between HBsAg positive women and estimated risk factors using cross-tabulation, and confirmed validity through chi-square test. Only the statistically significant variables were used to constitute the variable model, which was then analyzed using multiple logistic regression model. All the statistical analyses were run on SPSS version 18.0 (IBM, New York, NY, USA).
RESULTS
1. HBsAg positive rate by general characteristics
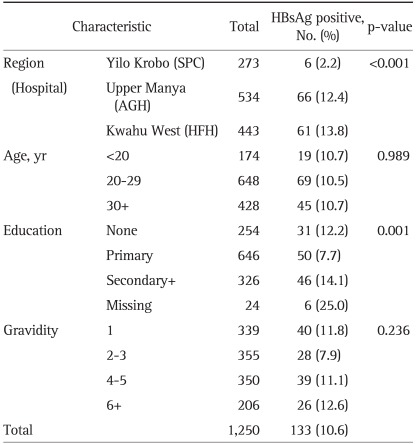
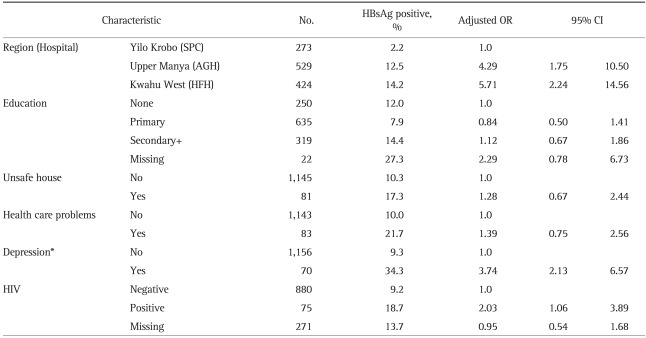
The mean HBsAg positive rate for these subjects was 10.6%. It was different by the districts (p<0.001). The HBsAg positive rates of AGH and HFH were 12.4% and 13.8%, respectively, which were higher than that of SPC's (2.2%). There were no significant age-related differences, but there was a significant difference according to education level of the subjects. Only 7.7% of those who received primary education were positive for HBsAg, but among those who received education at secondary school or higher, 14.1% proved to be HBsAg positive. There was no statistically significant difference in gravidity (Table 1).
Table 1.
The Hepatitis B Surface Antigen (HBsAg) Positive Rate Based on General Characteristics in Pregnant Women in Eastern Region of Ghana
2. HBsAg positive rate by self-reported social support and mental health status
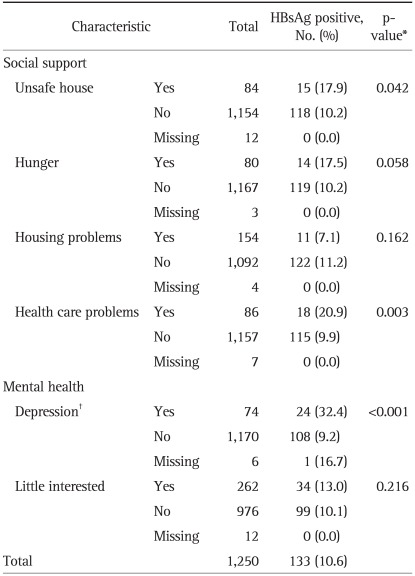
The pregnant women who answered 'yes' to the question asking whether they felt unsafe in their houses showed a higher HBsAg positive rate (p=0.042). No significant HBsAg positive rate difference existed depending on hunger, which was researched through the questionnaire asking whether they had missed any meals during the past month due to shortage of food supply. Also, no significant HBsAg positive rate difference was noticed between the groups who answered 'yes' and 'no' to the question asking whether they have had housing problems during the past 3 months. HBsAg positive rate of the group who answered 'yes' to the question 'Do you have transportation, child care, or other problems that prevent you from receiving health care?' was 20.9%, which was statistically higher than that of those who answered 'no' (p=0.003). The subjects who said they had depression and/or had received counseling or medication for mental health concerns ranked significantly higher in HBsAg positivity compared with subjects who said they had not. The question asking whether they had little interest in doing things during the past month did not result in a statistically significant difference in HBsAg positive rates (Table 2).
Table 2.
The Hepatitis B Surface Antigen (HBsAg) Positive Rate Based on Self-Reported Social Support and Mental Health Status in Pregnant Women in Eastern Region of Ghana
*p-value obtained by chi-square test except missing cases; †Depression and/or had received counseling or medication for mental health concerns.
3. HBsAg positive rate by diseases
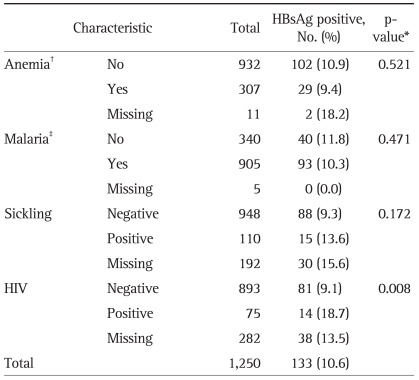
As shown in Table 3, there was no significant correlation in HBsAg positive rates between subjects who had anemia and those who did not. No statistically significant difference in HBsAg positivity was found due to history of malaria or sickle cell anemia. Of 75 HIV-positive pregnant women, 14 had HBsAg, making 18.7% of the group. Of the 893 HIV-negative pregnant women, 81 had HBsAg, marking 9.1% of the group. We found out that there is a statistically significant difference in HBsAg positive rates between HIV carriers and non-carriers. Missing group on HIV test located between HIV positive and negative group. (p=0.008, Table 3).
Table 3.
The Hepatitis B Surface Antigen (HBsAg) Positive Rate Based on Diseases in Pregnant Women in Eastern Region of Ghana
*p-value obtained by chi-square test except missing cases; †Anemia defined as <10 g/dL of hemoglobin level; ‡Self-reported disease history.
4. Multiple logistic regression analysis for HBsAg positivity
These results suggested chances of a causal relationship between several factors such as district, education, unsafe living conditions, health care problems, depression and HIV carrier and HBsAg positive rates. We reaffirmed the risk factors of hepatitis B through multiple logistic regression analyses.
Positivity of HBsAg was the highest in HFH, followed by that of AGH, which were 5.71 times and 4.29 times higher than that of SPC, respectively. Education, unsafe living conditions and health care problems had no statistical significance in HBsAg positive rates, unlike the results from cross-tabulation. The pregnant women with depression had HBsAg positive rate which was 3.74 times higher than that of those with no depression. The HIV positive group showed 2.03-times higher HBsAg positive rate compared to the HIV negative group, the difference of which was statistically significant (Table 4).
Table 4.
Multiple Logistic Regression Analysis for Hepatitis B Surface Antigen (HBsAg) Positivity in Pregnant Women in Eastern Region of Ghana
OR, odds ratio; CI, confidence interval; HIV, human immunodeficiency virus.
*Depression and/or had received counseling or medication for mental health concerns.
DISCUSSION
The study result highlights the fact that means HBsAg has a positive rate 10.6% for 1,500 subjects is higher than the WHO recommended birth-dose standard of 8%. Some other studies reveal that 15.8% of HBV seroprevalence in Ghana8 and a maximum of 20.9% of in antigen positive rate.6 Yet there is no policy of birth-dose vaccination in Ghana. Birth dose vaccination should be considered in these areas with high prevalence.
The prevalence rate of HBsAg among parturients or pregnant women in Accra, Ghana increased from 6.4% in 19949 to 10.5% in 2005,10 which was similar to this study result.
According to the recent population-based review study for the HBV seroprevalence, Ghana was grouped 1 (high endemicity, i.e., >8% affected) and AFR-D (Africa with high child and high adult mortality).11 Median seroprevalence estimate of HBsAg in pregnant women in group 1 region was 9.9%,11 which was lower than the current study result.
The present study also details the risk factors of HBV infection in pregnant women in Eastern Ghana. The HBsAg positive rates for AGH and HFH were 12.4% and 13.8%, respectively, which were higher than that of SPC (2.2%). According to the previous reports, HBV prevalence showed higher or similar rates in rural districts.12,13 In this study, HFH is in the capital of a municipality and SPC is in a more urban district than AGH; there is not much difference in the prevalence between urban and rural consistent with previous reports.12,13 According to our survey, inoculated rate of HBV vaccine in AGH, HFH, SPC were 0.7%, 0.3%, and 1.1%, respectively which were very slight. In Ghana, it was not until 2002 that vaccination against hepatitis B started at government level.2 In this study SPC district showed lower rates in variables which are related to HBsAg positvity compared to AGH and HFH (0.0%, 8.0%, and 6.4% for depression, 8.2%, 36.8%, and 11.8% for little interested, 1.3%, 9.5% and 7.2% for health care problems and 6.9%, 8.4%, and 9.4% for HIV positive, respectively).
There was no significant difference with regards to the age. This result demonstrates that the vertical transmission is dominant in these areas. Although the possibility of horizontal transmission cannot be excluded considering peculiarity of Africa,6 importance of vertical transmission should be considered. Our results correspond with the other article, suggesting the majority of hepatitis B infected children in Sub-Saharan Africa were infected before age 5 years.7 Vertical transmission is said to be caused by three predominant methods: 1) trans-placental intrauterine transmission; 2) transmission during delivery by contact with maternal infected fluids in the birth canal; and 3) postnatal transmission from mothers to infants during childcare or through breast-feeding.14,15
From our survey, 1,453 pregnant women out of 1,500 (98.2%) were planning to breastfeed their babies. Considering the high HBV prevalence in these areas, there is an urgent need to block off vertical transmission through these three passages with recommended HBV birth-dose vaccination.5
There was no significant education-related difference. This result is in contrast to the previous data, suggesting hepatitis B infection is inversely related to education.16 Our research needs further evaluation to figure out the accurate correlation between education and hepatitis B infection. How much hygiene education and prevention is being undertaken in elementary and higher education; and how much education itself is contributing to hygiene improvement in Ghana should be researched in future studies.
Mental health state correlated with HBsAg positivity. These findings correspond with the result of Atesci et al.17 They demonstrated that exposure to hepatitis B can influence patient's mental status like depression. But in our study, we have a limitation of knowing whether the subjects were aware of their HBV infection state as there is no routine HBsAg screening test during pregnancy in Ghana. Prenatal and antenatal checkup services including HIV test is free in Ghana; however, HBsAg testing is not included in this routine.
Further research will be required to explain this finding. In any case, supporting mental health needs to be considered when taking measures to prevent hepatitis B infection in pregnant women in Ghana.
HIV positive group showed significantly higher HBsAg positive rate than HIV negative group (odds ratio, 2.03; 95% confidence interval, 1.06 to 3.89). A study suggested that for HIV infected Africans, the rate of HBV co-infection is higher than European or Latin American.18 Of the 35 million people living with HIV worldwide, around 20% had HBV in some HBV endemic regions in sub-Saharan Africa whereas only 5% in Western countries.19 On Multicentre AIDS Cohort Study (MACS) cohort, co-infected group showed 8 times high liver-related mortality than HIV mono-infected group.20 Ghana, however, has a strong and comprehensive program aimed at virtual elimination of vertical transmission of HIV.21 We believe that integrating prevention of vertical transmission of HBV into the HIV/AIDS program will be effective considering that Eastern region is leading in prevalence of HIV in Ghana.22
The current study has limitations. Firstly, we only test HBsAg among serologic markers for hepatitis B viral infection; no data of serum HBV titer, hepatitis B e antigen/antibody to hepatitis B e antigen. Secondly, we use rapid test instead of Enzyme Immunoassay (EIA) for HBsAg; Accu-Tell® One Step HBsAg Whole Blood Test, which is a rapid, immunochromatographic assay for the qualitative detection of HBsAg in human whole blood, serum or plasma. However, the validity was acceptable level; when the test results were compared to EIA, the sensitivity was 98.89% and the specificity was 98.87%. When the test results were compared to Radioimmunoassay, they were 100.00%, and 99.43%, respectively.23
In spite of these limitations, this current study clearly demonstrates high HBV prevalence and risk factors in pregnant women in Eastern Ghana. Further studies will be required to take measures for each risk factor including traditional life style and folk medicine. It is our belief that HBV screening test for pregnant women and HBV vaccination after birth can be efficacious in Eastern Ghana.
ACKNOWLEDGEMENTS
This work was carried out for the program "Support for Mother and Child Health Care in Eastern Region, Ghana" which was supported by the KOICA.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kaiser Family Foundation. Washington, DC: Kaiser Family Foundation; c2011. [cited 2011 Oct 3]. U.S. global health policy [Internet] Available from: http://www.globalhealthfacts.org/ [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; c2011. [cited 2011 Aug 31]. Global health observatory data repository [Internet] Available from: http://apps.who.int/ghodata/?theme=country#. [Google Scholar]
- 3.Ghana districts [Internet] Accra: Ghana Districts; 2011. [cited 2011 Oct 3]. Available from: http://www.ghanadistricts.com/home/ [Google Scholar]
- 4.Adu-Sarkodie Y, Matke P, Tetteh C, Appiah-Denkyira E. Seroprevalence of hepatitis B markers in a rural community in Ghana. Trop Med. 1996;38:87–90. [Google Scholar]
- 5.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405–420. [Google Scholar]
- 6.Martinson FE, Weigle KA, Royce RA, Weber DJ, Suchindran CM, Lemon SM. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. Am J Epidemiol. 1998;147:478–487. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 7.Dumpis U, Holmes EC, Mendy M, et al. Transmission of hepatitis B virus infection in Gambian families revealed by phylogenetic analysis. J Hepatol. 2001;35:99–104. doi: 10.1016/s0168-8278(01)00064-2. [DOI] [PubMed] [Google Scholar]
- 8.Martinson FE, Weigle KA, Mushahwar IK, Weber DJ, Royce R, Lemon SM. Seroepidemiological survey of hepatitis B and C virus infections in Ghanaian children. J Med Virol. 1996;48:278–283. doi: 10.1002/(SICI)1096-9071(199603)48:3<278::AID-JMV11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Acquaye JK, Mingle JA. Hepatitis B viral markers in Ghanaian pregnant women. West Afr J Med. 1994;13:134–137. [PubMed] [Google Scholar]
- 10.Damale NK, Lassey AT, Bekoe V. Hepatitis B virus seroprevalence among parturients in Accra, Ghana. Int J Gynaecol Obstet. 2005;90:240–241. doi: 10.1016/j.ijgo.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Merrill RM, Hunter BD. Seroprevalence of markers for hepatitis B viral infection. Int J Infect Dis. 2011;15:e78–e121. doi: 10.1016/j.ijid.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Gust ID, Dimitrakakis M, Faaiuso S, Ainuu J, Zimmet P. The prevalence of hepatitis B infection amongst urban and rural populations in Western Samoa. J Hyg (Lond) 1981;86:87–93. doi: 10.1017/s0022172400068777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou J, Liu Z, Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2:50–57. doi: 10.7150/ijms.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 15.Ghendon Y. Perinatal transmission of hepatitis B virus in high-incidence countries. J Virol Methods. 1987;17:69–79. doi: 10.1016/0166-0934(87)90070-x. [DOI] [PubMed] [Google Scholar]
- 16.Duong TH, Nguyen PH, Henley K, Peters M. Risk factors for hepatitis B infection in rural Vietnam. Asian Pac J Cancer Prev. 2009;10:97–102. [PMC free article] [PubMed] [Google Scholar]
- 17.Atesci FC, Cetin BC, Oguzhanoglu NK, Karadag F, Turgut H. Psychiatric disorders and functioning in hepatitis B virus carriers. Psychosomatics. 2005;46:142–147. doi: 10.1176/appi.psy.46.2.142. [DOI] [PubMed] [Google Scholar]
- 18.Rivas González P. Viral hepatitis in newly diagnosed HIV immigrants in Spain: unexpected HIV rates of HCV antibody and "isolated anti-HBV core" among sub-Saharan Africans; Paper presented at: The 4th International Workshop on HIV and Hepatitis Coinfection; Madrid, Spain: 2008. [Google Scholar]
- 19.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85:303–315. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health; National AIDS Control Programme. National guidelines for prevention of mother to child transmission of HIV (PMTCT) Accra: Ministry of Health; 2008. [Google Scholar]
- 22.Ghana AIDS Commission; Joint United Nations Programme on HIV/AIDS; United Nations General Assembly Special Session. Ghana's progress report on the United Nations General Assembly Special Session (UNGASS) declaration of Commitment on HIV and AIDS. Geneva: Joint United Nations Programme on HIV/AIDS; 2010. Reporting period: January 2008 to December 2009. [Google Scholar]
- 23.AccuBioTech [Internet] ECPlaza Network. ECPlaza Network; 2011. [cited 2011 Aug 31]. Available from: http://accubiotech.en.ecplaza.net/ [Google Scholar]