Abstract
Background/Aims
Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) and peroxisome proliferator-activated receptor gamma (PPARγ) ligands can modulate cellular differentiation, proliferation, and apoptosis through various pathways. It has been shown that HMG-CoA reductase inhibitors and PPARγ agonists separately inhibit pancreatic stellate cell (PaSC) activation. We studied the effects of a combination of both types of drugs on activated PaSCs via platelet-derived growth factor (PDGF), which has not previously been reported. The present study was performed to elucidate the underlying mechanisms of these effects by focusing on the impact of the signaling associated with cell-cycle progression.
Methods
Primary cultures of rat PaSCs were exposed to simvastatin and troglitazone. Proliferation was quantified using the BrdU method, and cell-cycle analysis was performed using a fluorescent activated cell sorter. The protein expression levels of smooth muscle actin (SMA), extracellular signal-regulated kinase (ERK), and a cell cycle machinery protein (p27Kip1) were investigated using Western blot analysis.
Results
Simvastatin reversed the effects of PDGF on cell proliferation in a dose-dependent manner. The combination of a low concentration of simvastatin (1 mM) and troglitazone (10 mM) synergistically reversed the effects of PDGF on cell proliferation but had no effect on cell viability. The expression of a-SMA was markedly attenuated by combining the two drugs, which blocked the cell cycle beyond the G0/G1 phase by reducing the levels of phosphorylated ERK and reversed the expression of p27Kip1 interrupted by PDGF.
Conclusions
Simvastatin and troglitazone synergistically inhibited cell proliferation in activated PaSCs by blocking the cell cycle beyond the G0/G1 phase. This inhibition was due to the synergistic modulation of the ERK pathway and the cell cycle machinery protein p27Kip1.
Keywords: HMG-CoA reductase inhibitor, PPARγ agonist, Pancreatic stellate cells, Synergism
INTRODUCTION
Chronic pancreatitis is characterized by irreversible fibrosis from sustained inflammation, pain and loss of exocrine and endocrine functions.1 Like hepatic stellate cells (HSCs), pancreatic stellate cells (PaSCs) are considered to have a key role in pancreatic fibrosis, inflammation and the desmoplastic reaction in pancreatic cancer.2-8
Quiescent PaSCs which have a typical phenotype characterized by vitamin A-containing lipid droplet, changes to activated form in response to pancreatic injury or profibrogenic stimuli by growth factors (platelet-derived growth factor [PDGF] and TGF-β1), cytokines (IL-1, IL-6, IL-8, and TNF-α) or angiotensin II, and reactive oxygen species.2,5,7,9-12 Activated PaSCs have a fibroblast-like phenotypes including nuclear enlargement, enhanced prominence of the endoplasmic network, while vitamin A containing lipid droplet lost.2 Furthermore activated PaSCs express α-SMA and the extracellular matrix proteins such as collagen type I, collagen II and fibronectin and secrete proinflammatory cytokines and chemokines.5,7
Due to a pivotal role of PaSCs in the development of pancreatic fibrosis, the target treatment for the factors associated with the modulation of these cells can be a promising modality for pancreatic fibrosis.13 So, there have been many studies about antifibrotic therapies for targeting on treatment of activated PaSCs such as blockade of the receptors for PDGF, TGF-β, and angiotensin II as well as antioxidant.13-18
Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are widely used in clinical settings for their cholesterol-lowering properties. In addition to lowering cholesterol levels, many experimental studies have shown that statins have a pleotropic effect on the fibrogenic or cancer cells with antiproliferative, proapoptotic and antifibrogenic properties.19-23 Therefore, statins have been suggested as a potential therapeutic or preventive agents for the patients with fibroproliferative disorder.24
Peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor located in the nucleus membrane and has been known as a key transcription factor for adipocyte differentiation.25,26 Like statins, PPARγ ligand have been demonstrated to affect proliferation, differentiation and apoptosis of different cell types.27,28 PPARγ also mediate antifibroic effects in HSCs and PaSCs.29-33 Previous studies using thiazolidinedione derivatives have shown that troglitazone decreased proliferation of PaSCs and the expression of α-SMA by the modulation of PPARγ expression31 or PPARγ independent manners.34
Because both statins and PPARγ agonist have the characteristics of the suppressive fibrogenetic activities in PaSCs, we hypothesized that there may be a positive effects on the inhibition of PaSCs' activities between two drugs. Indeed, a synergistic effect was observed on the suppression of cancer cell proliferation through the combination treatment of such drugs.35,36 Also, statin has been reported to activate PPARγ receptor in immune cells.37,38 However, the effects of combined treatment of these drugs in PaSCs have been not yet been fully evaluated. Therefore, we tried to clarify whether combined treatment using a statin (simvastatin) and a PPARγ agonist (troglitazone) has the synergistic capacity to affect the proliferation/activation of PaSCs and to examine the mechanism underlying this effect.
MATERIALS AND METHODS
1. Study materials
We purchased simvastatin and troglitazone from Sigma-Aldrich Inc. (St. Louis, MO, USA). Each drug was dissolved in dimethylsulfoxide and was diluted in phosphate-buffered saline (PBS) before use. α-SMA and ERK monoclonal antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The cell growth media PDGF (recombinant bovine PDGF-BB) was purchased from Sigma-Aldrich Inc.
2. PaSCs isolation and culture
Pancreatic tissues from 6- to 8-week old male Sprague-Dawley rats weighing 200 to 250 g were extracted and finely ground. The ground pancreatic tissue was digested in Gey's balanced salt solution with 0.05% collagenase P, 0.02% proteinase and 0.05% DNAse and was shaken in a 37℃ water tub and centrifuged at 3,000 rpm for 5 minutes. After filtration through 150 µm mesh, cells were centrifuged in a 28.7% Nycodenz (Sigma-Aldrich Inc.) gradient at 1,400 g for 20 minutes. The band just above the interface of the Nycodenz solution was suspended in Dulbecco's modified Eagle's Medium containing 10% fetal bovine serum (FBS). Cell viability was assessed according to trypan blue staining, and cells were cultured at 37℃ in a 5% CO2 humidified atmosphere. After isolation, cells were cultured for 5 to 7 days until activated and confluent. Experiments were performed between passages 2 and 4.
3. Cell proliferation assay
PaSCs were treated with simvastatin (1, 2.5, 5 µM) or troglitazone (10 µM) at various concentrations for 1 hour and then stimulated with PDGF (10 ng/mL) for 48 hours. Cell proliferation was assessed using a commercial kit (Cell Proliferation ELIZA, BrdU; Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol. BrdU incorporation was quantified according to differences in absorbance at wavelengths 370 to 492 nm.
4. Assessment of cell viability
Cell viability was assessed using an LDH detection kit (Roche Diagnostics) which measures LDH activity released from the cytosol of damaged cells. Briefly, PaSCs were seeded in a 96-well tissue culture plate at a density of 1×104 cells per well, and reaction buffer and experimental drugs were added to each well. The mixtures were incubated for 30 minutes at room temperature. The reaction was stopped by additional 2 mol/L HCl, and absorbance was measured spectrophotometrically at 492 nm using an Anthos Labtec microplate reader (Labtec, Uckfield, Sussex, UK). Results were expressed as a percentage of the total LDH released from cells incubated with 1% (wt/vol) Triton X-100.
5. Cell cycle analysis
The cell cycles of PaSCs were analyzed using flow cytometry. Briefly, serum-derived SCs (60% to 70% density) were treated with simvastatin or troglitazone or control vehicle for 1 hour and then exposed to 10 ng/mL PDGF. After 24 hours, the cells were harvested and washed twice with PBS and suspended in PBS solution containing 40 µg/mL propidium iodide, 0.02% Triton X-100, and 50 µg/mL ribonuclease A. Cell fluorescence was measured using a FACSCaliber flow cytometer and analyzed using software to determine the distribution of cells in the various phases of the cell cycle.
The α-SMA, ERK, and p27Kip1 protein expression in PaSCs were determined by Western blot. PaSCs were collected by scraping and homogenized in lysis buffer containing 25 mMol/L Tris-HCl pH. 7.4, 1 mMol/L EDTA, 0.5% Triton X-100. Protein concentrations were measured with the use of Bradford's method (BioRad, Rockville, NY, USA). Equal amounts of proteins (20 µg) were separated on 12.5% SDS-polyacrylamide gel, transferred onto nitrocellulose membranes, and blocked with TBST (Tris-buffered saline [pH 7.4], 0.05% Tween-20) with 5% nonfat milk and 5% FBS. The primary antibodies were applied overnight at 4℃. Antibodies against α-SMA (1:200; Sigma-Aldrich Inc.), p27Kip1 (1:1,000; Cell Signaling Technology, Beverly, MA, USA), and ERK/pERK (1:1,000; Santa Cruz Biotechnology Inc.) were used, and β-actin-specific antibody (Santa Cruz Biotechnology Inc.) served as the sample loading control. After extensive washing with TBST, the membranes were incubated for 1 hour in corresponding horseradish peroxidase-coupled secondary antibodies (goat anti-mouse and donkey anti-rabbit 1:1,000; Santa Cruz Biotechnology Inc.). The chemiluminescent reaction was developed using a West Pico (Pierce) reagent.
6. Statistical analysis
Statistical significance of the LDH difference between treated and untreated samples was determined by t-test. For the dose response studies including proliferation, cell cycle study and Western blot, statistical significance between untreated samples and samples treated with simvastatin, troglitazone, and combined treatment was determined by Mann-Whitney U test. p-values <0.05 were considered statistically significant.
RESULTS
1. Neither simvastatin or troglitazone is cytotoxic to ratisolated PaSCs
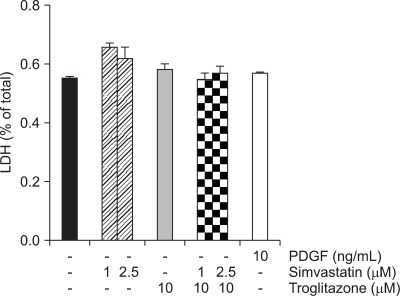
To determine whether simvastatin and troglitazone affect cell viability, primary cultured PaSCs were incubated for 24 hours with simvastatin (1, 2.5 µM) or troglitazone (10 µM). This observation was verified through an LDH assay. As depicted in Fig. 1, neither of the two drugs nor the combination of the two drugs were cytotoxic to PaSCs.
Fig. 1.
Cytotoxicity analysis. The cytotoxicities induced by simvastatin and troglitazone were analyzed by LDH measurement. Exposure to simvastatin and troglitazone for 24 hours did not result in cytotoxicity in primary pancreatic stellate cells (PaSCs).
PDGF, platelet-derived growth factor.
2. Simultaneous treatment with simvastatin and troglitazone induces phenotypic change in PaSCs
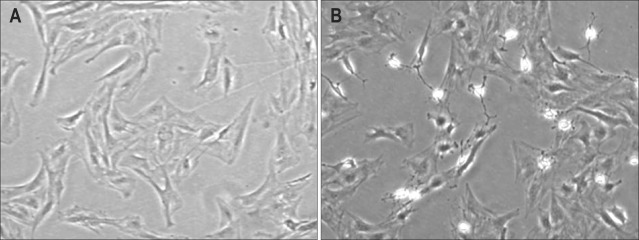
When primary PaSCs were exposed to PDGF (10 ng/mL) for 24 hours, these cells acquired a myofibroblast-like morphology with expanded cytoplasm (Fig. 2A). Increasing concentrations of simvastatin induced considerable morphological changes in activated PaSCs with PDGF. In contrast, no significant morphologic changes were noted in troglitazone-treated cells (data not shown). Next, we tested the effects of combined simvastatin and troglitazone on the phenotypic changes of PaSCs. As depicted in Fig. 2B, simultaneous treatment with these two drugs resulted in significant morphologic changes in PaSCs compared with activated PaSCs by PDGF.
Fig. 2.
The morphological changes in pancreatic stellate cells. PaSCs in the second passage were exposed to (A) platelet-derived growth factor (10 ng/mL) or (B) simvastatin at 1 µM plus troglitazone at 10 µM.
3. Simultaneous treatment with simvastatin and troglitazone inhibits cell proliferation and α-SMA expression in activated PaSCs
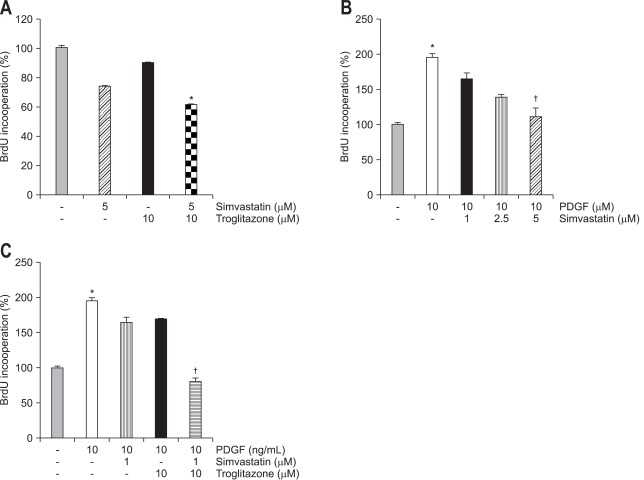
We tested whether each drug or combined treatment with these two drugs affect the proliferation in quiescent and activated PaSCs. In the quiescent state, pre-incubation with simvastatin (5 µM) showed inhibitory tendency of cell proliferation but troglitazone alone (10 µM) had no effect on cell proliferation. However, the combined treatment with two drugs significantly suppressed cell proliferation in quiescent state (Fig. 3A). Exposure to PDGF (10 ng/mL) for 24 hours increased the rate of proliferation of PaSCs, and pre-incubation with simvastatin (5 µM) significantly blunted the effect of PDGF on cell proliferation (Fig. 3B).
Fig. 3.
The cell proliferation assay in pancreatic stellate cells. The rate of cell proliferation was measured using a BrdU assay. (A) Cells were serum-starved for 24 hours and incubated with simvastatin or troglitazone for 6 hours without an activator. Incubation with simvastatin resulted in the dose-dependent inhibition of cell proliferation. (B) Cells were pre-incubated with simvastatin for 1 hour and exposed to 10 ng/mL of platelet-derived growth factor (PDGF) for 24 hours. Pre-incubation with simvastatin resulted in the dose-dependent inhibition of cell proliferation by PDGF. (C) The increased cell proliferation rate with PDGF was not inhibited by pre-incubation with simvastatin or troglitazone alone at concentrations of 1 µM or 10 µM, respectively. However, simvastatin plus troglitazone markedly suppressed the cell proliferation rate to the level of the control group (*p<0.05 compared with control groups, †p<0.05 compared with the PDGF treatment groups).
Next, we tested whether the combination of these drugs could affect cell proliferation in low concentration. A lower dose of simvastatin (1 µM) further decreased the PDGF-induced PaSCs proliferation with co-treatment of troglitazone (10 µM) (p<0.05) (Fig. 3C). Next, we studied whether this combination affected the expression of α-SMA. Both simvastatin (1 µM) and troglitazone (10 µM) decreased expression of α-SMA in PaSCs and the combination of both drugs further decreased its expression (Fig. 4).
Fig. 4.
Expression of α-smooth muscle actin (α-SMA). α-SMA expression was increased by platelet-derived growth factor (PDGF) (lane 2). This increased expression was reduced by pre-incubation with simvastatin (1 µM) and troglitazone (10 µM) and was further decreased by the combination of the two drugs.
4. Simultaneous treatment with simvastatin and troglitazone induces G1 arrest in activated PaSCs
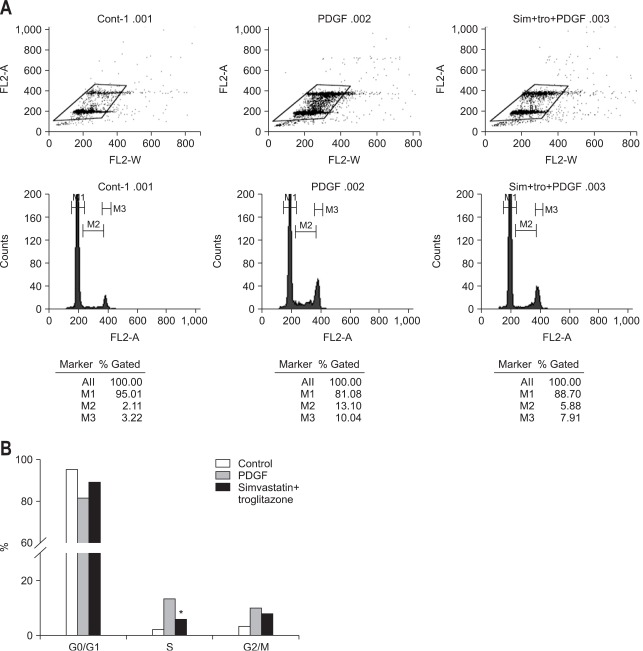
In subsequent experiment, we tested the effect of combined treatment (simvastatin 1 µM and troglitazone (10 µM) on cell-cycle progression using flow cytometry (Fig. 5A). Exposure to PDGF (10 ng/mL) resulted in an increased fraction of cells in the S phase and a corresponding decrease in the G0/G1 fraction, whereas exposure to combined treatment with troglitazone before PDGF reduced the fraction of cells in the S phase, suggesting that combined treatment synergistically induced cell-cycle arrest in activated PaSCs by PDGF at the G0/G1 stage (Fig. 5B).
Fig. 5.
(A, B) The analysis of cell-cycle progression. Pancreatic stellate cells were serum-starved for 24 hours, pre-incubated with troglitazone or vehicle for 1 hour, and exposed to 10 ng/mL of platelet-derived growth factor (PDGF) for 24 hours. The cells were collected and stained with 0.5 mg/mL propidium iodine, and cell fluorescence was measured with FAC-Scan. Compared with the control cells (dark), the cells exposed to PDGF showed a decrease in the number in the G0/G1 phase and an increase in the number in the S phase. Pre-incubation with simvastatin (1 µM) and troglitazone (10 µM) decreased the number of cells in the S phase, indicating an induction of cell-cycle arrest at G0/G1 (*p<0.05 compared with the PDGF treatment group).
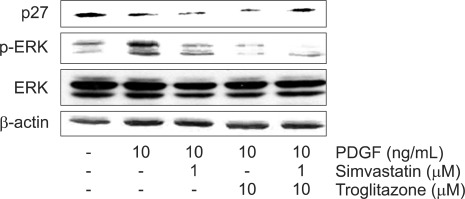
5. Simultaneous treatment of simvastatin and troglitazone afftects PDGF-induced ERK activation and p27Kip1 expression in activated PaSCs
The roles of ERK in the control of cell proliferation has been well established in may studies.39 We examined the effect of combined treatment with these two drugs on ERK activation. The result of Western blot revealed that simvastatin (1 µM) and troglitazone (10 µM) decreased the PDGF-induced ERK activation, and that combined treatment with the two drugs significantly suppressed the phosphorylation of ERK (Fig. 6).
Fig. 6.
The expression of ERK and p27Kip1 by Western blot analysis. p-ERK protein levels were significantly upregulated after the pancreatic stellate cells were stimulated by platelet-derived growth factor (PDGF). This increase was markedly reduced by pre-incubation with simvastatin (1 µM) and troglitazone (10 µM) and was further decreased by the combination of the two drugs. A significant elevation in the p27Kip1 protein level was induced by the combination of the two drugs, whereas neither simvastatin (1 µM) nor troglitazone (10 µM) alone had an effect on the p27Kip1 protein level.
p27Kip1 is a cell cycle machinery protein and is known as a universal cyclin-dependent kinase (CDK) inhibitor, a putative tumor suppressor. Here, we found that elevation of p27Kip1 was induced by combination treatment, whereas neither simvastatin (1 µM) nor troglitazone (10 µM) alone affected p27Kip1 protein level (Fig. 6).
DISCUSSION
The main findings of this study relate to the synergistic inhibition in proliferation of PaSCs and the expression of α-SMA by combining a statin and a PPARγ agonist. This mechanism was mediated by the modulation of the expressions of ERK and p27Kip1.
Statins and PPARγ agonists are widely used in the treatment of hypercholesterolemia and diabetes mellitus. In addition to their cholesterol-lowering and blood glucose-lowering functions, it is known that statins and PPARγ agonists have additional anti-cancer effects in various cancer cells.22,28 Also, it has been demonstrated that these drugs could inhibit the activation and proliferation of fibrogenic cells, including HSCs and PaSCs through the attenuation of extracellular matrix protein production and apoptosis induction.23,40-42
It is therefore expected that these drugs could serve as another therapeutic or preventive agent for various cancers and for fibrosis-associated diseases including chronic pancreatitis and liver cirrhosis. Accordingly, several clinical trials have been undertaken to verify both chemopreventive and antitumor effects of statins. However, these clinical trials have provided conflicting data as to whether statins have anti-cancer effects.43,44 Therefore, statin therapy as a supplement to or in conjunction with other drugs is currently being studied.
Combined treatment with statin and PPARγ agonists is clinically efficacious and safe. Previous studies have shown that the combination of these two drugs can be useful and has a synergistic effect on the treatment of dyslipidemia and cardiovascular diseases. Furthermore, additional anti-inflammatory effects of combined treatment with simvastatin and pioglitazone has been demonstrated in non-diabetic patients with cardiovascular disease and elevated high sensitive C-reactive protein levels.45 Recent study has shown that simultaneous treatment with these two drugs had synergistic anti-cancer effects in different cancer cells.35 Therefore, the theoretical basis of using a combination strategy has been demonstrated.
In this study, we have shown that a lower dose of simvastatin (1 µM) significantly potentiated antiproliferative effects in activated PaSCs and decreased the expression of α-SMA when combined with troglitazone. Previous studies showed that a lower concentration of lovastatin inhibited the proliferation and activation of PaSCs which is consistent with our findings.40 Our study showed that simvastatin mono-treatment could inhibit cell proliferation and show changes in phenotype in a dose-dependent manner. When combined with these drugs, cell proliferation could be inhibited with a lower dose of simvastatin (1 µM), a level which might be clinically available and shorter treatment period (24 hours).
Second, the combined effects of the two drugs are due to the synergistic effect in the regulation of cell proliferation and activation.39 In this study, both simvastatin (1 µM) and troglitazone (10 µM) decreased the phosphorylation of PDGF-induced ERK, and the combination of the two drugs synergistically suppressed the activation of ERK. This result suggests that the Ras-ERK cascade may be involved in the mediation of combination effects on PaSCs.
Statin and PPARγ agonists were shown to interfere with cell cycle progression by affecting cell cycle machinery proteins such as CDKs, p21, and p27Kip1.46-48 In this study, using FACS analysis, combination treatment was shown to induce cell cycle arrest in the G0/G1 phase. It participated in cell cycle regulation by controlling the expression of p27Kip1, which inhibits CDK activation. The Ras-ERK signaling system has a role in these pathways, and through the phosphorylation of ERK, cyclin D expression increases, whereas expression of p27Kip1 is suppressed, which further activates cell growth.49,50 We observed that both simvastatin (1 µM) and troglitazone (10 µM) failed to restore the suppression of PDGF-induced p27Kip1, but combined treatment increased the expression of p27Kip1 suppressed by PDGF. These results suggest that synergistic effect of these drugs on PaSCs was implemented by the cell cycle machinery protein p27Kip1.
In summary, our findings reveal a synergistic effect of simvastatin and troglitazone in the inhibition of PaSC proliferation. This effect is mediated by suppression of ERK and the modulation of the cell cycle machinery protein p27Kip1. Thus, the combination of a statin and PPARγ agonist might represent a novel therapeutic modality for the prevention of fibrosis of the pancreas and for treatment of chronic pancreatitis. However, the exact mechanism of other beneficial effect of this combined treatment need to be elucidated.
ACKNOWLEDGEMENTS
This work was supported by Korea University research grant (K1030551).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 2.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparmann G, Hohenadl C, Tornøe J, et al. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G211–G219. doi: 10.1152/ajpgi.00347.2003. [DOI] [PubMed] [Google Scholar]
- 5.Masamune A, Watanabe T, Kikuta K, Shimosegawa T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S48–S54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saotome T, Inoue H, Fujimiya M, Fujiyama Y, Bamba T. Morphological and immunocytochemical identification of periacinar fibroblast-like cells derived from human pancreatic acini. Pancreas. 1997;14:373–382. doi: 10.1097/00006676-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Luttenberger T, Schmid-Kotsas A, Menke A, et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47–55. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- 10.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hama K, Ohnishi H, Aoki H, et al. Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway. Biochem Biophys Res Commun. 2006;340:742–750. doi: 10.1016/j.bbrc.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 13.Talukdar R, Tandon RK. Pancreatic stellate cells: new target in the treatment of chronic pancreatitis. J Gastroenterol Hepatol. 2008;23:34–41. doi: 10.1111/j.1440-1746.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi H, Miyata T, Yasuda H, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem. 2004;279:8873–8878. doi: 10.1074/jbc.M309698200. [DOI] [PubMed] [Google Scholar]
- 15.Masamune A, Watanabe T, Kikuta K, Satoh K, Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G99–G108. doi: 10.1152/ajpgi.00272.2007. [DOI] [PubMed] [Google Scholar]
- 16.Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Kuno A, Ogawa K, et al. Combination therapy with an angiotensin-converting enzyme inhibitor and an angiotensin II receptor blocker synergistically suppresses chronic pancreatitis in rats. J Pharmacol Exp Ther. 2005;313:36–45. doi: 10.1124/jpet.104.077883. [DOI] [PubMed] [Google Scholar]
- 18.Nagashio Y, Asaumi H, Watanabe S, et al. Angiotensin II type 1 receptor interaction is an important regulator for the development of pancreatic fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2004;287:G170–G177. doi: 10.1152/ajpgi.00005.2004. [DOI] [PubMed] [Google Scholar]
- 19.Trebicka J, Hennenberg M, Odenthal M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Aprigliano I, Dudas J, Ramadori G, Saile B. Atorvastatin induces apoptosis by a caspase-9-dependent pathway: an in vitro study on activated rat hepatic stellate cells. Liver Int. 2008;28:546–557. doi: 10.1111/j.1478-3231.2008.01682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanase M, Ikeda H, Matsui A, et al. HMG-COA reductase inhibitor modulates collagen GEL-contraction by hepatic myofibroblast-like stellate cell line: involvement of geranylgeranylated proteins. Comp Hepatol. 2004;3(Suppl 1):S21. doi: 10.1186/1476-5926-2-S1-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–315. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 23.Rombouts K, Kisanga E, Hellemans K, et al. Effect of HMG-CoA reductase inhibitors on proliferation and protein synthesis by rat hepatic stellate cells. J Hepatol. 2003;38:564–572. doi: 10.1016/s0168-8278(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 24.Tien YW, Wu YM, Lin WC, Lee HS, Lee PH. Pancreatic carcinoma cells stimulate proliferation and matrix synthesis of hepatic stellate cells. J Hepatol. 2009;51:307–314. doi: 10.1016/j.jhep.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Kliewer SA, Lenhard JM, Willson TM, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 26.Forman BM, Tontonoz P, Chen J, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 27.Chou FS, Wang PS, Kulp S, Pinzone JJ. Effects of thiazolidinediones on differentiation, proliferation, and apoptosis. Mol Cancer Res. 2007;5:523–530. doi: 10.1158/1541-7786.MCR-06-0278. [DOI] [PubMed] [Google Scholar]
- 28.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 29.Marra F, Efsen E, Romanelli RG, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 30.Miyahara T, Schrum L, Rippe R, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 31.Masamune A, Kikuta K, Satoh M, et al. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- 32.Jaster R, Lichte P, Fitzner B, et al. Peroxisome proliferator-activated receptor gamma overexpression inhibits pro-fibrogenic activities of immortalised rat pancreatic stellate cells. J Cell Mol Med. 2005;9:670–682. doi: 10.1111/j.1582-4934.2005.tb00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivashchenko CY, Duan SZ, Usher MG, Mortensen RM. PPAR-gamma knockout in pancreatic epithelial cells abolishes the inhibitory effect of rosiglitazone on caerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G319–G326. doi: 10.1152/ajpgi.00056.2007. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu K, Shiratori K, Kobayashi M, Kawamata H. Troglitazone inhibits the progression of chronic pancreatitis and the profibrogenic activity of pancreatic stellate cells via a PPARgamma-independent mechanism. Pancreas. 2004;29:67–74. doi: 10.1097/00006676-200407000-00058. [DOI] [PubMed] [Google Scholar]
- 35.Yao CJ, Lai GM, Chan CF, et al. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int J Cancer. 2006;118:773–779. doi: 10.1002/ijc.21361. [DOI] [PubMed] [Google Scholar]
- 36.Mrówka P, Glodkowska E, Nowis D, et al. Ciglitazone, an agonist of peroxisome proliferator-activated receptor gamma, exerts potentiated cytostatic/cytotoxic effects against tumor cells when combined with lovastatin. Int J Oncol. 2008;32:249–255. [PubMed] [Google Scholar]
- 37.Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res. 2007;100:1442–1451. doi: 10.1161/01.RES.0000268411.49545.9c. [DOI] [PubMed] [Google Scholar]
- 38.Grip O, Janciauskiene S, Lindgren S. Atorvastatin activates PPAR-gamma and attenuates the inflammatory response in human monocytes. Inflamm Res. 2002;51:58–62. doi: 10.1007/BF02684000. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 40.Jaster R, Brock P, Sparmann G, Emmrich J, Liebe S. Inhibition of pancreatic stellate cell activation by the hydroxymethylglutaryl coenzyme A reductase inhibitor lovastatin. Biochem Pharmacol. 2003;65:1295–1303. doi: 10.1016/s0006-2952(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Yang CW, Park JH, et al. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004;286:F46–F57. doi: 10.1152/ajprenal.00428.2002. [DOI] [PubMed] [Google Scholar]
- 42.Wei JL, Ma CY, Zhang YD, Li Y. Synergistic effects of pravastatin and pioglitazone in renal tubular epithelial cells induced by transforming growth factor-beta1. Cell Biol Int. 2007;31:451–458. doi: 10.1016/j.cellbi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010;59:1572–1585. doi: 10.1136/gut.2009.190900. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton RJ, Freedland SJ. Review of recent evidence in support of a role for statins in the prevention of prostate cancer. Curr Opin Urol. 2008;18:333–339. doi: 10.1097/MOU.0b013e3282f9b3cc. [DOI] [PubMed] [Google Scholar]
- 45.Hanefeld M, Marx N, Pfützner A, et al. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. J Am Coll Cardiol. 2007;49:290–297. doi: 10.1016/j.jacc.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 46.Efuet ET, Keyomarsi K. Farnesyl and geranylgeranyl transferase inhibitors induce G1 arrest by targeting the proteasome. Cancer Res. 2006;66:1040–1051. doi: 10.1158/0008-5472.CAN-05-3416. [DOI] [PubMed] [Google Scholar]
- 47.Theocharis S, Margeli A, Vielh P, Kouraklis G. Peroxisome proliferator-activated receptor-gamma ligands as cell-cycle modulators. Cancer Treat Rev. 2004;30:545–554. doi: 10.1016/j.ctrv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Takeda I, Maruya S, Shirasaki T, et al. Simvastatin inactivates beta1-integrin and extracellular signal-related kinase signaling and inhibits cell proliferation in head and neck squamous cell carcinoma cells. Cancer Sci. 2007;98:890–899. doi: 10.1111/j.1349-7006.2007.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 50.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]