Abstract
Objective
The RhoA/Rho-kinase pathway plays a pivotal role in cold induced vasoconstriction, vascular smooth muscle cells function and vascular homeostasis. This study evaluates the efficacy of fasudil, a RhoA/Rho-kinase inhibitor, to reverse cold-induced vasospasm in patients with Raynaud’s phenomenon (RP) secondary to systemic sclerosis (SSc).
Methods
This is a single-center, double-blind, placebo-controlled, randomized, 3-period crossover study of oral fasudil (40 mg or 80 mg) or placebo administered 2 hours before a standardized cold challenge. The fall in skin temperature after the cold challenge and time to recover 50% and 70% of pre-challenge digital skin temperature were used as primary outcomes. Digital blood flow assessed by Laser Doppler, time to minimum skin temperature, and rate of skin cooling were also measured.
Results
Seventeen patients with SSc and RP completed the study. After cold challenge, skin temperatures and the average time (minutes) to recover 50% (placebo: 7.9, fasudil 40 mg: 7.5, and fasudil 80 mg: 8.2; p=.791) and 70% (placebo: 18.2, fasudil 40 mg: 15.0, and fasudil 80 mg: 17.1; p=.654) of pre-challenge skin temperature were not significantly different across the three groups. The digital blood flow measurements were higher in fasudil treated groups than placebo but differences were not significant (p=.693).
Conclusions
Fasudil administered at single oral dose of 40 mg or 80 mg was not associated with significant benefit in term of skin temperature recovery time and digital blood flow after cold challenge.
Keywords: Raynaud’s phenomenon, rho-kinase inhibitor, fasudil, systemic sclerosis
INTRODUCTION
Raynaud’s phenomenon (RP) is a reversible vasospastic disorder of digital arteries and cutaneous arterioles characterized by typical skin color changes and tissue ischemia (1). Avoidance of common triggers such as cold temperatures and emotional stress often leads to improvement of symptoms. When such strategy yields inadequate benefits, pharmacological therapy is needed.
Cutaneous vasoconstriction occurs through a general sympathetic adrenergic response and through local mechanisms in response to cold. While under normal conditions the vasomotor tone is regulated mainly by α2A-adrenoreceptors expressed on vascular smooth muscle cells (VSMC) (2), during cold exposure the normally “silent” α2C-adrenoreceptors (α2C-ARs) relocate from the Golgi complex to cell surface, driving the cold-induced vasoconstrictive response (3). Interestingly, the reactivity to α2-ARs stimulation is highly increased in cutaneous arteries of patients with systemic sclerosis (SSc) (4), and blockage of α2C-ARs has shown to shorten the time to recover digital skin temperature after cold challenge in patients with RP secondary to SSc (RP-SSc) (5).
The Rho/Rho-kinase pathway is activated by cooling and mediates vasoconstriction of cutaneous arteries by inducing α2C-AR relocation to the cell surface and by increasing calcium-dependent VSMC contractility (6). Rho-kinase inhibition has been shown to effectively reduce α2-AR-mediated response during cold-exposure and to prevent cold-induced vasoconstriction in human skin (6). Therefore, Rho/Rho-kinase inhibition may provide a highly selective intervention directed towards the mechanisms underlying thermo-sensitive vasomotor responses in the skin of RP patients.
Fasudil, a Rho/Rho-kinase signaling pathway inhibitor, has been used effectively in conditions characterized by vascular dysfunction including cerebral vasospasm after stroke (7), angina pectoris (8), and pulmonary hypertension (9).
In this study, we investigated the efficacy of fasudil to reverse cold-induced vasospasm by measuring the recovery of digital blood flow and skin temperature after a standard cold challenge in patients with RP-SSc.
PATIENTS AND METHODS
This study was a single-center, double-blind, placebo-controlled, randomized, three-period, crossover study of three single oral doses of fasudil 40 mg or 80 mg or placebo.
Men and women between the ages of 18–80 years with clinical diagnosis of RP secondary to SSc were eligible for the study. RP was defined as having a history of cold sensitivity associated with color changes of cyanosis or pallor in the digits, and at least two attacks daily during winter months. The diagnosis of SSc was defined by the American College of Rheumatology criteria (10) or by having at least three of five features of the CREST syndrome (calcinosis, RP, esophageal dysmotility, sclerodactyly, telangiectasias). Subjects were excluded if they had symptomatic orthostatic hypotension, evidence of malignancy, active ischemic digital ulcers, history of sympathectomy, upper extremity deep vein thrombosis or lymphedema (within 3 months), recent surgery requiring general anesthesia, current alcohol or illicit drug use, use of any investigational drug within 30 days of the study, current pregnancy or breast feeding. Approval from the Johns Hopkins Institutional Review Board was obtained prior to study initiation.
Each subject was randomly assigned to 1 of 6 possible treatment sequences (ABC, ACB, BAC, BCA, CAB, CBA) in a double-blind manner. Treatment consisted of a single oral dose of two tablets of placebo (A), one tablet of fasudil 40 mg (Ashai Kasei Pharma Corporation, Japan) and one of placebo (B) or two tablets of fasudil 40 mg (C). Randomization to receive a specific treatment sequence was performed at the screening/baseline visit based on a computer-generated code, with allocation on a 1:1:1:1:1:1 ratio for the six possible treatments. A single dose consisting of the two tablets was taken after fasting for 10 hours, once during each treatment period. A washout interval of at least 24 hours and no more than 28 days was maintained between treatments. All study sessions were completed within 35 days. Vasodilator therapy, if taken, was suspended for the day of the study.
Study measurements were obtained approximately two hours after oral ingestion of the study drug. The subjects sat in a recliner having undisturbed rest in a temperature stabilized room (27 °C ± 2 °C) for 30 minutes before any measurements to ensure a standard baseline.
The cold challenge was carried out by inserting the study hand up to the mid-forearm in the exposure chamber where temperature was previously lowered to −20°C. The exposure was continued for a maximum of 15 minutes until the skin temperature dropped to 12°C, or until the patient could no longer tolerate the cold. The hand was then removed from the chamber and passively rewarmed at ambient temperature (27°C). The digital skin temperature was measured with a thermistor (YSI, Yellow Springs, OH) affixed to the pad of the fourth distal phalanx before and at the end of the cold challenge, and then every 5 minutes for the first hour. One last measurement was performed 240 minutes after drug administration. The baseline digital skin temperature was defined as the pre-challenge digital skin temperature, expressed as the average of 3 pre-challenge temperature measurements. The digital skin temperature at the end of the cold challenge was defined as the minimum skin temperature.
The time to recover 50% and 70% of the drop from baseline (pre-challenge) skin temperature was derived for each subject for each cold challenge. The differences in digital skin temperatures and these recovery times served as the primary outcome variables.
The total digital blood flow (measured by Laser Doppler perfusion imaging [Motor Instrument, Devon, UK] on the dorsum of the 2nd, 3rd, 4th finger distally to the metacarpal-phalangeal joint after cold challenge), the time (minutes) to reach minimum digital skin temperature and the rate (°C/minute) of skin cooling during cold challenge served as secondary outcome variables.
A previous study (15) comparing nifedipine to placebo in RP patients showed a mean difference in finger skin temperature during cold challenge of 1.9°C (24.7±0.8 versus 22.8±0.6 respectively), yielding a power greater than 95% with a sample size as small as 10. Anticipating a possible smaller treatment effect such as a difference of 1.1 in mean skin temperature, we estimated a power of at least 82% with a total sample size of 16. Another study (5) using an experimental design and outcomes similar to ours found a significant difference in 50% and 70% recovery time after cold challenge for a total sample size of 12. Taken together, these data indicated that our sample of 17 patients was sensitive enough to detect any potential effect across treatment groups should any exist. No concern for type II errors was present.
Statistical analyses were completed using SPSS 17.0 (SPSS Inc, 2001). Given that patients received all three treatments in one of the six possible sequences, preliminary analyses examined the carryover effect (the extent to which the order of treatments affected primary and secondary outcomes). Tukey’s post hoc multiple comparison tests were completed using digital skin temperature at each time point as the outcome and the six possible sequences of treatment as the predictor. The same was done for digital blood flow measurements.
The second analysis step used one-way analysis of variance (ANOVAs) to examine mean differences in time to recover 50% and 70% of temperature drop from baseline with treatment group as the predictor.
The third analysis step used repeated measures tests to examine potential mean differences in digital skin temperature and digital blood flow by time point across treatments. Tukey’s post hoc analysis compared the mean digital skin temperature and mean digital blood flow across treatments.
The final analytic step used one-way ANOVAs to examine the potential mean differences in the duration of the cold challenge and mean differences in the rate of temperature drop during cold challenge with treatment group as the predictor. Significance levels for all analyses were set at .05.
RESULTS
The socio-demographic characteristics of study subjects are summarized in Table 1. A total of 17 patients were enrolled. These were mainly middle-aged (median age, 51 years), Caucasian (59%), women (88%), non smokers (73%), with limited SSc phenotype (59%). All participants completed the study. No adverse event was reported.
Table 1.
Sociodemographic and clinical characteristics of scleroderma patients
| Characteristic | Value (n=17) |
|---|---|
| Women, n (%) | 15 (88) |
| Ethnicity | |
| Caucasian, n (%) | 10 (59) |
| African American, n (%) | 6 (35) |
| Asian, n (%) | 1 (6) |
| Age, median (years) [range] | 51 [30–70] |
| Skin Subtype | |
| Limited, n (%) | 10 (59) |
| Diffuse, n (%) | 7 (41) |
| Smoking status | |
| Former, n (%) | 3 (18) |
| Current, n (%) | 1 (6) |
| Never, n (%) | 13 (76) |
| Current use of vasodilator therapy *, n (%) | 7 (41) |
| RP duration, mean±SD (years) | 11.9 ± 7.9 |
| Pulmonary arterial hypertension †, n (%) | 4 (33) |
| RP Severity Score, mean ‡ | 1.38 |
Therapy was suspended on the day of the study. All 17 patients were taking calcium channel blockers, with one subject being concurrently on oral sildenafil.
Pulmonary Arterial Hypertension defined as estimated Right Systolic Ventricular Pressure (RVSP) >40 mmHg (echocardiogram performed within 6 months). ECHO data was available for 12 of 17 patients.
Defined as 0 = no RP, 1 = RP alone, 2 = presence of digital pitting scars, 3 = active digital tip ulceration, 4 = active digital gangrene.
Preliminary analyses demonstrated that the order of the treatments had no significant effect on mean differences in digital blood flow or skin temperature at any given time point (no carryover effect).
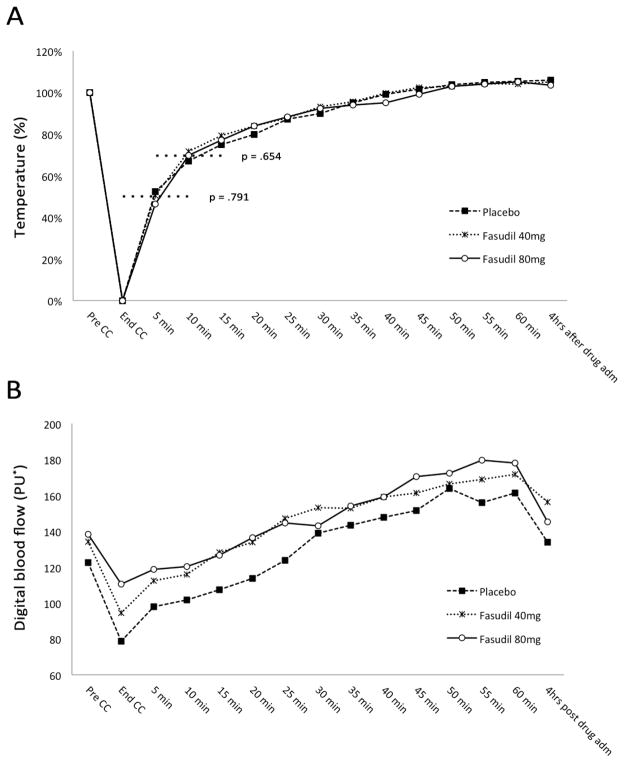
The changes of skin temperature during cold challenge are shown in Figure 1A. There were no statistically significant differences across the three treatments at any given time point (p=.718). In addition, the difference in average time to recover 50% and 70% of temperature drop from baseline was not statistically significant (p=.791 and p=.654 respectively), even though recovery times to 70% of baseline temperature were shorter in fasudil treated patients (40 mg, 15.0±9.4; 80 mg, 17.1±10.8) compared to placebo (18.2±10.7) (Table 2).
Figure 1. Temperature and digital blood flow curves following single dose fasudil administration versus placebo in SSc patients with Raynaud’s phenomenon.
* Perfusion units (PU) calculated by the instrument accounting for erythrocyte density and velocity in the sampled volume.
CC = cold-challenge; pre-CC = skin temperature measured right before the beginning of the CC; end-CC = skin temperature measured at the end of the CC (CC stopped when digital skin temperature reached 12 °C or the patient could no longer tolerate the cold).
(A) Recovery of digital skin temperature after cold challenge (CC) according to treatment group. Values are expressed as percentage of range from baseline (100%) to minimum digital skin temperature after CC (0%). Times to recover to 50% and 70% (dotted lines) represent the primary outcome.
(B) Mean digital blood flow during and after the cold-challenge (CC) measured with Laser Doppler perfusion imaging according to treatment group (values are reported in Table 2).
Table 2.
Primary and secondary outcomes of single dose fasudil administration versus placebo in SSc patients with Raynaud’s phenomenon.
| Placebo | Fasudil 40 mg | Fasudil 80 mg | p-value | |
|---|---|---|---|---|
| Recovery Time to 50% pre-CC Temperature* (min) | 7.9 ± 3.1 | 7.5 ± 3.2 | 8.2 ± 3.0 | 0.791 |
| Recovery Time to 70% pre-CC Temperature* (min) | 18.2 ± 10.7 | 15.0 ± 9.4 | 17.1 ± 10.8 | 0.654 |
| Minutes in the cold chamber | 6.94 ± 3.2 | 8.41 ± 4.7 | 7.06 ± 4.0 | 0.496 |
| Rate of skin cooling during CC (°C/min) | 2.74 ± 1.0 | 2.75 ± 1.6 | 3.09 ± 1.3 | 0.674 |
| Digital skin blood flow (PU)† | ||||
| Pre-CC | 122.6 ± 57.6 | 134.1 ± 73.4 | 138.3 ± 55.1 | 0.751 |
| End-CC | 78.6 ± 46.5 | 94.5 ± 64.0 | 110.5 ± 60.6 | 0.28 |
| 5 min | 97.8 ± 56.0 | 112.3 ± 67.9 | 118.6 ± 64.8 | 0.712 |
| 10 min | 101.6 ± 60.7 | 116.0 ± 79.2 | 120.3 ± 70.1 | 0.722 |
| 15 min | 107.4 ± 65.0 | 128.0 ± 84.4 | 126.7 ± 76.8 | 0.676 |
| 20 min | 113.8 ± 69.0 | 134.0 ± 91.9 | 136.5 ± 82.2 | 0.676 |
| 25 min | 123.7 ± 72.5 | 146.9 ± 94.1 | 144.4 ± 91.7 | 0.694 |
| 30 min | 138.7 ± 74.0 | 153.0 ± 96.2 | 143.0 ± 96.8 | 0.894 |
| 35 min | 143.3 ± 84.2 | 152.6 ± 86.9 | 154.0 ± 86.8 | 0.924 |
| 40 min | 147.6 ± 80.7 | 158.9 ± 95.7 | 158.9 ± 93.3 | 0.914 |
| 45 min | 151.7 ± 89.5 | 161.1 ± 100.2 | 170.6 ± 93.5 | 0.849 |
| 50 min | 163.8 ± 93.9 | 166.2 ± 104.3 | 172.4 ± 92.5 | 0.966 |
| 55 min | 155.8 ± 92.1 | 168.7 ± 103.2 | 179.5 ± 88.2 | 0.767 |
| 60 min | 161.3 ± 95.7 | 171.8 ± 97.4 | 177.9 ± 86.2 | 0.875 |
| 4 hrs (after drug administration) | 133.7 ± 63.5 | 156.1 ± 97.1 | 145.1 ± 43.6 | 0.708 |
Recovery times are defined as follows: 0.5 or 0.7 * [(pre-cold challenge digital skin temperature – end-cold challenge digital skin temperature) + end-cold challenge digital skin temperature]
Digital blood flow was measured on the dorsum of the 2nd, 3rd, 4th finger distally to the metacarpal-phalangeal joint. The mean skin surface area analyzed was 68.24 ± 12.88 cm2 CC = cold-challenge; pre-CC = skin temperature measured right before the beginning of the CC; end-CC = skin temperature measured at the end of the CC (CC stopped when digital skin temperature reached 12 °C or the patient could no longer tolerate the cold); PU = perfusion units calculated by the instrument accounting for erythrocyte density and velocity in the sampled volume.
The average time to reach the minimum digital skin temperature (i.e. cold challenge mean duration) was shortest for those on the placebo treatment, followed by those on 80 mg and then those on 40 mg of fasudil (Table 2), but the differences were not statistically significant (p=.496). In addition, no significant difference was found in rate of skin cooling during cold challenge between the three treatment groups (p=.674).
The mean digital blood flow was not statistically different at any given time point across the three treatment groups (p=.693) (Figure 1B). Table 2 provides details on post-hoc analyses examining series of one-way ANOVAs at each time point for all participants. Interestingly, the digital blood flow appears to be slightly higher in the fasudil treatment groups (particularly at 80 mg) during and immediately after the cold challenge, but with great variability and no statistical significance.
DISCUSSION
This is the first placebo-controlled trial of fasudil for treatment of RP in a selected population of SSc patients. Fasudil administered as a 40 mg or 80 mg single oral dose was not associated with significant benefit in term of skin temperature recovery time and digital blood flow after a standard cold challenge.
In SSc patients, therapy for RP is based on administration of systemic arterial vasodilators. Oral dihydropyridine calcium channel blockers are the most commonly used drugs, but their efficacy is highly variable and limited by the inability to reach ideal therapeutic dosages due to unpleasant side effects. Other vasodilators including nitrates, prostaglandins, phosphodiesterase-5 inhibitors and sympatholytic agents do not provide complete control of symptoms in severe RP.
The RhoA/Rho-kinase pathway represents an interesting therapeutic target for RP-SSc. Together with its primary role in cold-induced vasoconstriction, VSMC function and vascular homeostasis, Rho-kinase mediates several downstream effects of important vasoactive molecules (i.e. angiotensin II, thrombin, platelet derived growth factor), negatively regulates nitric oxide synthesis by endothelial cells and promotes VSMC proliferation in response to reactive oxygen species. In addition, Rho-kinase expression is enhanced by proinflammatory stimuli and can lead to higher endothelial production of inflammatory cytokines and adhesion molecules (11). In vivo inhibition of Rho-kinase with fasudil have shown to prevent ischemia-reperfusion injury (12), to suppress collagen synthesis in cardiac fibroblasts (13), and to reduce pulmonary vascular resistances in patients with PAH (9).
The placebo-controlled trial design and the administration of a standardized cold challenge are valuable tools to measure vascular responsiveness to pharmacological intervention in RP, but clearly do not allow any inference regarding the clinical efficacy. This study can only address whether acute dosing in a laboratory setting influences vascular reactivity after cold exposure. The impact of a real life situation on measurements of RP is not taken into account, and this is an important limitation of this investigation. Likewise, we did not measure drug levels in study patients. While previous pharmacokinetic studies in healthy subjects (14) have shown that 2 hours after ingestion of fasudil 40 mg the plasma concentration of its active metabolite was above the threshold levels to achieve Rho-kinase inhibition (7), it is possible that in SSc patients the absorption of this medication is altered, leading to inadequate peripheral concentration or to subclinical pharmacological effects. In addition, our study was focused on RP-SSc. Whereas primary RP is thought to be a functional abnormality of cutaneous thermoregulatory vessels with loss of vasomotor control, in RP secondary to SSc there are structural vascular abnormalities of peripheral arteries associated with endothelial damage, VSMC proliferation and intimal hyperplasia, ultimately leading to a more refractory vascular disease and irreversible chronic tissue ischemia, particularly in subjects with longer disease duration (1).
Since prolonged use of oral fasudil have shown an acceptable safety profile in previous studies (8), further trials of longer duration would be of great interest and will help to clarify if at higher doses or following chronic administration, fasudil or other rho-kinase inhibitors would be of benefit in the treatment of RP in SSc.
SIGNIFICANCE AND INNOVATION.
This is the first placebo-controlled trial evaluating the efficacy of fasudil, a RhoA/Rho-kinase inhibitor, to reverse cold-induced vasospasm in patients with Raynaud’s phenomenon secondary to systemic sclerosis.
Rho/Rho-kinase signaling pathway plays a prominent role in cold-induced vasospasm and mediates hypoxia-induced vascular smooth muscle cell proliferation, down-regulation of nitric oxide and secretion of vasoactive molecules.
RhoA/Rho-kinase inhibition is of interest for the treatment of vascular dysfunction in Raynaud’s phenomenon and systemic sclerosis-related vasculopathy.
Administration of fasudil was not associated with significant benefit in term of skin temperature recovery time and digital blood flow after a standard cold challenge.
Acknowledgments
Ashai Kasei Pharma Corporation (Japan), Peninsula Foundation, Scleroderma Research Foundation and the National Institute of Health (F.B. NIH grant AR-055667).
Footnotes
Financial disclosure: This study has been supported by Ashai Kasei Pharma Corporation (Japan). No further benefit from other commercial sources has been obtained for the work reported on this manuscript. None of the authors have financial benefits or conflict of interest to disclose relevant to this investigation.
Contributor Information
Andrea Fava, Johns Hopkins University School of Medicine, Baltimore, MD
Peter K Wung, Instructor of Medicine, Johns Hopkins, University School of Medicine, Baltimore, MD
Fredrick M Wigley, Professor of Medicine, Johns Hopkins, University School of Medicine, Baltimore, MD
Laura K Hummers, Assistant Professor of Medicine, Johns Hopkins, University School of Medicine, Baltimore, MD
Natalie R. Daya, Emory University, Biostatistics and Bioinformatics Department, Atlanta, GA
Sharon R. Ghazarian, Johns Hopkins University School of Medicine, Baltimore, MD.
Francesco Boin, Assistant Professor of Medicine, Johns Hopkins, University School of Medicine, Baltimore, MD
References
- 1.Boin F, Wigley FM. Understanding, assessing and treating Raynaud’s phenomenon. Curr Opin Rheumatol. 2005;17(6):752–60. doi: 10.1097/01.bor.0000179944.35400.6e. [DOI] [PubMed] [Google Scholar]
- 2.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. alpha-Adrenoceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol. 1988;255(5 Pt 2):H1000–3. doi: 10.1152/ajpheart.1988.255.5.H1000. [DOI] [PubMed] [Google Scholar]
- 3.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278(4):H1075–83. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 4.Flavahan NA, Flavahan S, Liu Q, Wu S, Tidmore W, Wiener CM, et al. Increased alpha2-adrenergic constriction of isolated arterioles in diffuse scleroderma. Arthritis Rheum. 2000;43(8):1886–90. doi: 10.1002/1529-0131(200008)43:8<1886::AID-ANR27>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA, Wigley FM, White B, Leatherman G, Zhong J, Krasa H, et al. Efficacy and tolerability of a selective alpha(2C)-adrenergic receptor blocker in recovery from cold-induced vasospasm in scleroderma patients: a single-center, double-blind, placebo-controlled, randomized crossover study. Arthritis Rheum. 2004;50(12):3994–4001. doi: 10.1002/art.20665. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94(10):1367–74. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E Fasudil Ischemic Stroke Study G. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238(1–2):31–9. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, et al. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46(10):1803–11. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, et al. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J. 2006;70(2):174–8. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- 10.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 11.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 12.Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Tanaka E, et al. Beneficial effect of hydroxyfasudil, a specific Rho-kinase inhibitor, on ischemia/reperfusion injury in canine coronary microcirculation in vivo. J Am Coll Cardiol. 2005;45(4):599–607. doi: 10.1016/j.jacc.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Zhang KX, Li YJ, Guo BY, Wang M. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses high glucose-induced proliferation and collagen synthesis in rat cardiac fibroblasts. Clin Exp Pharmacol Physiol. 2011;38(6):387–94. doi: 10.1111/j.1440-1681.2011.05523.x. [DOI] [PubMed] [Google Scholar]
- 14.Hinderling PH, Karara AH, Tao B, Pawula M, Wilding I, Lu M. Systemic availability of the active metabolite hydroxy-fasudil after administration of fasudil to different sites of the human gastrointestinal tract. J Clin Pharmacol. 2007;47(1):19–25. doi: 10.1177/0091270006293767. [DOI] [PubMed] [Google Scholar]
- 15.Wollersheim H, Thien T, van ‘t Laar A. Nifedipine in primary Raynaud’s phenomenon and in scleroderma: oral versus sublingual hemodynamic effects. J Clin Pharmacol. 1987;27(11):907–13. doi: 10.1002/j.1552-4604.1987.tb05587.x. [DOI] [PubMed] [Google Scholar]