Abstract
Purpose
This phase I trial evaluated intraperitoneal (IP) pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer.
Experimental Design
Dose escalation of d1 IP pemetrexed accrued 3 patients to each of 5 dose levels (60mg/m2 to 1000mg/m2), along with d2 IP cisplatin (75mg/m2), and d8 IP paclitaxel (60mg/m2). The goals were to determine maximum tolerated dose (MTD), 18-month progression-free survival (PFS), and pharmacokinetics (PK) of IP pemetrexed.
Results
Cycles, given every 21d, had an 80% 6-cycle completion rate. There was minimal grade 3 toxicity in the first 4 dose levels and remarkably an almost complete absence of peripheral neuropathy and alopecia. At the highest dose level, 2 of 3 patients experienced ≥grade 3 and dose-limiting toxicity (DLT) (hematologic, infection, gastrointestinal). There was a PK advantage for IP pemetrexed with a IP:plasma area under the concentration-time-curve ratio of 13-fold. Neither analysis of PK nor homocysteine levels explains the unexpected severity of toxicity in those 2 patients. Based on plasma C24 h levels, the 42 cycles at ≥500mg/m2 IP pemetrexed without DLT, the MTD appears to be 500 mg/m2. Median PFS is 30.1 months; 18-month PFS is 78.6% (median follow-up 22.4 months).
Conclusions
This IP only regimen in front-line ovarian cancer is feasible with PFS in line with recent literature. We suggest phase II trials of this regimen in this population with IP pemetrexed at 500mg/m2. The favorable toxicity profile at doses <1000mg/m2, which needs to be confirmed, appears to compare well with standard combination intravenous/IP platinum/taxane chemotherapy in this disease.
Keywords: intraperitoneal chemotherapy, pemetrexed
Introduction
Epithelial ovarian cancer continues to be the leading cause of gynecologic cancer death in the United States, with more than 21,990 new cases and 15,460 deaths expected in 2011 (1). Since the incorporation of taxanes into platinum-based therapy in the mid 1990's, there has been little progress in achieving a further significant reduction in overall mortality. However, in optimally debulked Stage III ovarian cancer, advances validated by several phase III trials and meta-analyses, include the introduction of intraperitoneal (IP) therapy with cisplatin which has increased survival (2-7). Acute toxicity has been a significant barrier to universal acceptance of the IP approach (4, 5). Investigation into how to lengthen progression free survivals (PFS), while lessening toxicity remains an area of active research.
Pemetrexed (Alimta®) is a multi-targeted anti-folate, which has the ability to interfere with the synthesis of three folate-dependent enzymes (thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase) involved in de novo biosynthesis of thymidine and purine nucleotides. Pemetrexed, when given intravenously (IV), is broadly active in a wide variety of solid tumors, including mesothelioma, non-small cell lung cancer, and platinum sensitive and resistant ovarian cancer (8-15). Prior to the start of this trial, preliminary data from a GOG phase II trial using IV pemetrexed suggested that there was considerable activity in platinum resistant ovarian cancer; this data was subsequently confirmed (12). A phase III trial demonstrated that the addition of IV pemetrexed to IV cisplatin in patients with pleural mesothelioma significantly increased overall survival (OS) as well as PFS and reduced disease-related symptoms (8). In trials of pemetrexed in other solid tumors, treatment with folate and vitamin B12 was shown to improve its toxicity profile and has significantly reduced grade 3 and 4 toxicities such as neutropenia and gastrointestinal toxicities, as well as mortality (8, 16).
At the time of conception and initiation of this trial, there were no previously published reports on the use of IP pemetrexed in humans. Preclinical data in rats showed that administration of IP pemetrexed led to a significantly higher (up to 40.8-fold) IP:IV area under the concentration time curve (AUC) ratio, than did an equivalent dose of IV pemetrexed (17). In addition, the knowledge that pemetrexed seeks out third spaces, such as the IP cavity, was felt to be an advantage that along with the higher AUC would increase the efficacy of this drug. Preclinical data showed that cisplatin does not affect the pharmacokinetics of pemetrexed (18); likewise those of total platinum are unaltered by pemetrexed administration (19). Therefore, the IP administration of pemetrexed and cisplatin was an appropriate choice.
In the design of this trial, the significant toxicity of the IV paclitaxel, IP cisplatin, and IP paclitaxel arm of the GOG-172 trial was taken into consideration (4). Because of the toxicity seen in the GOG trial many physicians were resistant to using IP chemotherapy, and off trial modifications, including reduction of the IP cisplatin dose from 100 mg/m2 to 75 mg/m2, were common. The AUC advantage of IP cisplatin in comparison to IV administration is at least 15; while remarkably the AUC advantage of IP paclitaxel is up to 1000 fold higher than an equivalent dose administered IV (20). Given this context, the inclusion of IV paclitaxel may add neurologic toxicity without substantially adding to treatment efficacy. We reasoned that by replacing the IV paclitaxel with non-cross-resistant IP pemetrexed, and giving the IP cisplatin at 75 mg/m2, we would decrease the significant incidence and severity of neurotoxicity and other toxicities, while maintaining the efficacy.
Our phase I front-line trial in optimally debulked Stage IIIC ovarian, peritoneal, and tubal cancer patients, includes IP pemetrexed given day 1 along with IP cisplatin on day 2. IP paclitaxel is given on day 8 as in the highly active GOG-172 regimen. Our trial serves as the first trial in this disease to include IP pemetrexed. It is the first front-line trial in this disease in which all drugs in the combination are given IP. The goal of the study was to determine the maximum tolerated dose (MTD) of this combination therapy, and to determine its toxicity. Secondary goals were to determine the PFS with a goal of 80% of patients being progression-free at 18 months, and to perform correlative pharmacokinetic analysis of IP pemetrexed.
Materials and Methods
Patient eligibility
Patients who had a histologically or pathologically confirmed diagnosis of Stage III carcinoma of the ovary, primary peritoneum, or fallopian tube were eligible if they: (1) had no prior treatment and were optimally debulked at primary surgery to individual tumor plaques of <1cm or (2) had received up to four cycles of IV carboplatin/taxane as neoadjuvant chemotherapy for advanced, un-resectable disease and at interval surgery were optimally debulked to <1cm.
Additional inclusion criteria included an ECOG performance status of 0 – 2, adequate hepatic function [defined as serum bilirubin ≤2 times the upper limit of normal (ULN) and alanine and aspartate transaminases ≤2.5 times the ULN], and adequate renal function, as defined by a creatinine clearance rate of ≥45mL/minute. Eligible patients were required to have a hemoglobin level of ≥9g/dL, white blood cell count ≥3500/μL, and platelets ≥100,000/μL. Patients with mild to moderate renal insufficiency (defined as a creatinine clearance between 45 and 79 mL/minute) needed to be able to interrupt non-steroidal anti-inflammatory treatment for at least 2 days before, the day of, and at least 2 days after the administration of pemetrexed. Finally, patients must have been able to receive folic acid, vitamin B12, and dexamethasone treatments.
Exclusion criteria included life-threatening complications of their malignancies, severe and/or uncontrolled concurrent medical disease (e.g. uncontrolled diabetes or heart disease, uncontrolled chronic renal or liver disease, or active uncontrolled infection). Additional exclusion criteria included presence of third space fluid not controllable by drainage, evidence of uncontrollable nausea, history of abdominal fistula, intra-abdominal abscess or other contraindication to IP therapy, pre-existing history of significant hearing loss, or known hypersensitivity to any component of pemetrexed, cisplatin, or paclitaxel. Eligible patients must not have a prior malignancy except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, adequately treated Stage I or II cancer from which the patient was in complete remission, or any other cancer from which the patient had been disease-free for 5 years.
All patients provided written informed consent before study enrollment. The study was approved by the University of Arizona Institutional Review Board (IRB), and was conducted in accordance with institutional and federal guidelines.
Study design and treatment
The study was an open label, escalating dose, phase I trial in patients with optimally debulked Stage III ovarian, peritoneal, or fallopian tube cancer.
IP pemetrexed was administered on Day 1, IP cisplatin dosed at 75 mg/m2 on Day 2, then IP paclitaxel dosed at 60 mg/m2 on Day 8 of each cycle with courses repeated every 21 days for 6 cycles. Rationale for sequencing pemetrexed day 1 and cisplatin day 2 comes from in vitro sequencing data suggesting that the preferred administration schedule to avoid antagonistic effects and to take advantage of their synergy, was to administer pemetrexed first followed by cisplatin (21). No dose reductions were required, and there were only 3 one-week dose delays. Patients were treated with IP pemetrexed at the following escalating dose levels: 60, 120, 500, 750, and 1000 mg/m2. If none of the initial 3 patients on a dose level experienced a dose-limiting toxicity (DLT) after the first cycle of therapy, then the dose was escalated to the next level. If 2 or more patients on any dose level experienced a DLT, then the MTD would be determined to be the next lower dose level. DLTs were defined as grade 3 or higher febrile neutropenia, thrombocytopenia with bleeding, neurologic toxicity, non-hematologic toxicities (not including fatigue, alopecia, nausea, vomiting, elevated liver transaminases) and grade 4 neutropenia lasting >7 days, thrombocytopenia, and increased liver transaminases. A total of 10 patients were to be treated at MTD.
Because there were no previously published reports in humans of IP pemetrexed, we started at a very low dose of 60 mg/m2, knowing at the outset that the IV dose of pemetrexed given in combination was 500 mg/m2 in other tumors (8,9,16). There were minimal grade 3 and no grade 4 toxicities observed at dose levels ≤ 500mg/m2 IP pemetrexed. During the conduct of the trial more mature data on IV pemetrexed in ovarian cancer subsequently became available, with doses up to 900mg/m2 being active and tolerated (12). We therefore obtained IRB approval to continue the dose escalation up to 1000mg/m2 IP pemetrexed. Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.0.
Patients were given supplemental folic acid and vitamin B12 to prevent the Grade 4 hematologic and Grade 3 and 4 non-hematologic toxicities associated with pemetrexed therapy. Folic acid (400-800ug) was taken daily beginning at least 7 days prior to the first dose of pemetrexed and continuing until 3 weeks after the last dose of pemetrexed. Higher doses of folic acid have not been shown to be more effective in the prevention of pemetrexed toxicity (22). Vitamin B12 (1000 μg) was administered as an intramuscular injection 1–2 weeks prior to the first dose of pemetrexed and repeated every 9 weeks until 3 weeks after the last dose of pemetrexed. Strict attention was made to patient adherence to folic acid and vitamin B12. In addition, for rash prophylaxis, dexamethasone (4mg oral or equivalent) was given twice daily the day before, the day of, and the day after pemetrexed administration.
Patient evaluation and definition of response
Prior to the beginning of each cycle, CA125, complete blood counts and serum chemistries were evaluated, creatinine clearance was calculated, and a physical examination was performed. After the end of treatment, patients’ CA125 levels were measured and computed tomography (CT) scans were performed. Response to treatment was evaluated with post-treatment CT scans and measured changes in CA125 levels 6 months after the initiation of the treatment regimen, or within one month after discontinuation of treatment if stopped early. CA125 response in evaluable patients (N=13) was analyzed using the modified Gynecologic Cancer Intergroup (GCIG) criteria (23). There was one evaluable patient by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. After completing the treatment, CA125 levels continued to be monitored and patients were seen for follow-up assessments of disease status. Consolidation treatment was given at the discretion of the individual provider after discussion with the patient.
IP port placement
All patients underwent IP port placement at the time of their primary laparotomy for ovarian cancer. The University of Arizona Cancer Center has several decades of experience in IP chemotherapy and the gynecologic oncologists who performed the surgeries have extensive experience in IP chemotherapy. Single-lumen implantable ports (Bard Access Systems, Inc, Salt Lake City, UT) were placed with permanent sutures anchoring the port in place. There were no dose delays or discontinuation of IP chemotherapy due to IP catheter complications. Only one IP catheter revision was necessary which occurred prior to start of the IP treatment regimen.
Statistical analysis
PFS was defined as the time from the start of therapy to the time of first documentation of progression, or death due to any cause; PFS and OS were estimated using the Kaplan-Meier method. Statistical analysis was carried out using the SAS statistical package, version 9.2 (Cary, NC).
Pemetrexed Assay
IP fluid samples were obtained when possible from the IP port for analysis. Cycle 1 was intended to be the cycle for pharmacokinetic sample draws, but later cycle draws were also utilized for these analyses due to the difficulty of obtaining IP fluid. Fortunately, the pharmacokinetics of pemetrexed have been observed to remain stable/consistent over multiple treatment cycles (Package insert for Alimta, Pharmacokinetics section).
Plasma and IP fluid pemetrexed concentrations were analyzed by a published liquid chromatography tandem mass spectrometry (LC-MS/MS) method (24) with minor modifications. Briefly, diluted plasma or IP fluid samples (0.1 ml) were mixed with the internal standard solution (0.02 ml of 1 μg/ml of methotrexate in saline) and ice-cold methanol (0.2 ml). An aliquot of the supernatant was injected onto the LC-MS/MS system. The LC-MS/MS system consisted of a Surveyor HPLC system and a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron). Chromatographic separation of pemetrexed and the internal standard was achieved on an Xterra MS C-18 column (3.9 × 50 mm, 3.5 μm, Waters) with a gradient mobile phase consisting of 10 mM formic acid and acetonitrile. The mass spectrometric analysis was done with the electrospray ionization interface operated in the positive polarity mode. The selected reaction monitoring transition was: m/z 428.1 → 281.1 for pemetrexed and m/z 455.2 → 308.1 for the internal standard. Calibration curves were prepared either in plasma diluted with phosphate buffered saline at the same dilution as the plasma samples or in phosphate buffered saline for the IP fluid samples. Linear calibration range was established for the pemetrexed concentrations of 1 – 1,500 ng/ml.
Homocysteine levels
Homocysteine levels (chemiluminescent immunoassay, Abbott Laboratories, Chicago, IL) were measured in the plasma samples prior to, and at 24h post IP pemetrexed administration for cycle 1, as a marker of functional folate status. Baseline homocysteine levels have been shown to correlate with severe toxicity from pemetrexed in pooled trials (25).
Results
Patients
Fifteen patients were enrolled and treated on this study, with 3 patients on each of the 5 dose levels. Baseline characteristics are shown in Table 1. All patients had stage IIIC ovarian, peritoneal, or tubal cancer. Three of the 15 patients had bulky disease and received neoadjuvant chemotherapy consisting of IV carboplatin and paclitaxel for 3 or 4 cycles prior to their debulking surgery. All 15 patients were optimally debulked to <1cm residual disease, with one third of patients undergoing small or large bowel resections as part of the debulking procedure. There was a median of 23 days (range 9-41) from surgery to the start of treatment. A total of 80 cycles of pemetrexed were administered, with 12 of 15 patients (80%) completing all 6 cycles of IP chemotherapy. One patient was in cycle 5 at dose level 2 (120 mg/m2) when she experienced a seizure of unclear etiology. Since the possibility of a relationship with this regimen could not be ruled out, the patient was switched to standard IV carboplatin and paclitaxel. The remaining 2 patients who were at cycle 1or 2 of dose level 5 (1000 mg/m2), experienced significant ≥ grade 3 toxicities, as described below, and did not complete the regimen on that basis. At the completion of this regimen, 5 patients, who had no evidence of disease (NED) by CT scan and CA125, received single agent consolidation with either a taxane or platinum agent. One patient who had a partial response (PR) by RECIST, received further treatment with IV carboplatin, paclitaxel, and bevacizumab.
Table 1.
Patient and disease characteristics
| Characteristic | Patients (N = 15) |
|---|---|
| Median age, y (range) | 65 (46 – 76) |
| ECOG performance status, n (%) | |
| 0 | 7 (46.7) |
| 1 | 8 (53.3) |
| Race/Ethnicity, n (%) | |
| White, Hispanic | 3 (20.0) |
| White, non-Hispanic | 12 (80.0) |
| Primary site, n (%) | |
| Ovarian | 10 (66.7) |
| Fallopian tube | 3 (20.0) |
| Peritoneal | 2 (13.3) |
| FIGO stage, n (%) | |
| IIIC | 15 (100.0) |
| Cell type, n (%) | |
| Serous | 13 (86.7) |
| Mixed epithelial | 1 (6.7) |
| Other | 1 (6.7) |
| Tumor grade, n (%) | |
| 2 | 1 (6.7) |
| 3 | 14 (93.3) |
| Primary site, n (%) | |
| Ovarian | 10 (66.7) |
| Fallopian tube | 3 (20.0) |
| Peritoneal | 2 (13.3) |
| Residual disease, n (%) | |
| 0 | 5 (33.3) |
| <1cm | 10 (66.7) |
| Neoadjuvant chemotherapy, n (%) | |
| Yes | 3 (20.0) |
| No | 12 (80.0) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics
Toxicity
There was little grade 3 and no grade 4 toxicities observed in the first 4 dose levels (Table 2). The most common grade 3 toxicity was fatigue (25%). The most common grade 1 or 2 toxicity was gastrointestinal (grade 2 nausea, 41.7%). Remarkable was the almost complete absence of neuropathy or alopecia, given that 100% of these subjects had completed 5 cycles, and 91%, 6 cycles.
Table 2.
Treatment-related toxicity for 60 -750mg/m2 dose levels by grade (N = 12)
| Patients With Event, n (%) |
|||||
|---|---|---|---|---|---|
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Auditory | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Constitutional | |||||
| Fatigue | 1 (8.3) | 2 (16.7) | 3 (25.0) | 0 (0) | 0 (0) |
| Insomnia | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Dermatologic | |||||
| Alopecia | 2 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pruritus | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal | |||||
| Anorexia | 0 (0) | 2 (16.7) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 0 (0) | 3 (25.0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 4 (33.3) | 5 (41.7) | 1 (8.3) | 0 (0) | 0 (0) |
| Oral mucositis | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 3 (25.0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Other – GI | 2 (16.7) | 3 (25.0) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | |||||
| Anemia | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Leukopenia | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Lymphopenia | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia | 0 (0) | 1 (8.3) | 1 (8.3) | 0 (0) | 0 (0) |
| Thrombocytopenia | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neurologic | |||||
| Dizziness | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sensory neuropathy | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Seizure | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) |
| Ocular | 2 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Pain | |||||
| Back Pain | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 1 (8.3) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) |
| Abdominal Pain | 0 (0) | 3 (25.0) | 0 (0) | 0 (0) | 0 (0) |
Table 3 represents the toxicities of the 3 patients observed at dose level 5 (1000 mg/m2 IP pemetrexed). 100% of the ≥ grade 3 toxicities observed were experienced by 2 of the patients (subjects 014 and 015). Their toxicities are those commonly attributed to pemetrexed, and include hematologic, infection, gastrointestinal toxicity, fatigue, and metabolic toxicities. In the 2 patients who experienced increased toxicity, we confirmed with the patient and family that they had been compliant with folic acid (the timing of the vitamin B12 injections having been confirmed to be in compliance). Subject 015, after receiving day1 (IP pemetrexed) of cycle 1, succumbed to the consequences of her opportunistic infection in the setting of grade 4 neutropenia, thrombocytopenia, and grade 3 diarrhea and oral mucositis. Subject 014, having received cycle 2, survived her infection in the setting of her grade 4 neutropenia, thrombocytopenia, anemia, and grade 3 diarrhea. Upon recovery from the acute event and without any further therapy, she remained NED 406 days from start of the IP pemetrexed regimen. The remaining patient (subject 012) treated at this dose level suffered only grade 1 or 2 toxicities similar to that seen at the lower dose levels.
Table 3.
Treatment-related toxicity for 1000mg/m2 dose levels by grade (N = 3)
| Patients With Event, n (%) |
|||||
|---|---|---|---|---|---|
| Adverse event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Constitutional | |||||
| Fatigue | 0 (0) | 2 (66.7) | 1 (33.3) | 0 (0) | 0 (0) |
| Dermatologic | |||||
| Alopecia | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other - Skin | 2 (66.7) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Gastrointestinal | |||||
| Anorexia | 0 (0) | 2 (66.7) | 1 (33.3) | 0 (0) | 0 (0) |
| Non-malignant ascites | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) | 0 (0) |
| Dysgeusia | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) |
| Oral mucositis | 0 (0) | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) |
| Rectal bleeding | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Hematologic | |||||
| Anemia | 0 (0) | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) |
| Leukopenia | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) |
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) |
| Thrombocytopenia | 0 (0) | 0 (0) | 0 (0) | 2 (66.7) | 0 (0) |
| Infection | |||||
| Febrile neutropenia | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Infection - abdomen | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Opportunistic infection | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) |
| Tachycardia | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| Metabolic | |||||
| Creatinine | 1 (33.3) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Hypokalemia | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Hypomagnesemia | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Musculoskeletal | |||||
| Weakness | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) |
| Pulmonary | 1 (33.3) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
Since at 1000 mg/m2 IP pemetrexed, 2 of 3 patients incurred DLTs, the MTD of this regimen may be 750 mg/m2. However, in light of the unexpected severity of these toxicities in 2 patients at this dose level, the trial was put on hold to allow pharmacokinetic analyses in an attempt to better understand the reasons for these events, and to help determine what lower dose level may be safe. No additional patients were accrued on this study.
Efficacy
Fourteen patients were evaluable for efficacy measurements. The majority of patients were evaluable by CA125 (N=13), only one patient by RECIST, as all patients had been optimally debulked at surgery. 12 patients were NED by CT scan at the end of the regimen. One additional patient was evaluable by RECIST and met criteria for a PR. The remaining patient (subject 014) had ascites only on her CT scan which proved to be non-malignant. Interestingly, following the acute toxicities, she was plagued with recurrent exudative non-malignant ascites, confirmed by serial paracenteses, for several months after discontinuation of the regimen. Out of 13 evaluable patients by CA125, there was 100% response, with 12 complete responses (CR) and 1 PR. Five of 14 patients have recurred, with 2 deaths.
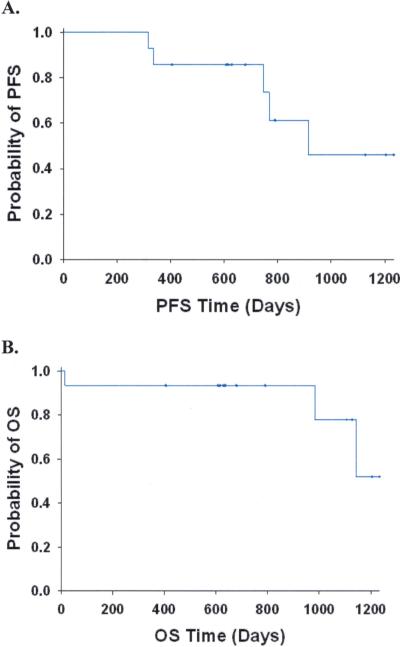
With a median follow-up time of 22.4 months, the median PFS was 30.1 months and the median OS has not been reached (Figure 1). The 6 month PFS was 100%, 12 month PFS, 85.7%, and 18 month PFS, 78.6%. Thus our secondary goal of PFS at 18 months of 80% was almost reached.
1.
Progression free (A; PFS, N=14) and Overall (B; OS, N=15) survival for all evaluable patients. Censored observations are designated with a “+” at the time of censoring.
Pharmacokinetic analysis
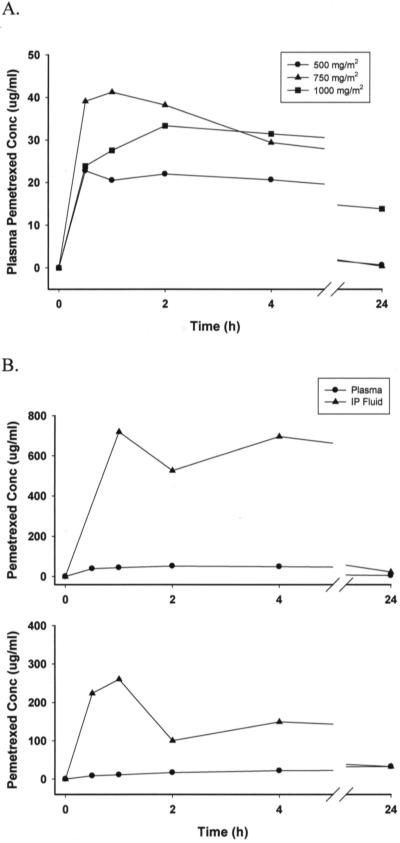
Figure 2A illustrates the average plasma concentration-time profile of pemetrexed after IP administration of 500, 750, and 1000 mg/m2. Between 0.5 to 4 h after initiation of IP administration, the plasma concentrations in most patients were maintained at relatively constant levels. Plasma concentrations declined significantly 24 h after initiation of IP administration. Figure 2B illustrates the plasma and IP fluid concentration-time profile of two patients after IP administration of 1000 mg/m2. The IP concentrations were consistently higher than the plasma concentrations in both patients. Similarly, the IP concentrations declined significantly 24 h after initiation of IP administration.
2.
(A) Average plasma concentration-time profile of pemetrexed after intraperitoneal administration of 500, 750, and 1000 mg/m2. (B) Plasma and intraperitoneal concentrations of pemetrexed from subjects 012 (top curve) and 015 (lower curve) after intraperitoneal administration of 1000 mg/m2.
Table 4 summarizes the pharmacokinetic parameters of pemetrexed after IP administration of 500, 750, and 1000 mg/m2. The average plasma Cmax showed a less than proportional increase as the dose was increased to 1000 mg/m2 (25.1 ± 1.3, 39.3 ± 7.3, and 38.7 ± 11.2 μg/ml for 500, 750, and 1000 mg/m2, respectively). The average plasma AUC0-24h increased proportionally as the dose was increased to 1000 mg/m2 (293.5 ± 42.0, 422.2 ± 57.9, 566.8 ± 182.1 μg/ml *h for 500, 750, and 1000 mg/m2, respectively). The plasma pemetrexed concentration at 24 h after initiation of dosing exhibited a more than proportional increase as the dose was increased to 1000 mg/m2 (0.674 ± 0.998, 0.418 ± 0.054, and 13.8 ± 16.6 μg/ml for 500, 750, and 1000 mg/m2 dose levels, respectively). IP fluid samples were not able to be collected for patients receiving 500 and 750 mg/m2 doses and were collected at only two time points for patient 014 receiving 1000 mg/m2. Serial IP fluid samples were available from the remaining two patients receiving the 1000 mg/m2 dose. The IP fluid to plasma pemetrexed Cmax ratio for these two patients was 13.9 and 7.9 and the AUC0-24h ratio was 13.0 and 4.0.
Table 4.
Pharmacokinetic parameters after intraperitoneal administration of pemetrexed
| Dose (mg/m2) | Plasma | IP Fluid | ||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | AUC0-24 h (μg/ml *h) | C24 h (μg/ml) | Cmax (μg/ml) | AUC0-24 h (μg/ml *h) | C24 h (μg/ml) | |
| 500 | ||||||
| Subject 006 | 25.6 | 301.0 | 0.119 | NDa | ND | ND |
| Subject 007 | 26.5 | 248.2 | 0.076 | ND | ND | ND |
| Subject 008 | 24.0 | 331.2 | 1.826 | ND | ND | ND |
| Mean ± SD | 25.1 ± 1.3 | 293.5 ± 42.0 | 0.674 ± 0.998 | ND | ND | ND |
| 750 | ||||||
| Subject 009 | 46.8 | 452.6 | 0.451 | ND | ND | ND |
| Subject 010 | 47.0 | 482.8 | 0.448 | ND | ND | ND |
| Subject 011 | 34.3 | 370.9 | 0.356 | ND | ND | ND |
| Mean ± SD | 39.3 ± 7.3 | 422.2 ± 57.9 | 0.418 ± 0.054 | ND | ND | ND |
| 1000 | ||||||
| Subject 012 | 51.6 | 722.8 | 5.42 | 719.4 | 9394.1 | 22.6 |
| Subject 014 | 31.6 | 366.6 | 3.07 | ND | ND | 22.3 |
| Subject 015 | 33.0 | 611.1 | 33.0 | 260.3 | 2430.3 | 32.7 |
| Mean ± SD | 38.7 ± 11.2 | 566.8 ± 182.1 | 13.8 ± 16.6 | 489.8 ± 324.7 | 5912.2 ± 4924.1 | 25.9 ± 5.95 |
These data demonstrate a pharmacokinetic advantage (by Cmax of up to 14-fold, and by AUC of up to 13-fold) for IP pemetrexed at the 1000 mg/m2 dose level. While there was a proportional increase in plasma AUC with increasing dose level, reflecting linear kinetics for AUC, what is striking is that at the 1000 mg/m2 dose, the 24h plasma pemetrexed concentration was still quite elevated, > 20-fold higher and out of line with that observed at the lower doses.
This analysis however did not explain why 2 patients (subjects 014 and 015) at the 1000 mg/m2 dose level experienced excess toxicity, while subject 012 at the same dose level did not. Figure 2B shows paradoxically, that the pemetrexed plasma concentrations in subject 012 (top curve) were significantly higher than for subject 015 (lower curve). Similarly Table 4 shows that the IP Cmax and AUC for subject 012 were higher than for subject 015, and the plasma AUC and C24h were higher than for subject 014. Subject 015, who died from toxicity, had the highest plasma C24h level of the whole study.
In a further attempt to find a basis for the increased toxicity in subjects 014 and 015, we next investigated plasma homocysteine levels, as levels >11.5 umol/L were associated with a high risk for severe toxicity from pemetrexed (25). Homocysteine can serve as a marker of functional folate status. At dose levels < 1000 mg/m2, homocysteine levels were normal both pre and 24 h post IP pemetrexed administration. At the 1000 mg/m2 dose subjects 012 and 015, but not subject 014, had high levels above this cut-off both pre IP pemetrexed and at the 24h time point. In subject 015, the pemetrexed treatment raised the homocysteine level further (e.g., lowered the functional folate level), when compared to the pre-treatment level. This may be secondary to the high plasma pemetrexed levels detected at this time point. This also suggests that her functional folate status was inadequate, and that she was at risk for excess toxicity. This finding was not observed for subject 014 who also experienced excess toxicity, but who appeared to have an adequate folate status at least for the first cycle when these samples were drawn.
Taken together, analyses of our pharmacokinetic and homocysteine levels do not clearly explain the excess toxicities observed in 2 of 3 patients at the highest dose level. The elevated plasma C24h levels do stand out, however, for this dose level. In contrast, at both the 500 and 750 mg/m2 dose levels, the plasma C24h levels were low at <1 ug/ml (Table 4). A total of 7 patients received IP pemetrexed for 6 cycles each (total 42 cycles), at dose levels ≥ 500 mg/m2, without excessive toxicity. Our available data therefore can support a MTD at 500 mg/m2 IP pemetrexed, although we cannot rule out a MTD as high as 750 mg/m2.
Discussion
Our trial represents the first regimen for front-line advanced stage optimally debulked ovarian (including peritoneal and tubal) cancer patients in which all the drugs in the combination are given IP. We show that this IP only approach is feasible front-line, as our median PFS of 30.1 months is in line with the literature of stage III ovarian cancer patients who receive front-line IV/IP cisplatin or carboplatin/ taxane chemotherapy (3, 4, 26-30). While the doses and drugs vary from study to study, and some of the studies include IV neoadjuvant chemotherapy as do ours, the median PFS in these studies ranges from 19-29 months. Our six cycle completion rate of 80% is similar to the 71% for GOG-114 and significantly better than the 42% for GOG-172 (3, 4). IP catheter complications in our study were minimal and did not result in any dose delays or discontinuations of treatment. While direct comparisons cannot be made and our results confounded in part by the use of consolidation therapy in some patients, our PFS rate at 6, 12, and 18 months were at least as good as for GOG-172 (4). At 18 months, our PFS rate was 78.6%, compared to approximately 60% for GOG-172.
This is also the first report of IP pemetrexed administration in ovarian cancer. For dose levels < 1000 mg/m2 IP pemetrexed, the relative lack of toxicity compared to IV/IP cisplatin or carboplatin/taxane chemotherapy was very apparent. There was minimal if any alopecia or neuropathy and almost no grade 3 or 4 hematologic toxicity from the replacement of IV paclitaxel with IP pemetrexed. Among grade 3 or 4 toxicities, only fatigue was comparable in frequency to those of the other IV/IP studies (3, 4, 26-33). We included as comparators in the literature front-line combination IV/IP chemotherapies for stage III ovarian cancer which included platinum agents (carboplatin, cisplatin) and taxanes (paclitaxel, docetaxel) at varying doses (e.g., cisplatin at either 75 or 100 mg/m2). Some of this literature allowed for neoadjuvant chemotherapy first, as did our study, and one of them included an additional agent, liposomal doxorubicin.
At the 1000 mg/m2 IP pemetrexed dose, we found a large pharmacokinetic advantage for IP administration (by Cmax of up to 14-fold, and by AUC of up to 13-fold). At this dose, the grade 3 or 4 hematologic, infection, and gastrointestinal toxicities predominate similar to that seen in the IV/IP literature (3, 4, 26-30). While again direct comparison is difficult and our numbers are small, it is possible that our gastrointestinal and infection toxicities may be higher at this dose of IP pemetrexed than in the IV/IP literature. Certainly the patient mortality and other near mortality in this small trial are notable.
The severe toxicities in the two patients at the highest dose level remain largely unexplained by pharmacokinetic analyses or homocysteine levels. The toxicities observed were identical to those seen in patients not supplemented with folate or vitamin B12. Folate/vitamin B12 supplementation does not completely protect from pemetrexed toxicity, however (16). Also, these toxicities did not appear to be ameliorated by leucovorin infusion in the one patient who was able to receive the rescue drug. The one pharmacokinetic finding which stood out was the >20-fold significantly elevated plasma pemetrexed concentrations 24 hours after IP drug delivery at this dose level, not seen at lower dose levels. In retrospect, although there were no DLTs, had we terminated the study early at 500 or 750 mg/m2 IP pemetrexed, we would likely have avoided the excess toxicity we observed. A limitation of this study is that the MTD was not definitively determined, in that the trial did not proceed to accrue additional patients at a dose level < 1000 mg/m2. Based on the C24h pemetrexed levels, the number of patients in this study who tolerated ≥ 500 mg/m2 without DLT, and in the context of the pharmacokinetic advantage of IP pemetrexed, we have evidence to suggest that the 500 mg/m2 IP pemetrexed dose could serve as the MTD of this regimen.
After we opened this trial, several reports appeared of IV pemetrexed, single agent and in combination, in ovarian cancer (10-15). In platinum sensitive recurrent ovarian cancer patients and in combination with carboplatin, IV pemetrexed doses up to 900 mg/m2 were well tolerated and active (10,11). In one of these studies the response rate was 84% (11). In platinum resistant ovarian cancer patients, IV single agent pemetrexed was tolerable up to 900 mg/m2 with response rates up to 21%, and stabilization rates up to 35% (12). There was however, more significant toxicity with 900 mg/m2 IV pemetrexed than at the 500 mg/m2 dose (13). Thus at this time, there is even a greater rationale for the pursuit of study of this regimen with IP pemetrexed at 500 mg/m2 in front-line platinum sensitive ovarian cancer patients, especially given the extremely low toxicity profile at this dose.
In support of the IP pemetrexed approach, is a phase II study of IP pemetrexed at 500 mg/m2 and IV cisplatin at 75 mg/m2 in patients with diffuse malignant peritoneal mesothelioma, presented in 2010 (34). These patients had been optimally debulked and treated intraoperatively with hyperthermic chemotherapy. In this phase II trial, 90% of patients completed all 6 cycles and there were no grade 3 or 4 toxicities. At this IP pemetrexed dose, preliminary pharmacokinetic analysis suggests a peritoneal fluid to plasma AUC ratio of 70, which is higher than what we obtained at 1000 mg/m2 IP dose of pemetrexed. We were unfortunately unable to measure IP AUC at the 500 mg/m2 dose level. In rats given IP pemetrexed, the peritoneal fluid to plasma AUC ratio of 19.2 to 40.8 (17) is intermediate to our results and those obtained in the mesothelioma study (34). Plasma concentrations in the mesothelioma study were approximately half of ours at the same dose level but similar to our data, the plasma levels were sustained 4 hours post IP administration of pemetrexed (34). In studies of patients with mucinous peritoneal carcinomatosis, several factors were studied which were found not to significantly affect pharmacokinetics of hyperthermic IP chemotherapy (35, 36). What is unknown is whether IP pemetrexed pharmacokinetics is altered in the setting of prior hyperthermic IP chemotherapy treated mesothelioma, as compared to IP chemotherapy naïve ovarian cancer. Regardless, the finding of such a large pharmacokinetic advantage for IP pemetrexed at 500 mg/m2, and the significant lack of toxicity, is encouraging.
In summary, the pharmacokinetic advantage of IP pemetrexed favors pursuit of this route for administration of pemetrexed in ovarian cancer. It is clear that this drug has considerable activity in this disease. The 500 mg/m2 dose of IP pemetrexed in combination with standard doses of IP cisplatin and IP paclitaxel appears to have a favorable toxicity profile, which needs to be confirmed by larger studies, compared to IV/IP cisplatin or carboplatin/taxane therapy. Our experience with this regimen strongly suggests that it is the IV paclitaxel which significantly contributes to grade 3 or 4 toxicities of IV/IP therapy, rather than the IP cisplatin (at a dose of 75 mg/m2) or IP paclitaxel.
Translational Relevance.
We report the first front-line trial in Stage III optimally debulked ovarian cancer patients in which all drugs in combination (pemetrexed, cisplatin, paclitaxel) are given intraperitoneally. Moreover, this is the first report of intraperitoneal pemetrexed in ovarian cancer. We show a pharmacokinetic advantage to intraperitoneal pemetrexed. At doses <1000 mg/m2 intraperitoneal pemetrexed, the combination is very well tolerated with no >grade 1 peripheral neuropathy or alopecia, and only 8.3% grade 3 hematologic toxicity. The median progression-free survival of 30.1 months is consistent with other reports of combination intravenous/intraperitoneal chemotherapy in this disease. Our findings suggest pursuit of larger trials of this regimen at 500 mg/m2 intraperitoneal pemetrexed in combination with intraperitoneal cisplatin and paclitaxel, which appears to have far less toxicity than standard intravenous/intraperitoneal cisplatin or carboplatin/ taxane regimens.
Acknowledgments
Paul H. Sugarbaker, MD for his advice and insight.
Supported in part by the NIH Cancer Center support grant CA-023074, including contributions from the Clinical Research, Biometry, and the Analytical Chemistry Shared Services and Women's Cancers of the University of Arizona Cancer Center, Tucson, AZ; and by an Investigator Initiated Grant from Lilly Oncology, Eli Lilly Co., Indianapolis, IN.
References
- 1.American Cancer Society . Cancer Facts & Figures 2011. American Cancer Society; Atlanta: 2011. [Google Scholar]
- 2.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. New Engl J Med. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson L, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Alberts DS, Chambers SK. Introduction. In: Alberts DS, Clouser MC, Hess LM, editors. Intraperitoneal therapy for ovarian cancer. Springer-Verlag; Heidelberg: 2010. pp. 1–6. [Google Scholar]
- 6.Trimble EL, Christian MC. Intraperitoneal chemotherapy for women with advanced epithelial ovarian carcinoma. Gynecol Oncol. 2006;100:3–4. doi: 10.1016/j.ygyno.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Hess LM, Benham-Hutchins M, Herzog TJ, Hsu CH, Malone DC, Skrepnek GH, et al. A meta-analysis of the efficacy of intraperitoneal cisplatin for the front-line treatment of ovarian cancer. Intl J Gynecol Cancer. 2007;17:561–70. doi: 10.1111/j.1525-1438.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- 8.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 9.Hanna N, Shepherd FA, Fossella FV, Pereira JR, de Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 10.Matulonis UA, Horowitz NS, Campos SM, Lee H, Lee J, Krasner CN, et al. Phase II study of carboplatin and pemetrexed for the treatment of platinum-sensitive recurrent ovarian cancer. J Clin Oncol. 2008;26:5761–66. doi: 10.1200/JCO.2008.17.0282. [DOI] [PubMed] [Google Scholar]
- 11.Sehouli J, Camara O, Mahner S, Bauknecht T, Lichtenegger W, Runnebaum I, et al. A phase-I trial of pemetrexed plus carboplatin in recurrent ovarian cancer. Cancer Chemother Pharmacol. 2010;66:861–8. doi: 10.1007/s00280-009-1230-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, et al. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol. 2009;27:2686–91. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vergote I, Calvert H, Kania M, Kaiser C, Zimmermann AH, Sehouli J. A randomised, double-blind, phase II study of two doses of pemetrexed in the treatment of platinum-resistant, epithelial ovarian or primary peritoneal cancer. Eur J Cancer. 2009;45:1415–23. doi: 10.1016/j.ejca.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Hensley ML, Larkin J, Fury M, Gerst S, Tai DF, Sabbatini P, et al. A phase I trial of pemetrexed plus gemcitabine given biweekly with B-vitamin support in solid tumor malignancies or advanced epithelial ovarian cancer. Clin Cancer Res. 2008;14:6310–6. doi: 10.1158/1078-0432.CCR-08-0338. [DOI] [PubMed] [Google Scholar]
- 15.Gasent Blesa JM, Alberola Candel V, Provencio Pulla M, Esteban González E, Martín Algarra S. Management of platinum-resistant ovarian cancer with the combination of pemetrexed and gemcitabine. Clin Transl Oncol. 2009;11:35–40. doi: 10.1007/s12094-009-0308-z. [DOI] [PubMed] [Google Scholar]
- 16.Scagliotti GV, Shin D-M, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol. 2003;21:1556–61. doi: 10.1200/JCO.2003.06.122. [DOI] [PubMed] [Google Scholar]
- 17.Pestieau SR, Stuart OA, Sugarbaker PH. Multi-targeted antifolate (MTA): pharmacokinetics of intraperitoneal administration in a rat model. Eur J Surg Oncol. 2000;26:696–700. doi: 10.1053/ejso.2000.0983. [DOI] [PubMed] [Google Scholar]
- 18.Thodtmann R, Depenbrock H, Dumez H, Blatter J, Johnson RD, van Oosterom A, et al. Clinical and pharmakokinetic Phase I study of multitargeted antifolate (LY231514) in combination with cisplatin. J Clin Oncol. 1999;17:3009–16. doi: 10.1200/JCO.1999.17.10.3009. [DOI] [PubMed] [Google Scholar]
- 19.Dickgreber NJ, Fink TH, Latz JE, Hossain AM, Musib LC, Thomas M. Phase I and pharmacokinetic study of pemetrexed plus cisplatin in chemonaive patients with locally advanced or metastatic malignant pleural mesothelioma or non-small cell lung cancer. Clin Cancer Res. 2009;15:382–9. doi: 10.1158/1078-0432.CCR-08-0128. [DOI] [PubMed] [Google Scholar]
- 20.Howell SB. Selection of drugs for intraperitoneal chemotherapy for ovarian cancer. In: Alberts DS, Clouser MC, Hess LM, editors. Intraperitoneal therapy for ovarian cancer. Springer-Verlag; Heidelberg: 2010. pp. 77–88. [Google Scholar]
- 21.Kano Y, Akutsu M, Tsunoda S, Kobayashi H, Inoue K, Mori K, et al. Schedule-dependent interactions between pemetrexed and cisplatin in human carcinoma cell lines in vitro. Oncol Res. 2006;16:85–95. doi: 10.3727/000000006783981215. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto CH, Hammond-Thelin LA, Latz JE, Forero L, Beeram M, Forouzesh B, et al. Phase I and pharmacokinetic study of pemetrexed with high-dose folic acid supplementation or multivitamin supplementation in patients with locally advanced or metastatic cancer. Clin Cancer Res. 2007;13:2675–83. doi: 10.1158/1078-0432.CCR-06-2393. [DOI] [PubMed] [Google Scholar]
- 23.Rustin GJS, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 24.Stapleton S, Reid J, Thompson P, Ames M, McGovern R, McGuffey L, et al. Plasma and cerebrospinal fluid pharmacokinetics of pemetrexed after intravenous administration in non-human primates. Cancer Chemother Pharmacol. 2007;59:461–6. doi: 10.1007/s00280-006-0285-7. [DOI] [PubMed] [Google Scholar]
- 25.Niyikiza C, Baker SD, Seitz DE, Walling JM, Nelson K, Rusthoven JJ, et al. Homocysteine and methylmalonic acid: markers to predict and avoid toxicity from pemetrexed therapy. Mol Cancer Ther. 2002;1:545–52. [PubMed] [Google Scholar]
- 26.Tiersten AD, Liu PY, Smith HO, Wilczynski SP, Robinson WR, III, Markman M, et al. Phase II evaluation of neoadjuvant chemotherapy and debulking followed by intraperitoneal chemotherapy in women with stage III and IV epithelial ovarian, fallopian tube or primary peritoneal cancer: Southwest Oncology Group Study S0009. Gynecol Oncol. 2009;112:444–9. doi: 10.1016/j.ygyno.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith HO, Moon J, Wilczynski SP, Tiersten AD, Hannigan EV, Robinson WR, et al. Southwest Oncology Group Trial S9912: Intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small-volume residual stage III ovarian cancer. Gynecol Oncol. 2009;114:206–9. doi: 10.1016/j.ygyno.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seamon LG, Carlson MJ, Richardson DL, Cohn DE, Fowler JM, Copeland LJ, et al. Outpatient platinum-taxane intraperitoneal chemotherapy regimen for ovarian cancer. Intl J Gynecol Cancer. 2009;19:1195–98. doi: 10.1111/IGC.0b013e3181b33d5b. [DOI] [PubMed] [Google Scholar]
- 29.Kim S-W, Paek J, Nam E-J, Kim S-H, Kim J-H, Kim Y-T. The feasibility of carboplatin-based intraperitoneal chemotherapy in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2010;152:195–9. doi: 10.1016/j.ejogrb.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Konner JA, Grabon DM, Gerst SR, Iasonos A, Thaler H Pezzulli SD, et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. 2011;29:4662–68. doi: 10.1200/JCO.2011.36.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry E, Matthews KS, Singh DK, Buttin BM, Lurain JR, Alvarez RD, et al. An outpatient intraperitoneal chemotherapy regimen for advanced ovarian cancer. Gynecol Oncol. 2009;113:63–7. doi: 10.1016/j.ygyno.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Dizon DS, Sill MW, Gould N, Rubin SC, Yamada SD, DeBernardo RL, et al. Phase I feasibility study of intraperitoneal cisplatin and intravenous paclitaxel followed by intraperitoneal paclitaxel in untreated ovarian, fallopian tube, and primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123:182–6. doi: 10.1016/j.ygyno.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oaknin A, Desamparado R, Gonzalez-Martin A, Chiva L, Garcia-Donas J, de Juan A, et al. Feasibility of a modified outpatient regimen of intravenous/intraperitoneal chemotherapy in optimally debulked stage III ovarian cancer patients: a GEICO study. Intl J Gynecol Cancer. 2011;21:1048–55. doi: 10.1097/IGC.0b013e31821ee777. [DOI] [PubMed] [Google Scholar]
- 34.Bijelic L, Stuart OA, Sugarbaker PH. Intraperitoneal pemetrexed combined with intravenous cisplatin is well tolerated as an adjuvant treatment for diffuse malignant peritoneal mesothelioma.. Presented at the International Workshop for Peritoneal Surface Malignancy; Uppsala, Sweden. September 2010. [Google Scholar]
- 35.Jacquet P, Averbach A, Stephens AD, Stuart OA, Chang D, Sugarbaker PH. Heated intraoperative intraperitoneal mitomycin-C and early postoperative intraperitoneal 5-fluorouracil: pharmacokinetic studies. Oncology. 1998;55:130–8. doi: 10.1159/000011847. [DOI] [PubMed] [Google Scholar]
- 36.de Lima Vasquez V, Stuart OA, Mohamed F, Sugarbaker PH. Extent of parietal peritonectomy does not change intraperitoneal chemotherapy pharmacokinetics. Cancer Chemother Pharmacol. 2003;52:108–12. doi: 10.1007/s00280-003-0626-8. [DOI] [PubMed] [Google Scholar]