Abstract
Vitiligo is an autoimmune disease of the skin causing disfiguring patchy depigmentation of the epidermis and, less commonly, hair. Therapeutic options for vitiligo are limited, reflecting in part limited knowledge of disease pathogenesis. Existing mouse models of vitiligo consist of hair depigmentation but lack prominent epidermal involvement, which is the hallmark of human disease. They are thus unable to provide a platform to fully investigate disease mechanisms and treatment. CD8+ T cells have been implicated in the pathogenesis of vitiligo and expression of interferon-gamma (IFN-γ) is increased in the lesional skin of patients, however it is currently unknown what role IFN-γ plays in disease. Here, we have developed an adoptive transfer mouse model of vitiligo using melanocyte-specific CD8+ T cells, which recapitulates the human condition by inducing epidermal depigmentation while sparing the hair. Like active lesions in human vitiligo, histology of depigmenting skin reveals a patchy mononuclear infiltrate and single-cell infiltration of the epidermis. Depigmentation is accompanied by accumulation of autoreactive CD8+ T cells in the skin, quantifiable loss of tyrosinase transcript, and local IFN-γ production. Neutralization of IFN-γ with antibody prevents CD8+ T cell accumulation and depigmentation, suggesting a therapeutic potential for this approach.
Introduction
Vitiligo is a skin disease that causes patchy depigmentation of the epidermis and afflicts approximately 0.5% of the population, without preference for race or gender. It commonly affects the central face and genitals, often localizes to the hands and feet, and less commonly presents on the trunk and proximal extremities (Taieb and Picardo, 2009). Hair pigmentation is often spared within lesional skin, and the successful treatment of vitiligo results in repigmentation that usually begins as small dark macules around the hair follicles, presumably because follicular melanocytes are protected by immune privilege (Falabella, 2009).
Previous studies have implicated autoreactive CD8+ T cells in disease pathogenesis. For example, the frequency of anti-melanocyte CD8+ T cells in both the blood and skin of patients with vitiligo correlate with the severity of disease, and lesional CD8+ T cells induce melanocyte apoptosis in unaffected skin ex vivo (Ogg et al., 1998; van den Boorn et al., 2009), an observation that supports a direct role for cytotoxic T lymphocytes in melanocyte destruction in human vitiligo. The role of inflammatory cytokines is not yet fully defined, although IFN-γ has been the most extensively studied. In human patients, IFN-γ is expressed in lesional skin and can be produced by autoreactive CD8+ T cells (van den Boorn et al., 2009), but its role in depigmentation is not known.
Existing models of vitiligo have been established in wild-type C57BL/6 mice, which have black hair but light skin due to the few melanocytes found in the epidermis. Consequently, the majority of these models focus on depigmentation of the hair rather than the epidermis, and because hair depigmentation predicts a poor response to treatment, they may represent more severe forms of vitiligo (van den Boorn et al., 2009). Here we report a mouse model of vitiligo using adoptive transfer of melanocyte-specific CD8+ T cells into mice with increased epidermal melanocytes, which results in black skin and black hair. Similar to common forms of human vitiligo, depigmentation in these mice affects the epidermis but spares the hair, and the skin histologically resembles active human disease. Mechanistic studies in this model reveal that depigmentation requires IFN-γ, which induces the local accumulation of melanocyte-specific CD8+ T cells within the skin. These results support a critical role for IFN-γ in vitiligo and suggest that it acts to recruit CD8+ T cells to the skin. The ability of antibody-mediated neutralization of IFN-γ to prevent CD8+ T cell accumulation in the skin as well as depigmentation in this model suggests a therapeutic potential for this approach.
Results
Vitiligo induction through the adoptive transfer of melanocyte-specific CD8+ T cells consists of epidermal depigmentation sparing the hair
To develop a mouse model of vitiligo with prominent epidermal depigmentation, mice with black skin and hair were used as hosts for the adoptive transfer of melanocyte-specific CD8+ T cells. KRT14-Kitl*4XTG2Bjl (Krt14-Kitl*) transgenic mice contain melanocytes in the epidermis due to overexpression of Kit ligand (KITL) protein that is noncleavable due to mutations in the proteolytic domains, and is therefore membrane-bound (KITL*) (Majumdar et al., 1994); expression is driven from the human keratin 14 promoter (Krt14), resulting in retention of melanocytes in the epidermal basal layer and follicular epithelium. These mice have black hair and black skin with an elevated number of melanocytes in the epidermis but other cell populations are normal, representative of human skin (Kunisada et al., 1998). PMEL-specific TCR transgenic CD8+ T cells (PMEL CD8+ T cells) recognize both mouse and human PMEL (also known as gp100), a melanocyte-specific differentiation antigen (Overwijk et al., 1998). Using these cells, we modified a protocol optimized for melanoma immunotherapy (Antony et al., 2005) to induce vitiligo in our system. Naïve CD8+ T cells from the spleens of PMEL TCR transgenic mice were purified by negative selection, and one million cells were transferred i.v. into sublethally irradiated (500 rads) Krt14-Kitl* hosts. To track PMEL CD8+ T cells after transfer, PMEL TCR transgenic mice were crossed with mice that express GFP driven by the DPE promoter, which consists of the distal and proximal Cd4 enhancers and promoter, but lacks a silencer element that prevents CD4 expression in mature CD8+ T cells; this results in GFP expression in both CD4+ and CD8+ T cells (Mrass et al., 2006). On the day of transfer, Krt14-Kitl* hosts were infected i.p. with 106 pfu of recombinant vaccinia virus that expresses human PMEL (rVV-hPMEL), a potent antigenic stimulus for PMEL CD8+ T cells that results in their expansion in vivo (Overwijk et al., 2003). In summary, Krt14-Kitl* hosts are sublethally irradiated, GFP+ PMEL CD8+ T cells are adoptively transferred i.v., and hosts are infected i.p. with rVV-hPMEL to activate transferred T cells in vivo.
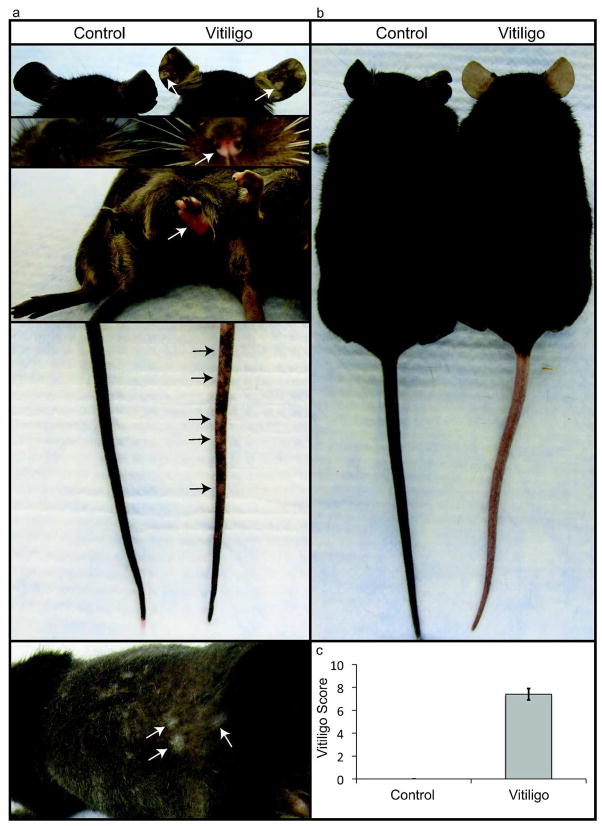
In all experiments, control mice were irradiated but did not receive rVV-hPMEL or PMEL CD8+ T cells. In preliminary experiments, when either irradiation or rVV-hPMEL was eliminated from the protocol, depigmentation occurred only inconsistently, and treatment with rVV-hPMEL without PMEL CD8+ T cell transfer did not result in depigmentation (not shown). In all mice that received the combination of sublethal irradiation, PMEL CD8+ T cells, and rVV-hPMEL, epidermal depigmentation appeared 4–5 weeks after transfer. Depigmentation consistently involved the ears, rear footpads, tail and, less commonly, the nose; occasionally, the trunk skin under the hair was affected (Fig. 1a), as well as the front footpads and genitals (not shown). Depigmentation was initially patchy but often progressed to confluence, involving the entire epidermal surface, while depigmentation of the hair was not observed, even months after transfer (Fig. 1b). When wild-type C57BL/6J mice with minimal epidermal pigment were used as hosts, tail depigmentation occurred but the hair remained unaffected (not shown). As in human vitiligo, there was no evidence of skin inflammation, including redness, swelling or epidermal scale. While changes related to normal physiology or aging of the Krt14-Kitl* mouse strain occasionally included either mild lightening of the tail root or focal depigmentation at the tip, the appearance was distinct from the patchy depigmentation in mice with vitiligo.
Figure 1. Depigmentation targets the epidermis and spares the hair.
(a) Within 4–5 wks after treatment, hosts developed patchy depigmentation (arrows) at visible epidermal surfaces, including the ears, nose, feet, tail, and occasionally on the trunk under the hair (photographed at 7 wks). (b) Chronic vitiligo resulted in widespread depigmentation of the epidermis but spared the hair (photographed at 18 wks). (c) Mice with vitiligo were assigned a Vitiligo Score based on the extent of involvement, and a representative result at 5 weeks is shown (Error bars = mean +/− SEM, n=3 control, n=5 vitiligo).
The extent of depigmentation was objectively quantified by an observer blinded to the treatment groups, using a point scale based on the extent of depigmentation at 4 easily visible locations, including the ears, nose, rear footpads, and tail. Each location was examined and the extent of depigmentation estimated as a percentage of the anatomic site; both ears and both rear footpads were estimated together and therefore evaluated as single sites. Points were awarded according to the following scheme: no evidence of depigmentation (0%) received a score of 0, >0 – 10% = 1 point, >10 – 25% = 2 points, >25 – 75% = 3 points, >75 – <100% = 4 points, 100% = 5 points. The “Vitiligo Score” was the sum of the scores at all 4 sites, with a maximum score of 20 points. The Vitiligo Score of an experimental group is reported as the average individual score of each mouse within that group; a typical example comparing control mice (irradiated only) and a group with vitiligo is presented in Fig. 1c.
Autoreactive T cells accumulate in the skin during vitiligo, which correlates with melanocyte loss
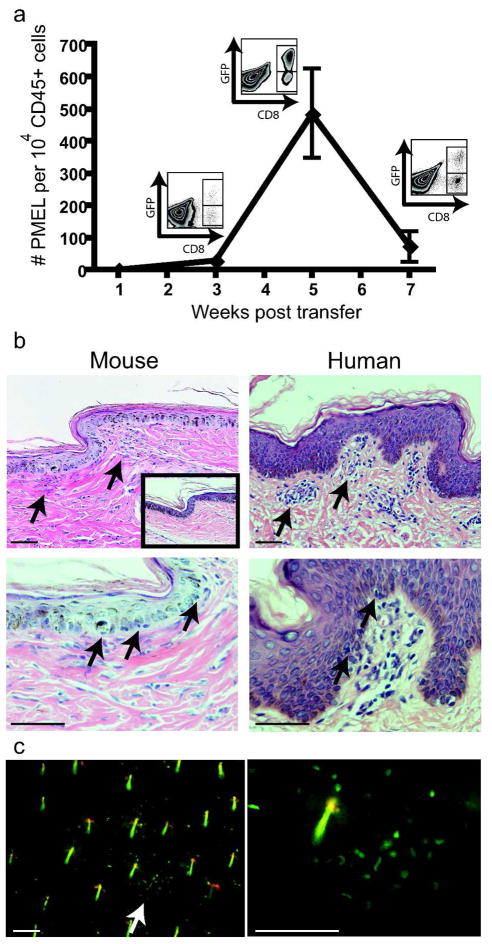
Consistent with the hypothesis that vitiligo occurs following skin infiltration of autoreactive T cells, PMEL CD8+ T cells gradually accumulated in the ear skin of mice over the course of disease. Total numbers of GFP+ (PMEL) T cells in the ear skin were measured by flow cytometry. Accumulation of these cells began 1–3 weeks after transfer, reached a maximum at 5 weeks when depigmentation became evident, and declined thereafter (Fig. 2a). As expected, depletion of CD8+ T cells 2 weeks after induction of vitiligo diminished depigmentation (not shown). Other immune cell populations commonly present in the skin were measured by flow cytometry, and total numbers of bone marrow-derived cells (CD45+), CD4+ T cells (CD4+), dendritic epidermal T cells (CD3+gamma/deltaTCR+), total dendritic cells (CD11c+), langerin+ dendritic cells (langerin+CD11c+), and macrophages (MHCII+, CD11b+, CD11c−) were not statistically different between lesional skin and controls (not shown).
Figure 2. T cells accumulate in the skin during vitiligo.
(a) GFP-labeled CD8+ T cells were quantified by flow cytometry of ear skin at the indicated times (error bars = mean +/− SEM, n=3 per time point). Example flow plots of the CD45+ gate are shown. (b) Tail skin from affected mice harvested 5 wks after transfer (mouse) is shown with affected skin from a vitiligo patient (human), revealing a patchy mononuclear infiltrate at the dermal-epidermal junction and epidermal infiltration (arrows). Normal mouse skin is shown at inset, scale bars=50μm. (c) Ears were harvested from mice with vitiligo at 5 wks after induction and GFP+ T cells were visualized with fluorescent microscopy (arrow). Scale bars=100μm. Hairs are visible as short, linear yellow structures due to autofluorescence.
In both mouse and human skin, melanocytes primarily reside in the basal layer of the epidermis, a zone that forms a narrow plane located just superficial to the dermal-epidermal (DE) junction. Hematoxylin and eosin-stained sections of lesional skin from affected mice 5 weeks after induction of vitiligo revealed a patchy mononuclear infiltrate at the DE junction where melanocytes reside, as well as single-cell infiltration of the epidermis. The similarity of these findings to human disease is illustrated by comparison to histological sections from a patient with vitiligo (Fig. 2b), a presentation that is consistent with previous reports (Ahn et al., 1994; Wankowicz-Kalinska et al., 2003). Lastly, fresh, whole-mounted ears from mice with vitiligo at 5 weeks after induction were visualized using fluorescent microscopy, a technique that permits en face examination of the distribution pattern of autoreactive GFP+ PMEL CD8+ T cells across this plane. In areas of macroscopically normal pigmentation, PMEL CD8+ T cells within the ear were distributed both as single cells as well as aggregated into distinct clusters (Fig. 2c).
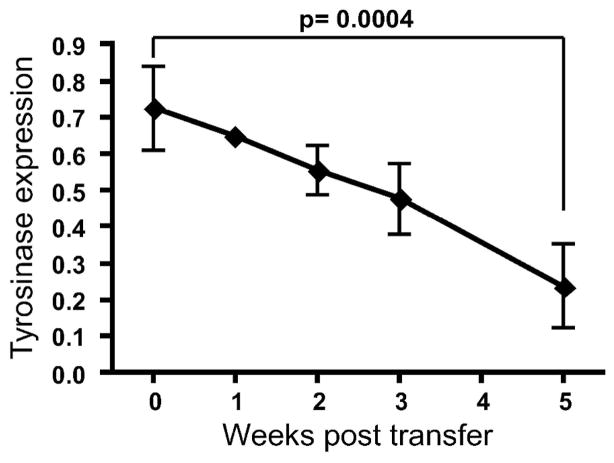
Melanocyte loss in lesional mouse ear skin was quantified by measuring tyrosinase RNA expression in the whole ear at regular times after induction of vitiligo. Tyrosinase expression in each sample was first normalized to beta-actin (ACTB) RNA transcript, and this result was divided by normalized tyrosinase expression in control mice, representing the fraction of remaining tyrosinase expression over the course of vitiligo. When compared to control (irradiated only) mice (0 weeks), expression slowly declined over the course of disease, beginning soon after induction and becoming significantly decreased by 60–70% after 5 weeks (Fig. 3). Similar results were observed using primers for PMEL (not shown).
Figure 3. Depigmentation correlates with loss of melanocytes.
Ear skin from affected mice was harvested at the indicated times. qRT-PCR revealed a quantifiable decrease in tyrosinase, reflecting the progressive loss of melanocytes during disease (error bars = mean +/− SEM, n=3 per time point).
Depigmentation critically depends on IFN-γ, as does skin-specific accumulation of autoreactive CD8+ T cells
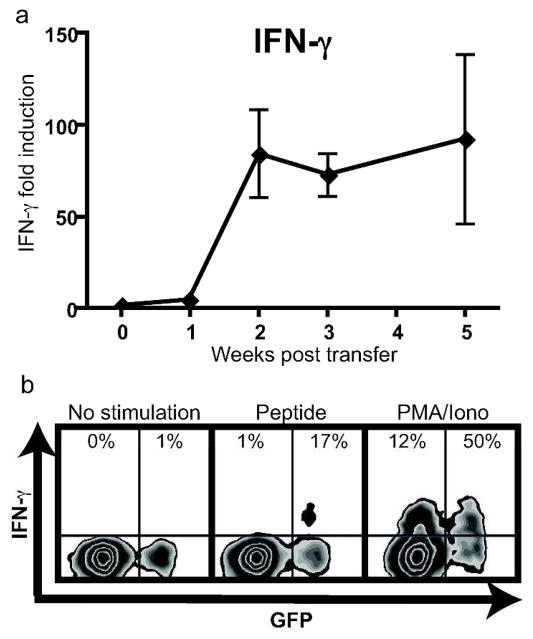
Consistent with human disease, IFN-γ expression was elevated within the depigmenting skin of mice with vitiligo, and was expressed from autoreactive CD8+ T cells. This expression, measured within the ear skin of mice using qRT-PCR, was significantly elevated starting 2 weeks after transfer and was maintained throughout the course of disease (Fig. 4a). IFN-γ expression in each sample was first normalized to ACTB RNA transcript, and this result was divided by normalized IFN-γ expression in control mice (irradiation only), representing the fold induction of IFN-γ over the course of vitiligo. The expression of GFP from transferred PMEL CD8+ T cells allowed the identification of the transferred population as distinct from endogenous CD8+ T cells using flow cytometry. A proportion of PMEL CD8+ T cells from skin-draining lymph nodes, but not endogenous T cells, produced IFN-γ following stimulation with their cognate antigen human PMEL peptide, while both populations produced IFN-γ in response to stimulation with PMA and ionomycin (Fig. 4b). ELISA assay of cells from skin-draining lymph nodes confirmed secretion of IFN-γ protein following stimulation with human PMEL peptide in mice with vitiligo but not controls, which included stimulated cells from irradiated-only mice and unstimulated cells from mice with vitiligo (not shown).
Figure 4. IFN-γ expression is induced in the skin during vitiligo and is produced by autoreactive CD8+ T cells.
(a) qRT-PCR for IFN-γ was performed on ear skin of mice at the indicated times (error bars = mean +/− SEM, n=3 mice per time point). (b) Single-cell suspensions were isolated from skin-draining lymph nodes 7 weeks after vitiligo induction and either left unstimulated, or stimulated with PMEL peptide or PMA and ionomycin as indicated. Following stimulation, cells were stained for IFN-γ, and flow cytometry revealed IFN-γ expression in transferred melanocyte-specific CD8+ T cells (GFP+) and endogenous CD8+ T cells (GFP−). Cells shown are gated on the total CD8+ population, and the percentage shown is of either the total GFP− or GFP+ gate. A representative result is shown.
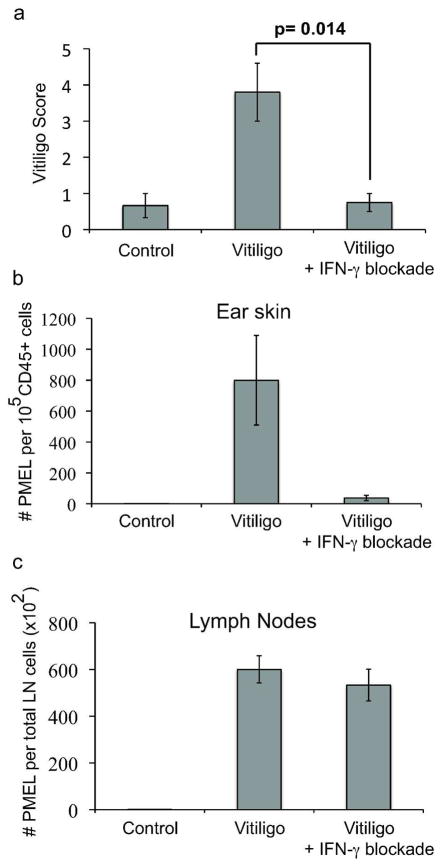
The expression of IFN-γ correlates with depigmentation in both human vitiligo and in this mouse model. In order to determine if depigmentation requires IFN-γ, vitiligo was induced as before and host mice were treated either with control antibody or IFN-γ neutralizing antibody (XMG-6) at a dose of 100–500 μg i.p. twice weekly. Initial studies revealed that the administration of IFN-γ neutralizing antibody beginning 1 day after vitiligo induction significantly inhibited the development of depigmentation (not shown). In order to rule out an effect on T cell priming during rVV-hPMEL infection, and to test whether IFN-γ neutralization might be useful as a treatment, IFN-γ neutralizing antibody treatment was initiated 2 weeks after vitiligo induction. Even at this later time point, the administration of IFN-γ neutralizing antibody significantly abrogated depigmentation evaluated 5 weeks after induction (Fig. 5a), suggesting a specific effect on autoimmunity. There was no difference in efficacy between the two treatment schedules (not shown).
Figure 5. IFN-γ neutralization prevents depigmentation and melanocyte-specific CD8+ T cell accumulation in the skin.
IFN-γ was neutralized through intraperitoneal injection of IFN-γ neutralizing antibody (500 μg) beginning 2 weeks after induction and twice weekly until scored at 5 weeks (error bars = mean +/− SEM, n=4 control, n=5 vitiligo, n=4 vitiligo + IFN-γ blockade). (a) Vitiligo Score is decreased by IFN-γ neutralization. (b–c) The effects of IFN-γ neutralizing antibody on the total numbers of melanocyte-specific CD8+ T cells in the ear (b) and in skin-draining lymph nodes (c).
IFN-γ has widely variable effects on the immune response, including the polarization of T cells, upregulation of MHC I in peripheral tissues, direct target cell cytotoxicity, the induction of adhesion molecules on endothelial cells, and expression of chemokines for homing to peripheral tissues (Andersen et al., 2006; Friedl and Weigelin, 2008; Marelli-Berg and Jarmin, 2004). In order to determine whether autoreactive T cell numbers were directly affected by treatment with IFN-γ neutralization, total numbers of GFP+ (PMEL) T cells from the skin and from skin-draining lymph nodes were quantified using flow cytometry. The accumulation of PMEL CD8+ T cells within ear skin was significantly diminished after IFN-γ neutralization (Fig. 5b), however the total numbers of PMEL CD8+ T cells in skin-draining lymph nodes was unaffected (Fig. 5c), indicating that IFN-γ is required specifically for CD8+ T cell accumulation in the skin, and therefore may play a site-specific role in the development of vitiligo.
Discussion
This study describes, to our knowledge, a previously unreported mouse model of vitiligo that targets the epidermis but spares the hair. A recent study in humans with vitiligo reported a direct correlation among the severity of vitiligo, involvement of the hair, and autoreactive CD8+ T cell response to stimulation in vitro (van den Boorn et al., 2009). Two other models of melanocyte-targeted immunity resulted in prominent loss of the hair (Becker et al., 1996; Gilhar et al., 2001), however we do not observe this in our model. Studies in a mouse model of melanoma immunotherapy report hair depigmentation with a treatment protocol that includes PMEL CD8+ T cell adoptive transfer into RAG1−/− hosts (Antony et al., 2005), a result that we have reproduced (not shown). These data demonstrate that melanocytes within the hair follicle can serve as targets for PMEL CD8+ T cells under certain immunologic conditions, arguing against altered PMEL antigen presentation by follicular melanocytes as a cause for sparing of the hair in vitiligo. Because the hair follicle is an immune privileged organ (Gilhar et al., 2007) and is often protected from depigmentation in patients, the involvement of the hair in existing mouse models of vitiligo suggests a more robust T cell response than the model presented here. Thus, these models are complimentary, and may provide an opportunity to further dissect the mechanisms of immune tolerance in the hair follicle.
Flow cytometry of GFP+ T cells in the skin revealed that autoreactive T cells accumulated gradually after transfer and became maximal at 5 weeks. The loss of melanocytes paralleled this accumulation, as levels decreased beginning soon after transfer and continued for 5 weeks. These data reveal an early loss of melanocytes prior to visible depigmentation, suggesting subclinical disease induced by very few infiltrating T cells. Fluorescence microscopy in our model revealed microscopic clusters of T cells in areas of grossly normal pigmentation, a pattern that is reminiscent of patchy depigmentation but on a microscopic scale. A previous report by Wankowicz-Kalinska, et al. described the microscopic infiltration of T cells in clinically normally pigmented skin of patients with vitiligo, which was accompanied by focal melanocyte loss by immunohistochemistry. They labeled this phenomenon “microdepigmentation”, and hypothesized that it may represent the earliest stage in the development of macroscopic depigmented lesions (Wankowicz-Kalinska et al., 2003). Our data describing the early accumulation and microscopic clustering of autoreactive T cells in the skin with a quantifiable decrease in melanocytes, all prior to macroscopic depigmentation, support this concept.
Similar to human disease (van den Boorn et al., 2009), the mouse model of vitiligo described here revealed that IFN-γ RNA transcript was elevated in affected skin and that autoreactive CD8+ T cells produced IFN-γ. Furthermore, treatment with anti-IFN-γ antibody abrogated depigmentation in this model, which was associated with a prominent deficiency in T cell accumulation in the skin. Additional studies including BrdU incorporation and staining for Ki-67 reveal limited evidence for proliferation within the skin (not shown), suggesting that migration is a major component of the T cell accumulation during disease, and that IFN-γ may induce T cell recruitment to the skin during vitiligo. A study conducted by Gregg, et al. reported that depigmentation of the hair and epidermis in their TCR transgenic mouse model targeting tyrosinase was impaired in IFN-γ−/− mice (Gregg et al., 2010), consistent with our results. IFN-γ-dependent mechanisms related to T cell homing to peripheral tissues include the local induction of chemokines and the expression of adhesion molecules on endothelial cells (Bromley et al., 2008). Their studies identified a role for the chemokine receptors CCR5 and CXCR3 in depigmentation. However their contribution, revealed using individual receptor-deficient mice, was mild compared to IFN-γ-deficient mice, suggesting that additional IFN-γ-dependent mechanisms contributed to disease. Future studies will further define the role of IFN-γ in T cell recruitment to the skin, and determine whether IFN-γ neutralization can reverse existing skin depigmentation, which depends not only on inhibiting the autoimmune response, but on the ability of melanocytes to repigment the epidermis.
The clinical significance of the treatment response to IFN-γ neutralization reported here lies in its potential use as a new therapy for vitiligo. However, this must be balanced with the understanding that IFN-γ is required for clearance of a number of pathogens (Zhang et al., 2008), and thus future studies to implicate critical events downstream of IFN-γ signaling may provide more targeted, and therefore safer, systemic treatments for vitiligo and related autoimmune diseases. In addition to testing systemic treatments for vitiligo, future studies will take advantage of the prominent epidermal depigmentation observed in this model of vitiligo to test new topical treatments.
Materials and Methods
Mice
KRT14-Kitl*4XTG2Bjl (Krt14-Kitl*) mice were a gift from B.J. Longley, University of Wisconsin. GFP-PMEL TCR transgenic mice were produced by crossing DPEGFP mice that express GFP in T cells (provided by U. von Andrian, Harvard Medical School, Boston, MA) with the PMEL TCR transgenic mouse strain [The Jackson Laboratory, Bar Harbor, ME, stock# 005023, B6.Cg-Thy1a/CyTg(TcraTcrb)8Rest/J]. All mice were on a C57BL/6J background, were maintained in pathogen-free facilities (monitored regularly for the pathogens listed in the HM Plus panel, Charles River Laboratories, Wilmington, MA) at the University of Pennsylvania, and procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Induction of vitiligo through adoptive transfer of purified PMEL CD8+ T cells
GFP-PMEL CD8+ T cells were isolated from the spleen of GFP-PMEL TCR transgenic mice through negative selection on microbeads (Miltenyi Biotech, Auburn, CA) according to the manufacturer’s instructions. Purified CD8+ T cells (1 × 106) were injected i.v. into sublethally irradiated (500 rads one day prior to transfer) Krt14-Kitl* hosts (12–16 wk of age). Recipient mice also received i.p. injection of 1 × 106 pfu rVV-hPMEL (N. Restifo, NCI, NIH) on the same day of transfer. IFN-γ blockade was through i.p. injection of 100–500 μg of IFN-γ neutralizing antibody (XMG-6) beginning at the indicated times and twice weekly for the duration of the observation period (5–7 wks). Mice used as controls for vitiligo were sublethally irradiated but did not receive rVV-hPMEL or PMEL CD8+ T cell transfer. Mice with vitiligo used as treatment controls for IFN-γ blockade were treated with either no antibody or with an equal quantity of Rat IgG.
Flow cytometry
Ears and lymph nodes were harvested at the indicated times. The protocol to obtain single-cell suspensions of ear skin was modified from (Suffia et al., 2005). The ventral and dorsal sheets of the ears were separated and deposited dermal side down in skin digest media [RPMI containing 0.5% DNase I (Sigma-Aldrich, St. Louis, MO) and 0.5 mg/ml of liberase CI enzyme blend (Roche, Indianapolis, IN)] and were incubated for 90 min at 37° C. The sheets were dissociated in skin digest media plus 3% serum and 5mM EDTA using a medimachine (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions, and filtered using a 70-μm cell strainer. For the analysis of intracellular cytokines, single cell suspensions from the lymph nodes were stimulated with 1 μM soluble hPMEL25-33 peptide or PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (10 μg/ml) for 5h at 37°C with 5% CO2. The cells were incubated with Fc block in PBS containing 0.1% BSA, 1 mM EDTA, and then stained for the surface markers CD8β (YTS156.7.7, PE-labeled) and CD45.2 (104, APC-efluor780-labeled) as well as Live/Dead Aqua fixable viability dye (Invitrogen, Carlsbad, CA). When indicated, the cells were then fixed, permeabilized and stained for the cytokine IFN-γ (XMG1.2, PerCP-Cy5.5-labeled). Incubations were performed for 30 min on ice. For each ear, the entire suspension was analyzed. The total number of CD8β+ GFP+ cells per ear was calculated by gating on live, CD45.2+, CD8β+, GFP+ cells and reported as total CD8β+GFP+ cells per total CD45.2+ cells. The frequency of IFN-γ producers was determined by gating on live, CD8β+ cells, setting a GFP+ gate and reporting the frequency as a percentage of total GFP+ (transferred) or GFP− (endogenous) cells. For each lymph node sample, at least 100,000 cells were analyzed and the total number of GFP+ cells was calculated by dividing the GFP+ count by the singlet count and then multiplying by the total number of lymph node cells. The data were collected and analyzed using CELLQuestTM software and a FACSCaliburTM flow cytometer (BD Biosciences, San Jose, CA).
Microscopy
For microscopic examination of affected skin, mouse tails were excised and fixed in 10% neutral-buffered formalin. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H+E). For human samples, 8–10 μm formalin-fixed, paraffin-embedded biopsy specimens were obtained from the Penn Skin Disease Research Center Tissue Bank. De-identified samples were obtained from a patient with a vitiligo diagnosis and a T cell infiltrate. Sections were stained with H+E according to standard procedures.
For fluorescence microscopy of fresh tissue, ears were harvested, depilated, split into anterior and posterior halves, mounted on a glass slide with PBS and visualized on a fluorescent microscope. All images were captured on a Nikon Eclipse E600 microscope (Nikon, Melville, NY) equipped with a Photometrics Cool Snap EZ CCD camera for fluorescent images (Photometrics, Tucson, AZ), or a Nikon DS-Fi1 camera for light microscopy (Nikon, Melville, NY), using Nikon NIS Elements software.
Quantitative RT-PCR
Ears were minced into <5mm strips, stored in RNAlater (Ambion, Austin, TX) overnight at 4°C and then transferred to −20°C until RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. cDNA was generated using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, and real-time PCR was performed using primers for murine IFN-γ [previously described in (Elso et al., 2004)] or murine tyrosinase (Applied Biosystems, Carlsbad, CA) and normalized to ACTB RNA transcript (Qiagen, Germantown, MD). Real-time PCR was performed using either 2X Power SYBR Green PCR master mix (for IFN-γ and ACTB) or Taqman 2X PCR master mix (for tyrosinase) on a 7500 Fast Real time PCR system (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s instructions. Gene expression is reported relative to the average expression at time 0 after normalization to expression of ACTB.
Statistical analysis
Statistical analysis was performed using Prism software (GraphPad Software, La Jolla, CA). Dual comparisons were made using the unpaired Student’s t test and longitudinal comparison determined using linear regression.
Acknowledgments
The authors would like to thank Mohammed Ali for excellent technical assistance. The Penn Skin Disease Research Center Core was responsible for identification and processing of human tissue samples, and was funded by NIH grant 5-P30-AR057217-03. This project was supported by NIH grants AI041521 (to L.A.T.) and AI071302 (to C.A.H.). J.E.H. was supported by NIH training grant T32-AR007465 and research grants from the La Roche-Posay Research Foundation, AMBI Research Foundation/Skin of Color Society, and the Dermatology Foundation.
Abbreviations
- DE
dermal-epidermal
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein
- H+E
hematoxylin-eosin
- IFN-γ
interferon gamma
- Krt14-Kitl*
KRT14-Kitl*4XTG2Bjl mouse strain
- MHC
major histocompatibility complex
- rVV-hPMEL
recombinant vaccinia virus-human PMEL (also known as gp100)
- TCR
T cell receptor
Footnotes
Work was performed in Philadelphia, PA, USA
Conflict of Interest
The authors declare no conflict of interest.
References
- Ahn SK, Choi EH, Lee SH, et al. Immunohistochemical studies from vitiligo-- comparison between active and inactive lesions. Yonsei Med J. 1994;35:404–10. doi: 10.3349/ymj.1994.35.4.404. [DOI] [PubMed] [Google Scholar]
- Andersen MH, Schrama D, Thor Straten P, et al. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. Journal of immunology (Baltimore, Md: 1950) 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JC, Varki N, Brocker EB, et al. Lymphocyte-mediated alopecia in C57BL/6 mice following successful immunotherapy for melanoma. J Invest Dermatol. 1996;107:627–32. doi: 10.1111/1523-1747.ep12584237. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- Elso CM, Roberts LJ, Smyth GK, et al. Leishmaniasis host response loci (lmr1–3) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 2004;5:93–100. doi: 10.1038/sj.gene.6364042. [DOI] [PubMed] [Google Scholar]
- Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313–8. doi: 10.4103/0019-5154.57604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Landau M, Assy B, et al. Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on Prkdc(scid) mice. J Invest Dermatol. 2001;117:1357–62. doi: 10.1046/j.0022-202x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. The Journal of clinical investigation. 2007;117:2019–27. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RK, Nichols L, Chen Y, et al. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–17. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada T, Lu SZ, Yoshida H, et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J Exp Med. 1998;187:1565–73. doi: 10.1084/jem.187.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar MK, Feng L, Medlock E, et al. Identification and mutation of primary and secondary proteolytic cleavage sites in murine stem cell factor cDNA yields biologically active, cell-associated protein. J Biol Chem. 1994;269:1237–42. [PubMed] [Google Scholar]
- Marelli-Berg FM, Jarmin SJ. Antigen presentation by the endothelium: a green light for antigen-specific T cell trafficking? Immunol Lett. 2004;93:109–13. doi: 10.1016/j.imlet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Mrass P, Takano H, Ng LG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–61. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg GS, Rod Dunbar P, Romero P, et al. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. The Journal of experimental medicine. 1998;188:1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. The Journal of experimental medicine. 2003;198:569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffia I, Reckling SK, Salay G, et al. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–55. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360:160–9. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–32. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83:683–95. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Boisson-Dupuis S, Chapgier A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]