Abstract
Objective
In 2005 the Cystic Fibrosis (CF) Foundation recommended that children with CF maintain a body mass index (BMI) ≥50th percentile. Our study evaluated if gastrostomy (GT) placement increases the likelihood of reaching that goal compared to a standardized nutrition protocol.
Study design
Retrospective study of 20 children with CF ages 2–20 years with GTs placed from 2005–2010. Each case was pair-matched on age, sex, pancreatic status, BMI and lung function with a non-GT child with CF. Outcome measures included nutritional status and lung function at 6 months and 1 year.
Results
At baseline, mean±SD BMI Z-scores were similar (cases −1.19±0.60, controls −1.10±0.50; p=0.10). Cases had a significant 6-month increase in mean BMI Z-score to −0.29±0.84 compared to −1.02±0.67 for controls (p<0.001). By 1 year, the change in mean BMI Z-score was less different (cases −0.41±0.76, controls −0.71±0.51; p=0.07). Both groups had stable lung function. From exact logistic regression analysis, the odds ratio for cases compared to controls of reaching BMI≥50th percentile was 9.70 (95% CI:1.05–484.7;p=0.04) at 6 months and 3.65 (95%CI:0.69–25.86;p=0.16) at 1 year.
Conclusion
Our study suggests that children with CF who receive GTs are more likely to achieve BMI≥50th percentile than matched children without GTs.
Keywords: growth, body mass index, lung function
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease that affects approximately 70,000 individuals worldwide. It is marked by progressive decline in lung function and malnutrition. In 2005 an expert panel convened by the CF Foundation recommended that body mass index (BMI) percentiles be used to assess growth and that children and adolescents ages 2–20 years maintain BMI≥50th percentile for age 1. The latter recommendation is based on data demonstrating that patients with CF who maintain their growth within a normal range are more likely to have better pulmonary function and improved survival 2–4. Additionally, a faster rate of weight gain over a 2-year period has been shown to lead to better lung function 5.
According to the CF Foundation’s 2009 Annual Patient Registry Data Report 6, the median BMI percentile for patients 2–20 years of age remains below the desired goal at 48.7%. A variety of strategies have been investigated to improve the nutritional status of individuals with CF, including nutrition education, oral dietary supplementation and behavioral interventions, with variable success 7–13. Supplemental feedings via a gastrostomy (GT) have also been widely used in CF, and a number of retrospective case reports have suggested that GTs lead to increases in BMI 14–20. However, GT placement is invasive and carries multiple risks, including local organ perforation, infection and pain 21. To date, there have been no studies comparing the growth rates of CF patients with GTs to those without GTs.
The primary aim of this study was to evaluate if children with CF who have a BMI<50th percentile and receive supplemental feeds via a GT are more likely to achieve BMI≥50th percentile than matched children who are managed according to a standardized nutrition protocol but do not receive GTs. The secondary aims were to evaluate the effects of GT placement and supplemental nutrition on lung function and the number of hospitalizations required for pulmonary exacerbation and to describe the occurrence of complications during and after GT placement in this patient population.
Methods
This is a retrospective cohort study of patients 2–20 years of age who received health care at our CF Center and had GTs placed between January 2005 and April 2010. To be included in the study, the patients also needed to have at least 1 year of post-GT data. Children were excluded if they had GTs placed for reasons other than nutritional supplementation. Each of these “cases” was pair-matched with a child with CF who was also followed at our CF Center but did not have a GT (“controls”). The cases and controls were matched at the time the case received a GT based on the following criteria: age ± 2.5 years, sex, pancreatic status, BMI percentile ± 10% and, if available, percent predicted forced expiratory volume in one second (FEV1) ± 20%. The clinic visit just prior to GT placement for cases or at the time of match for controls is referred to as the “index visit”. The CF Foundation Patient Registry database and hospital medical records were used for data collection. This study was approved by the Johns Hopkins Institutional Review Board. The need for informed consent was waived.
Demographic data obtained from the records included the following: ethnicity, date of CF diagnosis, CF genotype (number of F508del mutations), pancreatic status, and history of an airway infection with Pseudomonas aeruginosa, Burkholderia cepacia or methicillin-resistant Staphylococcus aureus. In general, pancreatic status was determined by a combination of CF genotype and fecal elastase values. The presence or absence of CF-related diabetes and CF-related liver disease was also indicated.
At our institution, the nutrition of all children with CF is evaluated using a standardized protocol as published by Leonard et al. 22 The protocol includes a systematic approach to classifying nutritional status, patient and family education, and evaluation of caloric intake, nutrient absorption and CF-related diabetes. For the controls, in addition to the standard nutritional evaluation and counseling, it was specified if they received oral nutritional supplementation, an appetite stimulant and/or a gastroenterology (GI) referral for GT placement at any time during a 1-year follow-up period.
For each of the cases, the following GT data were collected: technique used for GT placement, length of hospital stay following the procedure with reason for prolonged hospitalization if required, type of supplemental formula given via the GT and primary schedule of administration, and complications encountered at the time of the procedure and during the year of follow up. The examined complications were perforation of surrounding organs, gastric wall separation, GT site lesion (i.e. granuloma), local GT site infection requiring antibiotics, tube malfunction and pain at the GT site.
Nutritional and lung function data (including height, weight, BMI and percent predicted FEV1) were obtained at the index visit, 6-month (±3 months) follow up and 1-year (±3 months) follow up. Height, weight and BMI Z-scores were calculated using CDC reference equations. The number of hospitalizations required for pulmonary exacerbation during the 1-year follow-up period was also noted.
Statistical analysis
Demographic and clinical characteristics were summarized with frequencies and percentages, means and standard deviations (SDs) or medians and ranges. Cases were compared to controls using appropriate matched-pairs analysis methods, i.e. McNemar’s test for categorical measures and paired t-test or Wilcoxon signed rank test for continuous measures. The proportion of cases and controls reaching the outcome of BMI≥50th percentile at the 6-month and 1-year follow ups was compared using Fisher’s exact test, and exact logistic regression analysis was used to estimate the odds ratio (OR) and confidence interval (CI). Analysis was performed using SAS version 9.22 (SAS Institute, Inc., Cary, NC). All reported p values are two-sided.
Results
Twenty cases and controls were included in the study. Their demographic and clinical characteristics at the time of the index visit are summarized in Table I. For each of the listed characteristics, there were no statistically significant differences between the two groups. FEV1 percent predicted data was available for 14 cases and 13 controls at the time of the index visit; this subgroup of subjects had respective mean±SD ages of 11.1±3.4 years and 11.9±3.3 years.
Table I.
Demographic and clinical characteristics of the cases and controls at the time of the index visit.
| Characteristic | Cases | Controls | p-value |
|---|---|---|---|
| Number of patients | 20 | 20 | - |
| Male sex, No. | 8 | 8 | - |
| Caucasian ethnicity, No. | 17 | 19 | 0.16 |
| Age in years at CF diagnosis, median (range) |
0.74 (0–6.58) |
1.74 (0–9.41) |
0.11 |
| F508del mutations, No.: | |||
| ○ One | 12 | 11 | |
| ○ Two | 4 | 6 | 0.83 |
| Pancreatic insufficient, No. | 20 | 20 | - |
| History of airway infection, No.: | |||
| ○ Pseudomonas aeruginosa | 18 | 13 | 0.10 |
| ○ Burkholderia cepacia | 2 | 0 | 0.16 |
| ○ Methicillin-resistant Staphylococcus aureus | 4 | 7 | 0.32 |
| CF-related diabetes, No. | 2 | 2 | 0.99 |
| CF-related liver disease, No. | 3 | 1 | 0.32 |
| At the index visit, mean±SD: | |||
| ○ Age in years | 9.0±4.4 | 9.1±4.7 | 0.54 |
| ○ Height Z-score | −0.94±0.50 | −0.51±1.06 | 0.10 |
| ○ Weight Z-score | −1.40±0.55 | −1.06±0.74 | 0.07 |
| ○ BMI Z-score | −1.19±0.60 | −1.10±0.50 | 0.10 |
| ○ FEV1 percent predicteda | 76.0±19.5 | 75.7±19.0 | 0.90 |
Data available for 14 cases and 13 controls
Percutaneous endoscopic gastrostomies (PEGs) were placed in 19 cases by pediatric gastroenterologists, and 1 child had an open gastrostomy performed by the pediatric surgical service. PEGs were completed using the standard “pull technique” under general anesthesia with endotracheal intubation; the EndoVive 18-French One-Step Button kit (Boston Scientific Corporation, Natick, MA) was used for 10 patients and the Bard Ponsky 20-French Pull kit (Bard Access Systems, Salt Lake City, UT) for the remainder. Fifteen children were admitted following GT placement for our standard protocol lasting 3 days and 2 nights. Five patients required an extended hospitalization: 4 needed additional management of pain at the GT site and 1 developed a pulmonary exacerbation following the procedure.
Eighteen cases were supplemented with a whole-protein formula, 1 with a partially hydrolyzed formula, and 1 with an elemental formula. All of the cases received the formula continuously overnight. A mealtime dose of pancreatic enzymes was given at the beginning and the end of the feeds. At our institution, we follow the recommendation of Stallings et al. 1 with goal energy intake at 110–200% of the Dietary Reference Intake. When supplemental feedings via a GT are initiated, approximately 50% of the estimated caloric needs are delivered via the GT. Adjustments are made as needed based on the individual’s weight gain and tolerance.
No patient suffered perforation or gastric wall separation during GT placement. Between the time of placement and the 6-month follow up, 7 patients developed a GT site lesion such as a granuloma, 2 patients had a local infection requiring antibiotics, and 1 patient had a tube malfunction; 50% of the cases experienced no complications during this period. All cases reported still using the GT for formula supplementation as of the 6-month visit. Between 6 months and 1 year, 3 patients were noted to have a GT site lesion and 1 patient complained of pain at the GT site; 80% of the patients developed no complications in this time frame. By 1 year, 1 child was no longer receiving supplemental nutrition via the GT at the family’s discretion because of a sustained improvement in BMI.
As of the 6-month visit, in accordance with the nutrition protocol, 6 controls had been advised to take oral supplements and 4 were prescribed an appetite stimulant. One patient was referred to GI for GT placement but did not follow through with a clinic visit. Between 6 months and 1 year, 6 controls were encouraged to take oral supplements and 2 were given an appetite stimulant; no patients were referred to GI. Of note, 1 of these patients was offered both oral nutritional supplementation and an appetite stimulant.
Table II summarizes height, weight and BMI Z-scores and FEV1 percent predicted data for the cases and controls at each of the follow-up visits. The distribution of BMI Z-scores at the index visit, 6-month follow up and 1-year follow up are presented in Figure I. At 6 months, the mean±SD BMI Z-score for the cases had improved 0.90±0.89 from baseline compared to only 0.08±0.56 for controls (p<0.001). The mean weight Z-score increased 0.68±0.61 for cases and 0.05±0.31 for controls (p<0.001), while the change in mean height Z-score was similar between the two groups (0.05±0.23 for cases vs. 0.03±0.17 for controls; p=0.77). At 1 year, the cases continued to have a higher mean BMI Z-score than the controls, but the difference from baseline was attenuated and no longer achieved statistical significance (0.78±0.90 vs. 0.38±0.52; p=0.07). The change in mean weight Z-score was statistically significant (cases 0.65±0.63 vs. controls 0.20±0.29; p=0.01), but the change in mean height Z-score was again comparable (cases 0.11±0.26 vs. controls 0.00±0.25; p=0.29).
Table II.
Mean±SD height, weight and BMI Z-scores and FEV1 percent predicted at the 6-month and 1-year follow ups for the cases and controls.
| 6 months | 1 year | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | p-valuea | Cases | Controls | p-valuea | |
| Height Z-score | −0.89±0.56 | −0.48±1.00 | 0.77 | −0.84±0.53 | −0.51±1.00 | 0.29 |
| Weight Z-score | −0.73±0.79 | −1.01±0.76 | <0.001 | −0.76±0.73 | −0.86±0.70 | 0.01 |
| BMI Z-score | −0.29±0.84 | −1.02±0.67 | <0.001 | −0.41±0.76 | −0.71±0.51 | 0.07 |
| FEV1 | 74.7±22.0% | 78.9±24.0% | 0.46 | 74.4±21.4% | 82.3±22.9% | 0.17 |
The p-value is from a paired t-test comparing the difference between the cases and controls in the change in the parameter from the time of the index visit to the specified follow-up visit.
Figure 1.
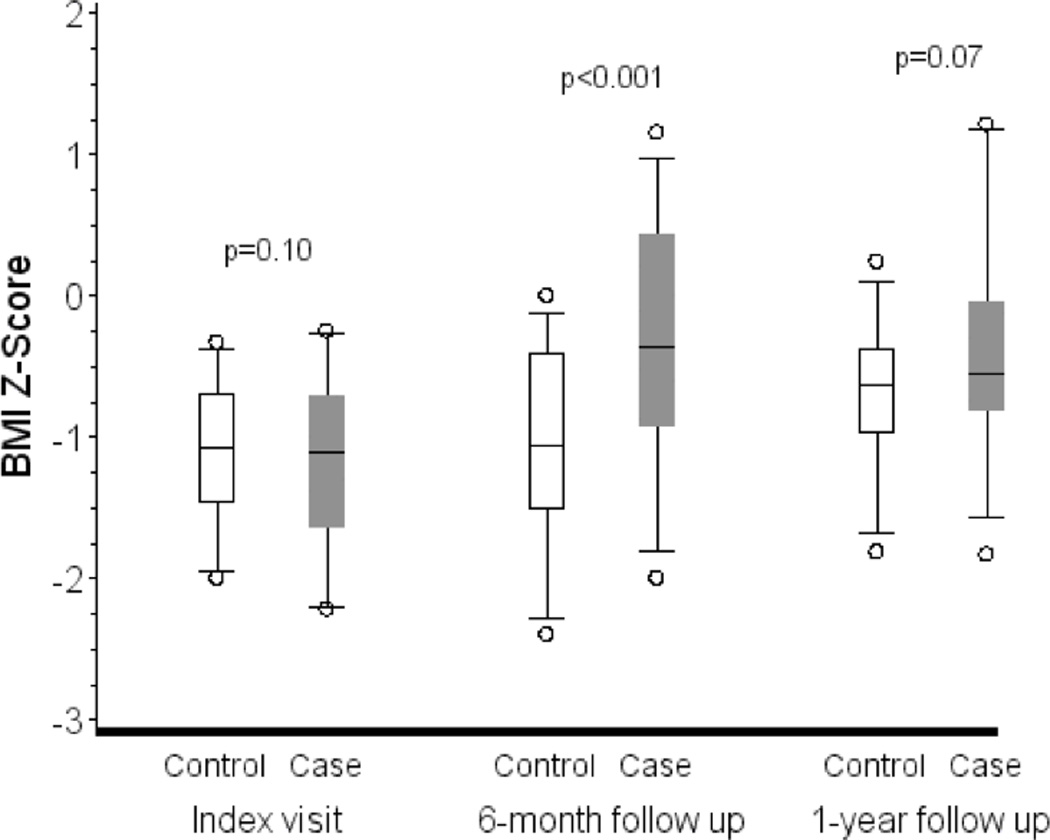
BMI Z-scores by visit and case-control status. The boxes represent the median and interquartile ranges, and the whiskers are the 5th and 95th percentiles. The p-values are from paired t-tests of the difference between the cases and controls in Z-scores at the index visit and the change from the index visit to the follow-up visits.
At the 6-month follow-up visit, 7 (35%) of the cases and 1 (5%) of the controls reached BMI≥50th percentile (p=0.04). Cases were almost 10 times as likely to reach BMI≥50th percentile at 6 months compared to controls (OR=9.70; 95% CI:1.05–484.7). At the 1-year follow up, 8 (40%) of the cases and 3 (15%) of the controls had reached BMI≥50th percentile (p=0.16), resulting in an OR of 3.65 (95% CI:0.69–25.86).
The groups were similar in the change in mean FEV1 percent predicted from the index visit to the follow-up visits (p=0.46 at 6 months, p=0.17 at 1 year). Additionally, there were no statistically significant differences between the cases and controls in the number of hospitalizations required for pulmonary exacerbation. From the index visit to the 6-month follow up, 4 cases and 6 controls each required 1 hospitalization while the rest had no hospitalizations (p=0.69). Between the 6-month and 1-year visits, 5 cases each had 1 hospitalization, 1 case had 2 hospitalizations, and 2 controls had 1 hospitalization (p=0.23).
Discussion
To our knowledge, this is the first pair-matched study evaluating the outcomes of children with CF who receive GTs. Our results indicate that children with CF who have a BMI<50th percentile for age and receive a GT are almost 10 times as likely to reach BMI≥50th percentile at 6 months compared to matched patients who do not receive GTs. The patients with GTs are still 3.65 times as likely to reach BMI≥50th percentile by 1 year, although this result does not achieve statistical significance. Our findings are noteworthy because studies have suggested that more rapid and consistent weight gain in CF is associated with improved lung function. Peterson et al. 5 followed children with CF for 2 years and evaluated how their weight gain patterns affected their pulmonary function. The authors demonstrated that children who had a faster 2-year rate of weight gain tended to have better FEV1 values. McPhail et al. 23 analyzed the effects of BMI on lung function in another cohort of children with CF. Baseline BMI percentile and rate of change of the BMI percentile were both positively associated with the slope of FEV1 percent predicted as calculated for ages 6 to 12 years.
Optimization of growth and nutrition is essential to the management of CF 2. Although there have been numerous studies looking at methods by which nutrition in patients with CF can be enhanced, the results have not been that encouraging. For example, Stark et al. 7 evaluated both behavioral intervention and nutrition education. The combination of interventions was more effective than nutrition education alone at increasing weight over a 9-week period, but there was no significant difference in weight between the treatment groups at the end of a 2-year period. Additionally, Kalnins et al. 8 compared the use of dietary supplements with dietary counseling in adolescents and adults with CF and found no significant difference in nutritional parameters between the groups after 3 months.
Our data is consistent with several retrospective studies that have demonstrated that the administration of supplemental feedings via a GT can improve weight gain in patients with CF. For example, Efrati et al. 14 reviewed 21 children with CF who had GTs placed between 1992 and 2001. In the first 2 years following placement, the mean weight Z-score increased significantly. While the mean BMI Z-score also increased significantly during the first year, there was minimal change during the second year of follow up. Additionally, mean FEV1 decreased significantly during the first year of gastrostomy feeding, but it showed a trend towards improvement during the second year. Truby et al. 15 reviewed the outcomes following GT placement for 14 children who had the procedure performed between 1999 and 2005. Similar to the previous study, there was a significant increase in mean BMI Z-score during the first year of follow up with no significant change during the second year. Furthermore, mean FEV1 remained stable over 2 years. These results suggest that GTs can lead to significant improvements in BMI in at least the first year after placement in patients with CF. However, these studies are limited because they are retrospective and lack a comparison group.
A major strength of our study is that it compares the outcomes of children with CF who received GTs for nutritional supplementation to matched patients who did not receive GTs but were managed using a rigorous, structured nutrition protocol shown to significantly improve BMI 22. Use of this protocol increased the number of children with BMI≥50th percentile by 11.8% over a 15-month period at our CF center. The children in our study who received GTs were already assessed and treated according to this protocol, yet GT placement gave them a considerably increased chance of reaching the national goal of BMI≥50th percentile for age after 6 months. Another strength of our study is that, unlike prior studies on GTs in CF, it uses BMI≥50th percentile for age as an outcome measure in accordance with the CF Foundation’s current care guidelines on nutrition 1.
Several results of our study warrant further consideration. At the 1-year follow up, the cases had an apparent waning of benefit from the GTs since the mean BMI Z-score was lower than at the 6-month follow up. This finding is similar to what was seen in the above-mentioned retrospective studies. While medical records indicate that only one patient discontinued GT feeds at 1 year, we speculate that the other patients also became less adherent with their prescribed supplemental nutrition regimen as their BMIs improved. In contrast, the control group had a greater improvement in mean BMI Z-score at the 1-year visit than at the 6-month visit, likely because their nutrition was still aggressively managed according to the standardized protocol used at our institution. Finally, it appears the cases had a slight decrease in FEV1 over the course of the study while the controls had an increase in FEV1. However, the difference in FEV1 between the two groups was not statistically significant at any of the time points.
While supplemental nutrition via a GT may help improve growth in children with CF, the invasive nature of the procedure and its associated complications may deter patients from agreeing to GT placement. A study by Gunnell et al. 24 reviewed the attitudes of patients with CF towards GTs and showed that patients were hesitant about the procedure because they feared embarrassment, pain and interference with normal activities. In our study, none of the children that underwent GT placement experienced major complications such as perforation or gastric wall separation and 75% did very well during our standard post-GT hospital admission. At 1 year, 80% of patients were not experiencing any complications from their GT. The complications that did occur, including GT site lesions, local cellulitis and tube malfunction, were easily treatable. It is reassuring that only one patient discontinued the use of enteral nutrition at 1 year. While more patient education about GTs is necessary, our study suggests that GT placement in CF patients is a generally safe and well-tolerated procedure.
There were several limitations of our study. Because it was retrospective, it did not allow for an exact comparison between the GT and non-GT groups. Additionally, the short follow-up period of 1 year prevented us from commenting on long-term maintenance of the observed weight gain. We also did not follow the patients for a sufficient amount of time to fully evaluate changes in such parameters as height, lung function and frequency of pulmonary exacerbations. Finally, while the children in our study seemed to have few complications following GT placement, we were unable to ask them any specific quality-of-life questions that would have provided us with more detailed information.
Our study offers support for the placement of GTs in children with CF who fail to reach the goal of BMI≥50th percentile for age despite rigorous management. Since early and aggressive nutritional interventions are needed in this patient population before a significant decline in lung function occurs, more specific guidelines regarding the appropriate timing of GT placement would be useful. Additionally, a long-term, prospective study would allow us to further evaluate the effects of GTs and supplemental feedings on growth and lung function in CF.
Acknowledgements
Gia M. Bradley’s work on the manuscript was supported by NIH grant 5 T32 HD 44355-8, and Kathryn A. Carson’s work was supported by National Center for Research Resources grant UL1 RR 025005.
Abbreviations
- CF
Cystic fibrosis
- BMI
Body mass index
- GT
Gastrostomy
- FEV1
Forced expiratory volume in one second
- GI
Gastroenterology
- SD
Standard deviation
- OR
Odds ratio
- CI
Confidence interval
- PEG
Percutaneous endoscopic gastrostomy
Footnotes
The authors have no conflicts of interest to disclose.
Data from this manuscript was presented in abstract form at the NASPGHAN Annual Meeting, October 2011, Orlando, FL.
References
- 1.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Matel JL, Milla CE. Nutrition in cystic fibrosis. Semin Respir Crit Care Med. 2009;30(5):579–586. doi: 10.1055/s-0029-1238916. [DOI] [PubMed] [Google Scholar]
- 3.Lai HJ, Shoff SM, Farrell PM. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–722. doi: 10.1542/peds.2007-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137(3):374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 5.Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics. 2003;112(3 Pt 1):588–592. doi: 10.1542/peds.112.3.588. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation Patient Registry. Bethesda, MD: Annual Data Report; 2009. [Google Scholar]
- 7.Stark LJ, Quittner AL, Powers SW, Opipari-Arrigan L, Bean JA, Duggan C, Stallings VA. Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2009;163(10):915–921. doi: 10.1001/archpediatrics.2009.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. J Pediatr. 2005;147(3):399–401. doi: 10.1016/j.jpeds.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Adde FV, Rodrigues JC, Cardoso AL. Nutritional follow-up of cystic fibrosis patients: the role of nutrition education. J Pediatr (Rio J) 2004;80(6):475–482. [PubMed] [Google Scholar]
- 10.Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. BMJ. 2006;332(7542):632–636. doi: 10.1136/bmj.38737.600880.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark LJ, Opipari-Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatr Pulmonol. 2011;46(1):31–35. doi: 10.1002/ppul.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers SW, Jones JS, Ferguson KS, Piazza-Waggoner C, Daines C, Acton JD. Randomized clinical trial of behavioral and nutrition treatment to improve energy intake and growth in toddlers and preschoolers with cystic fibrosis. Pediatrics. 2005;116(6):1442–1450. doi: 10.1542/peds.2004-2823. [DOI] [PubMed] [Google Scholar]
- 13.Nasr SZ, Drury D. Appetite stimulants use in cystic fibrosis. Pediatr Pulmonol. 2008;43(3):209–219. doi: 10.1002/ppul.20766. [DOI] [PubMed] [Google Scholar]
- 14.Efrati O, Mei-Zahav M, Rivlin J, Kerem E, Blau H, Barak A, Bujanover Y, Augarten A, Cochavi B, Yahav Y, Modan-Moses D. Long term nutritional rehabilitation by gastrostomy in Israeli patients with cystic fibrosis: clinical outcome in advanced pulmonary disease. J Pediatr Gastroenterol Nutr. 2006;42(2):222–228. doi: 10.1097/01.mpg.0000189348.09925.02. [DOI] [PubMed] [Google Scholar]
- 15.Truby H, Cowlishaw P, O'Neil C, Wainwright C. The long term efficacy of gastrostomy feeding in children with cystic fibrosis on anthropometric markers of nutritonal status and pulmonary function. Open Respir Med J. 2009;3:112–115. doi: 10.2174/1874306400903010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld M, Casey S, Pepe M, Ramsey BW. Nutritional effects of long-term gastrostomy feedings in children with cystic fibrosis. J Am Diet Assoc. 1999;99(2):191–194. doi: 10.1016/S0002-8223(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 17.Steinkamp G, von der Hardt H. Improvement of nutritional status and lung function after long-term nocturnal gastrostomy feedings in cystic fibrosis. J Pediatr. 1994;124(2):244–249. doi: 10.1016/s0022-3476(94)70312-4. [DOI] [PubMed] [Google Scholar]
- 18.Williams SG, Ashworth F, McAlweenie A, Poole S, Hodson ME, Westaby D. Percutaneous endoscopic gastrostomy feeding in patients with cystic fibrosis. Gut. 1999;44(1):87–90. doi: 10.1136/gut.44.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best C, Brearley A, Gaillard P, Regelmann W, Billings J, Dunitz J, Phillips J, Holme B, Schwarzenberg SJ. A pre-post retrospective study of patients with cystic fibrosis and gastrostomy tubes. J Pediatr Gastroenterol Nutr. 2011;53(4):453–458. doi: 10.1097/MPG.0b013e3182250c43. [DOI] [PubMed] [Google Scholar]
- 20.Conway SP, Morton A, Wolfe S. Enteral tube feeding for cystic fibrosis. Cochrane Database Syst Rev. 2008;(2):CD001198. doi: 10.1002/14651858.CD001198.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Fortunato JE, Troy AL, Cuffari C, Davis JE, Loza MJ, Oliva-Hemker M, Schwarz KB. Outcome after percutaneous endoscopic gastrostomy in children and young adults. J Pediatr Gastroenterol Nutr. 2010;50(4):390–393. doi: 10.1097/MPG.0b013e3181aed6f1. [DOI] [PubMed] [Google Scholar]
- 22.Leonard A, Davis E, Rosenstein BJ, Zeitlin PL, Paranjape SM, Peeler D, Maynard C, Mogayzel PJ., Jr Description of a standardized nutrition classification plan and its relation to nutritional outcomes in children with cystic fibrosis. J Pediatr Psychol. 2010;35(1):6–13. doi: 10.1093/jpepsy/jsp029. [DOI] [PubMed] [Google Scholar]
- 23.McPhail GL, Acton JD, Fenchel MC, Amin RS, Seid M. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr. 2008;153(6):752–757. doi: 10.1016/j.jpeds.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Gunnell S, Christensen NK, McDonald C, Jackson D. Attitudes toward percutaneous endoscopic gastrostomy placement in cystic fibrosis patients. J Pediatr Gastroenterol Nutr. 2005;40(3):334–338. doi: 10.1097/01.mpg.0000154656.64073.e1. [DOI] [PubMed] [Google Scholar]


