Abstract
Background
Currently, no pharmacological treatments for bipolar depression exist that exert rapid (within hours) antidepressant or antisuicidal effects. We previously reported that intravenous administration of the N-methyl-D-aspartate (NMDA) antagonist ketamine produced rapid antidepressant effects in patients with treatment-resistant bipolar depression. The present study sought to replicate this finding in an independent sample.
Methods
In this double-blind, randomized, crossover, placebo-controlled study, 15 subjects with DSM-IV bipolar I or II depression maintained on therapeutic levels of lithium or valproate received a single intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo on two test days two weeks apart. The primary outcome measure was the Montgomery-Asberg Depression Rating Scale (MADRS), which was used to rate overall depressive symptoms at baseline, at 40, 80, 110, and 230 minutes post-infusion, and on Days 1, 2, 3, 7, 10, and 14 post-infusion.
Results
Within 40 minutes, depressive symptoms as well as suicidal ideation significantly improved in subjects receiving ketamine compared to placebo (d=0.89, 95% C.I. = 0.61–1.16 and 0.98, 95% C.I. = 0.64–1.33, respectively); this improvement remained significant through Day 3. Seventy-nine percent of subjects responded to ketamine and 0% responded to placebo at some point during the trial. The most common side effect was dissociative symptoms, which occurred only at the 40-minute time-point.
Conclusion
This study replicated our previous finding that patients with bipolar depression who received a single ketamine infusion experienced a rapid and robust antidepressant response. In addition, we found that ketamine rapidly improved suicidal ideation in these patients.
Keywords: antidepressant, bipolar disorder, depression, glutamate, ketamine, NMDA, suicidal ideation
Introduction
Bipolar disorder (BPD) is one of the most severe and lethal of all psychiatric disorders (1, 2). While a number of treatments are frequently prescribed for bipolar depression, none rapidly improve depressive symptoms—including suicidal ideation—within a matter of hours or a few days (3–5). Such limitations significantly contribute to the life disruptions experienced by individuals with this disorder, and the delay in treating suicidal behavior is an issue of particular concern in this already vulnerable population (5). Hence, a treatment for bipolar depression that exerted rapid antidepressant and antisuicidal effects would have a major impact on quality of life for individuals with bipolar depression, as well as on public health in general.
Over the past decade, findings from preclinical, postmortem, and clinical studies have consistently implicated the glutamatergic system as a prime target system for developing the next generation of antidepressants, particularly by targeting the N-methyl-D-aspartate (NMDA) receptor complex (6). Postmortem studies reported decreased NR1 and NR2A transcript expression in the postmortem hippocampal tissue of individuals with BPD (7). In addition, decreased NMDA receptor expression was found to be associated with cytoplasmic postsynaptic density (PSD) signaling proteins in individuals with BPD (8); for instance, decreased PSD95 levels were observed in the dentate of individuals with BPD (9). Furthermore, polymorphisms of the GRIN1, GRIN2A, and GRIN2B genes appear to confer susceptibility to BPD (10–12); however, not all studies replicated this finding (13).
The role of the glutamatergic system in suicide has received considerably less attention; virtually no studies have examined this relationship in BPD. However, a link between treatment-emergent suicidal ideation and the glutamate system has been suggested in major depressive disorder (MDD)—specifically the involvement of the GRIA3 and GRIK2 genes (14, 15). Postmortem studies have also reported NMDA receptor alterations in brain regions in suicide victims (16, 17).
Preclinical studies have demonstrated the antidepressant and anxiolytic-like effects of the NMDA antagonist ketamine (18–22). In humans, an initial trial in subjects with treatment-resistant MDD found that ketamine improved depressive symptoms within 72 hours post-infusion (23). Subsequently, a double-blind, placebo-controlled, crossover study confirmed this finding, showing that a single ketamine infusion in patients with treatment-resistant MDD had rapid (within 2 hours post-infusion) and relatively sustained antidepressant effects (lasting 1 to 2 weeks) (24). This finding has since been reported in several other studies of MDD patients MDD (25–28); notably, the magnitude and time-frame of onset and duration of response to ketamine is remarkably similar across studies.
Building on this work, we recently conducted a clinical trial showing that a single intravenous ketamine infusion had antidepressant effects in 18 patients with treatment-resistant bipolar depression who were concomitantly receiving either lithium or valproate (4). In that double-blind, placebo-controlled, crossover study, subjects received a single infusion of ketamine or saline solution. Depressive symptoms were significantly reduced within 1 hour in those patients receiving ketamine, and these improvements lasted well beyond the expected duration of ketamine’s direct action at NMDA receptors; in contrast, the placebo group showed no significant change in severity of depressive symptoms.
It is also interesting to note that other studies have demonstrated that ketamine has significant and rapid antisuicidal effects in MDD patients (29–31). In one study, 26 patients with treatment-resistant MDD had significantly reduced Montgomery Asberg Depression Rating Scale (MADRS) suicide subscale scores 24 hours after a single ketamine infusion (30). In another study of 33 subjects with treatment-resistant MDD who received a single ketamine infusion, suicidal ideation scores decreased significantly within 40 minutes, an effect that remained significant for 4 hours post-infusion (29). No study to date has reported rapid antisuicidal effects associated with ketamine in patients with bipolar depression.
The objectives of the present controlled trial were to replicate—in an independent sample—our original finding that ketamine exerts rapid antidepressant effects in patients with bipolar depression. Based on our previous work, we hypothesized that a single intravenous ketamine infusion would exert a rapid and clinically significant improvement in depressive symptoms within 230 minutes post-infusion in these patients. In order to assess these issues as carefully as possible, only patients with BPD currently experiencing a major depressive episode were included in the patient sample.
Methods and Materials
Participants were male and female, aged 18 to 65 years, diagnosed with BPD-I or II without psychotic features, and currently experiencing a major depressive episode of at least 4 weeks duration as diagnosed by the Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Version (SCID-P) (32). Final diagnoses of bipolar depression and other Axis I disorders were made as a general consensus decision by 3 clinicians using all available clinical information (SCID-P, clinical interviews, and—in most cases—interviews with someone who knew the patient well). Subjects were studied as inpatients at the National Institute of Mental Health (NIMH) Mood Disorders Research Unit in Bethesda, Maryland between July 2009 and August 2011. Subjects were required to have a MADRS score of ≥20 at screening and at the start of each ketamine/placebo infusion. Patients were also required to have previously failed at least 1 adequate antidepressant trial (as assessed by the Antidepressant Treatment History Form, ATHF-modified (33)), and to have failed a prospective open trial of a mood stabilizer while at the NIMH (lithium or valproate for at least 4 weeks at therapeutic levels (serum lithium 0.6–1.2 mEq/L or valproic acid 50–125 µg/mL)).
All participants were in good physical health as determined by medical history, physical examination, blood labs, electrocardiogram (ECG), chest x-ray, urinalysis, and toxicology. No comorbid substance abuse or dependence for at least 3 months prior to enrolling in the study was allowed. A comorbid Axis I diagnosis of anxiety disorder was permitted if the disorder had not been the primary focus of treatment within 12 months before screening.
Exclusion criteria included: any serious unstable medical condition; previous treatment with ketamine; or concomitant treatment with psychotropic medications other than lithium or valproate in the 2 weeks before randomization (5 weeks for fluoxetine). Female subjects could not be pregnant or nursing. The study was approved by the Combined Neuroscience IRB at the National Institutes of Health (NIH). All subjects provided written informed consent and were assigned a Clinical Research Advocate from the NIMH Subjects Protection Unit to monitor the consent process as well as research participation throughout the study.
The effect size (Cohen’s d) comparing ketamine and placebo at Day 1 from the initial study in patients with BPD was 0.67. Making standard assumptions of 80% power and p≤.05, 2-tailed, we estimated that a minimum of 20 patients would be required to replicate this effect. An interim analysis was allowed after 60% of the patients finished the study.
Study design
This single-center, double-blind, randomized, crossover, placebo-controlled study was conducted to assess the efficacy and safety of a single intravenous infusion of the NMDA antagonist ketamine combined with lithium or valproate therapy for the treatment of BPD I or II depression. Patients were required to take either lithium or valproate within the specified range during the entirety of the study; this was regardless of whether they were already at therapeutic levels of lithium or valproate at admission. They received no other psychotropic medications (including benzodiazepines) or structured psychotherapy. Lithium and valproate levels were obtained weekly. Vital signs and oximetry were monitored during the ketamine infusion and for 1 hour post-infusion. ECG, complete blood counts (CBC), electrolyte panels, and liver function tests were obtained at baseline and study endpoint.
After non-response to open treatment with lithium or valproate as well as a 2-week drug-free period (except for treatment with lithium or valproate), subjects received intravenous infusions of ketamine hydrochloride (0.5 mg/kg) and saline solution 2 weeks apart using a randomized, double-blind, crossover design. The study design and the dose of ketamine used were based on our previous study of patients with bipolar depression (4). Patients were randomly assigned to the order in which they received the 2 infusions via a random numbers chart. Study solutions were supplied in identical 50-ml syringes containing either 0.9% saline or ketamine with the additional volume of saline to total 50 ml; when dissolved in 0.9 percent saline, ketamine forms a clear solution. Infusions were administered via a Baxter infusion pump (Deerfield, Illinois) over 40 minutes by an anesthesiologist in the peri-anesthesia care unit. All staff, including the anesthesiologist, were blind to whether placebo or drug was being administered.
Outcome Measures
Subjects were rated 60 minutes before the infusion, and at 40, 80, 110, and 230 minutes post-infusion. They were also rated on Days 1, 2, 3, 7, 10, and 14 post-infusion. The MADRS was the primary outcome measure. Secondary outcome measures included: the 17-item Hamilton Depression Rating Scale (HDRS) (34), the Beck Depression Inventory (BDI) (35), the Visual Analogue Scale (VAS) (36), the Hamilton Anxiety Rating Scale (HAM-A) (37), the Brief Psychiatric Rating Scale (BPRS) (38), the Clinician Administered Dissociative Scale (CADSS) (39), and the Young Mania Rating Scale (YMRS) (40). The HAM-A was obtained at all time points except for 40, 80, and 110 minutes post-infusion. Ratings for symptoms that could not change over brief periods of time (e.g., sleep, appetite) were carried forward from the initial ratings for those time points. Patient ratings were performed by research nurses and/or psychologists who trained together to establish reliability. High inter-rater reliability was obtained for the MADRS (ICC = 0.82), HDRS (ICC=0.91), and YMRS (ICC=0.90). Throughout the study, the same rater conducted most ratings for an individual patient.
Statistical Analyses
The primary intent to treat (ITT) analysis included all available data.
Linear mixed models were used to examine the effect of ketamine compared to placebo over the course of 2 weeks for each phase of the crossover study. Time, drug, and infusion were within-subjects factors. Fixed main effects and an interaction were included for time and drug along with a main effect of infusion. Restricted maximum likelihood estimates were used with a first order autoregressive covariance structure. The fixed intercept was included, but no random effects contributed to the model. Bonferroni-corrected simple effects tests were used to determine the timing of drug differences with significant interactions. Cohen’s d is the reported effect size with confidence intervals following Bonferroni correction. Significance was evaluated at p ≤.05, 2-tailed.
Kaplan-Maier proportional hazards survival analysis was used to determine the length of time to response and remission. Rates of response (50% improvement from baseline on MADRS) and remission (defined as a MADRS score < 10) were examined at each time point.
Results
Patients
Fifteen patients with bipolar depression were randomized. Demographic and clinical characteristics, as well as treatment history of the patient sample, are summarized in Tables 1 and 2. Patients received their first infusion an average of 53.9 (SD=12.7) days after entering the hospital. During that time, MADRS scores did not change significantly (p=.26).
Table 1.
Demographic and Clinical Characteristics (n=15)
| Mean | SD | |
|---|---|---|
| Age (Years) | 46.7 | 10.4 |
| Length of Illness (Years) | 30.6 | 11.2 |
| Length of Current Episode (Months) | 20.9 | 27.5 |
| Psychotropic Medications at Admission | 3.7 | 1.5 |
| Length of Therapeutic Mood Stabilizer Prior to Randomization (Days) | 63.3 | 98.7 |
| Lifetime Antidepressant Trials | 9.7 | 4.3 |
| N | % | |
| Sex (Female) | 8 | 53 |
| Education | ||
| • College/Graduate School | 5 | 33 |
| • Incomplete College | 9 | 60 |
| • High School | 1 | 7 |
| Disability | 8 | 53 |
| Unemployed | 12 | 80 |
| Family History | ||
| • Mood Disorder | 13 | 87 |
| • Alcohol Abuse or Dependence | 11 | 73 |
| Life History | ||
| • Suicide Attempt | 6 | 40 |
| • Sexual Abuse | 2 | 13 |
| Lifetime Diagnosis | ||
| • Alcohol Abuse or Dependence | 9 | 60 |
| • Substance Abuse or Dependence | 6 | 40 |
| • Anxiety Disorder | 11 | 73 |
| • Psychosis | 5 | 33 |
| Bipolar subtype | ||
| • I | 9 | 60 |
| • II | 6 | 40 |
| Rapid cycling | ||
| • Past 12 months | 0 | 0 |
| • Lifetime | 9 | 60 |
| Mood Stabilizer | ||
| • Lithium | 11 | 73 |
| • Valproate | 4 | 27 |
Table 2.
Medication use during current major depressive episode and lifetime (N=15)
| Current | Lifetime | |||
|---|---|---|---|---|
| Medication/Treatment | N | % | N | % |
| Lithium* | 11 | 73 | 15 | 100 |
| Antidepressants* | ||||
| • SSRI | 5 | 33 | 14 | 93 |
| • Bupropion | 5 | 33 | 12 | 80 |
| • Duloxetine | 3 | 20 | 11 | 73 |
| • Mirtazapine | 3 | 20 | 9 | 60 |
| • Venlafaxine | 1 | 7 | 11 | 73 |
| • TCA | 1 | 7 | 10 | 67 |
| • MAOI | 1 | 7 | 7 | 47 |
| • Trazodone | 1 | 7 | 7 | 47 |
| • Nefazodone | 1 | 7 | 5 | 33 |
| Antipsychotics | ||||
| • Aripiprazole | 3 | 20 | 10 | 67 |
| • Olanzapine*† | 3 | 20 | 7 | 47 |
| • Quetiapine* | 2 | 13 | 10 | 67 |
| • Risperidone | 1 | 7 | 9 | 60 |
| • Ziprasidone | 0 | 0 | 5 | 33 |
| • Conventional Antipsychotics | 0 | 0 | 2 | 13 |
| Anticonvulsants | ||||
| • Lamotrigine* | 5 | 33 | 12 | 80 |
| • Valproate | 4 | 27 | 8 | 53 |
| • Carbamazepine* | 2 | 13 | 6 | 40 |
| • Oxcarbazepine | 0 | 0 | 4 | 27 |
| • Gabapentin | 0 | 0 | 3 | 20 |
| • Topiramate | 0 | 0 | 1 | 7 |
| Somatic Treatments | ||||
| • ECT* | 1 | 7 | 6 | 40 |
| • VNS | 0 | 0 | 1 | 7 |
| Other | ||||
| • Thyroid Augmentation | 4 | 27 | 7 | 47 |
| • Stimulant | 2 | 13 | 6 | 40 |
| • Modafinil* | 0 | 0 | 5 | 33 |
| • Pramipexole* | 0 | 0 | 2 | 13 |
Abbreviations: ECT, electroconvulsive therapy; MAOI, monoamine oxidase inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; VNS, vagal nerve stimulation.
Adequate trial based on the Antidepressant Treatment History Form-modified (ATHF-modified), modified to include adequate trial definition for quetiapine, newer antidepressants, modafinil, pramipexole, and atypical antipsychotic drugs (modified from (33)).
6 subjects had failed olanzapine + SNRI/SSRI (fluoxetine/or other SSRI).
Eleven (73%) of the 15 randomized patients completed both phases of the protocol. All 4 patients who dropped out of the study did so in the first phase; at the time, 3 had received ketamine and 1 had received placebo. Two of the dropouts who received ketamine responded briefly and then began to lose improvement, one after 7 days and the other after 3 days. The other 2 dropouts did not reach response criteria and left the study after 3 days.
Fourteen (93%) patients received the ketamine infusion and 12 (80%) received the placebo infusion. Eleven of 14 (79%) completed the ketamine phase, and 11 of 12 (92%) completed the placebo phase.
Nine of 11 (82%) patients receiving lithium completed the full study; one dropped out during the ketamine phase, and one dropped out during the placebo phase. Two of the 4 (50%) patients receiving valproate completed the study; both dropped out during the ketamine phase. Mean lithium levels were 0.8 mEq/l (SD=0.1) and mean valproate levels were 78.0 µg/mL (SD=15.5).
Efficacy
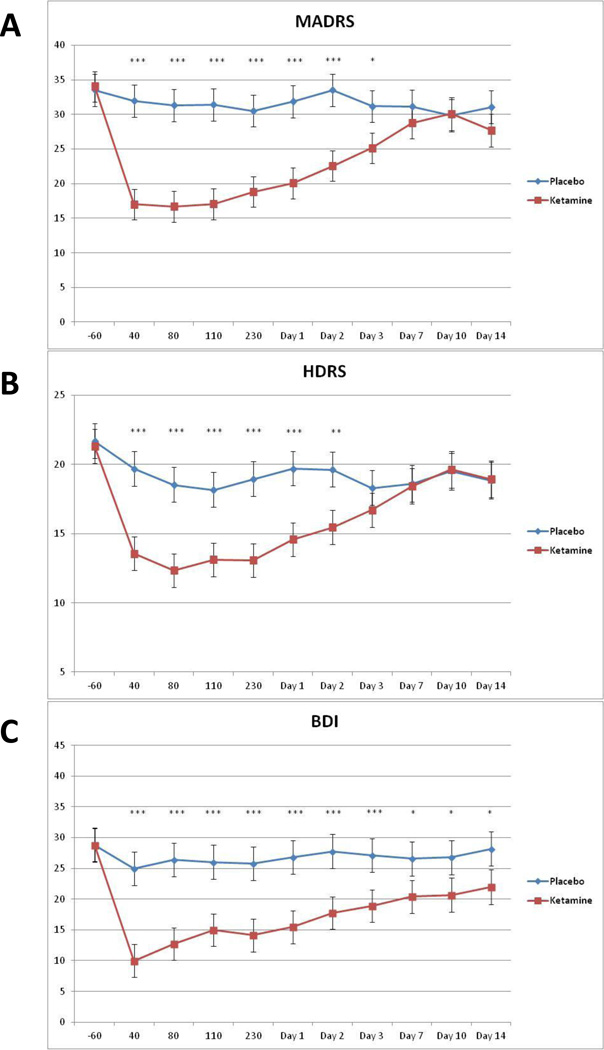
With the ITT sample, the linear mixed model with the MADRS showed a significant drug by time interaction (F10,187=5.94, p<.001). Post-hoc tests indicated significantly fewer depressive symptoms in patients who received ketamine versus those who received placebo from 40 minutes to 3 days post-infusion (Figure 1). After correction for multiple comparisons, no significant difference between the drugs was observed at baseline, or at Days 7, 10, or 14 (p=.83, p=.34, p=.93, and p=.19, respectively). The effects were moderate to large at 40 minutes (d=0.89, 95% C.I.=0.61–1.16) through 230 minutes (d=0.85, 95% C.I.=0.57–1.14), and moderate to large at Day 1 (d=0.70, 95% C.I.=0.42–0.98) and Day 2 (d=0.65, 95% C.I.=0.37–0.93). The biggest effect size was observed at 40 minutes post-infusion.
Figure 1.
Change in MADRS, 17-item HDRS, and BDI scores over 2 weeks (n=15). Values are expressed as generalized least square means and standard errors for the Intent to Treat (ITT) analysis. *indicates p<0.05, ** p <0.01, *** p <0.001.
When only those who completed the study were examined, the drug by time interaction was significant (F10,145=4.95, p<.001). Differences between the drugs were observed from 40 minutes to Day 3. The effect at Day 1 was similar to that observed with the full sample (d=0.68, 95% C.I.=0.38–0.98).
Because this was a crossover study, several additional statistical models were run to examine potential problems associated with carryover effects. A linear mixed model compared baseline MADRS scores for each drug and drug order. No significant effects were noted for either drug (p=.65) or order of drug administration (order: p=.43; order×drug: p=.10). No significant difference was noted in baseline MADRS and the first and second infusions, regardless of whether patients received ketamine first (first infusion mean=34.1, SE=2.0; second infusion mean=30.8, SE=2.5; F1,11=2.13, p=.17) or placebo first (first infusion mean=35.6, SE=1.9; second infusion mean=33.7, SE=2.0; F1,10=1.22, p=.30). To examine whether carryover may have affected the results, a linear mixed model was run using only the first phase of the study, and a significant drug by time interaction was noted (F10,66=5.46, p<.001). Post-hoc tests indicated significant drug differences from 40 minutes through Day 3; the effect size was very large at Day 1 (d=1.34, 95% C.I.=0.60–2.07).
When evaluating individual symptoms on the MADRS, 8 of 10 symptoms were significantly improved on ketamine compared to placebo. Only reduced appetite and decreased sleep were not significantly improved on ketamine.
Time to response and number of responders
The median time to ketamine response was 40 minutes. The median time to relapse (25% improvement from baseline following response for one day) was 2 days. The mean time to relapse was 4.5 (SE=1.3) days. Two patients maintained response for at least 1 week, a third patient responded for at least 10 days, and a fourth patient responded until the final rating on Day 14.
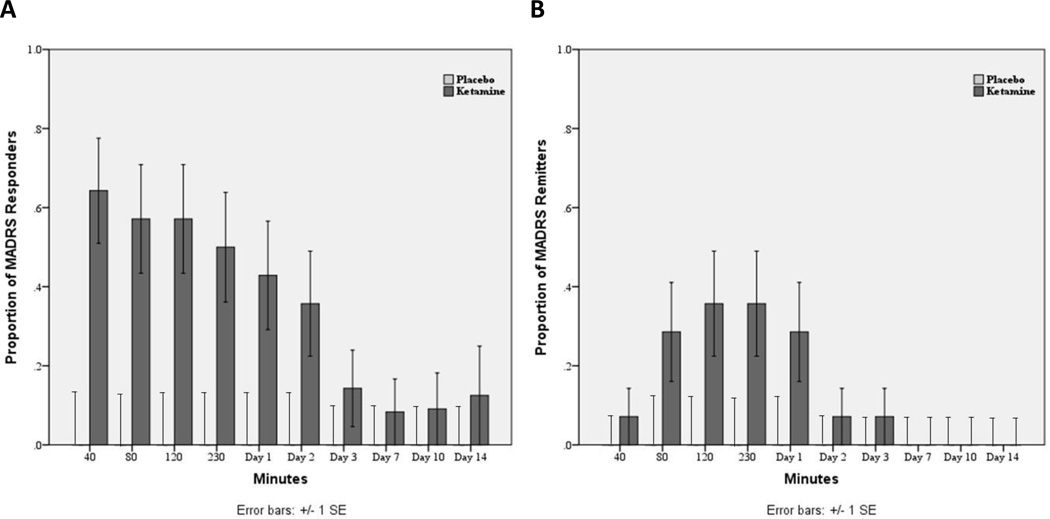
Using 50% change in MADRS as the response criteria, 64% of patients receiving ketamine responded at 40 minutes, 50% responded at 230 minutes, and 43% responded at Day 1 (Figure 2). Remission was defined as a MADRS score ≤10. Under those criteria, 7% of patients receiving ketamine experienced remission at 40 minutes, 36% met remission criteria at 230 minutes, and 29% met remission criteria at Day 1 (Figure 2). Altogether, 79% of subjects responded to ketamine at some point during the study; 0% responded to placebo.
Figure 2.
(A) Proportion of patients responding (50% improvement on MADRS) to ketamine and placebo from 40 minutes to Day 14 post-infusion. (B) Proportion of patients experiencing remission (MADRS <10) to ketamine and placebo from 40 minutes to Day 14 post-infusion.
Compared to baseline, patients receiving placebo improved an average of 5% at 40 minutes, 9% at 230 minutes, and 1% at Day 1. In contrast, patients receiving ketamine improved an average of 50% at 40 minutes, 45% at 230 minutes, and 41% at Day 1.
The type of mood stabilizer that patients received concomitantly did not affect response (see Supplemental Information).
Secondary outcome measures
To confirm results obtained using the primary outcome measure, similar models were used for the HDRS, BDI, and VAS-Depression rating scales. The drug by time interactions were significant for each of these scales (HDRS: F10,197=3.08, p=.001 (Figure 1); BDI: F10,140=3.08, p=.001 (Figure 1); VAS-Depression: F10,168=4.02, p<.001). The drug difference was significant from 40 minutes through Day 2 for the HDRS, and 40 minutes through Day 14 for the BDI and VAS-Depression scales.
When data from the HAM-A and VAS-Anxiety subscales were analyzed, significant drug by time interactions were observed showing lower ratings in patients receiving ketamine as early as the 40-minute time point (see Supplemental Information). No significant drug effect or interaction was observed when data from the YMRS or BPRS were analyzed (Figure S1 in the Supplement). A significant interaction between drug and time was found on the CADSS, showing higher values in patients receiving ketamine only at the 40-minute time point (Figure S1 in the Supplement).
Effects on suicidal ideation
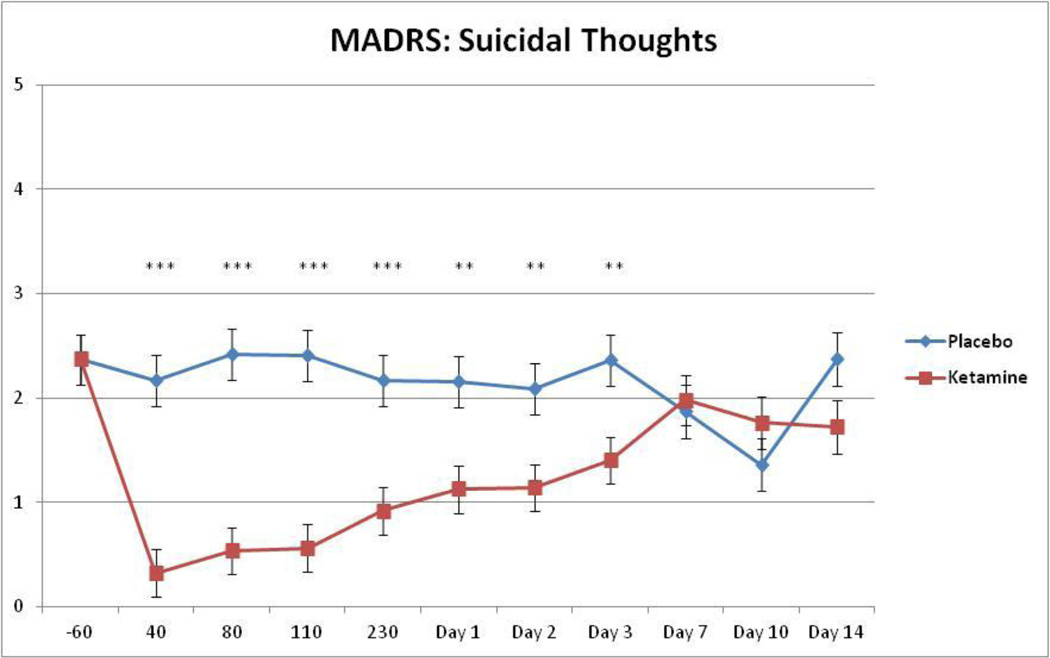
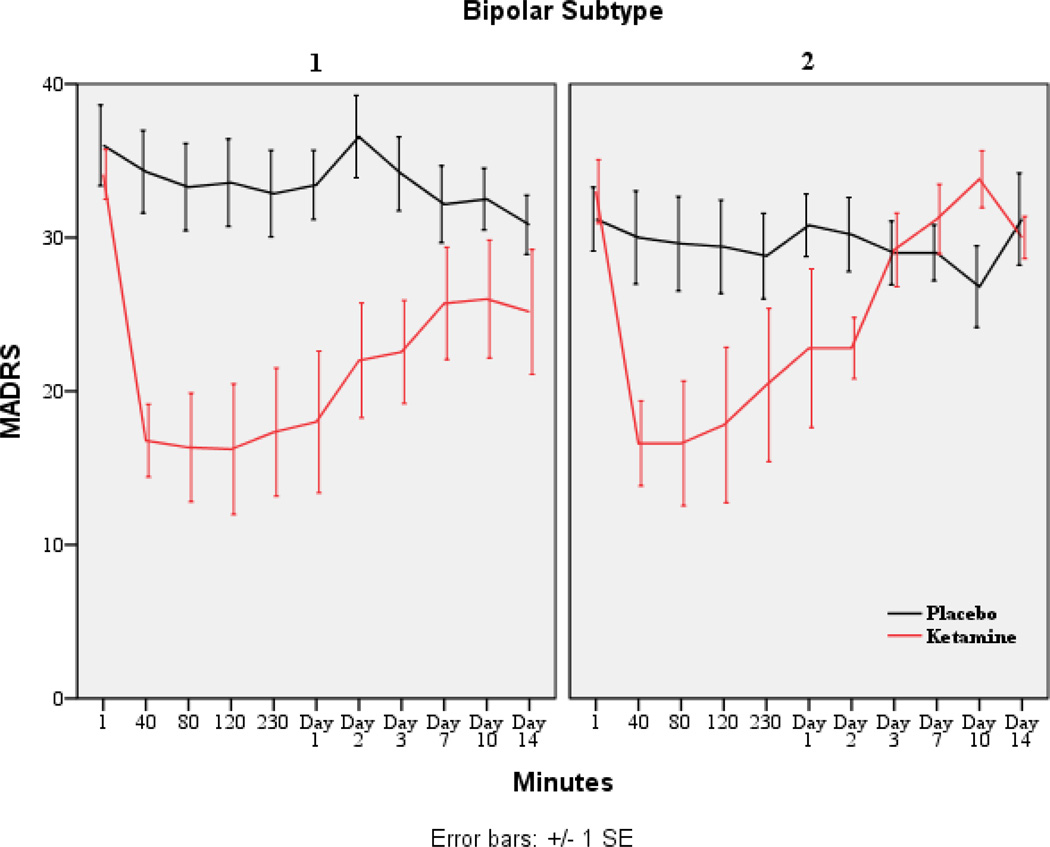
Given previous findings showing that ketamine reduced suicidal ideation in individuals with MDD, we examined changes in suicide item scores on the MADRS, HDRS, and BDI using linear mixed models. Each model used the baseline as a covariate to address concerns about confounding baseline and drug differences. All measures showed significant main effects for drug; specifically, ratings were higher in patients who received placebo versus those who received ketamine (MADRS: F1,58=63.21, p<.001, d=2.09; HDRS: F1,77=16.86, p<.001, d=0.94; BDI: F1,78=71.11, p<.001, d=1.91). The interaction between drug and time was significant for the suicide items on the MADRS, HDRS, and BDI (MADRS: F9,130=4.68, p<.001; HDRS: F9,163=1.94, p=.049; BDI: F9,165=1.98, p=.045). On the MADRS, patients who received ketamine had lower suicidal ideation scores from 40 minutes to Day 3 (Figure 3). On the HDRS, patients who received ketamine had lower suicidal ideation scores from 40 to 80 minutes and at Day 2. On the BDI, patients who received ketamine had lower suicidal ideation scores from 40 minutes to Day 2 and at Day 10. Figure 4 presents response by BPD subtype (I vs. II) and shows the similarity in treatment response.
Figure 3.
Change in suicidal thoughts (suicide item from MADRS) over 2 weeks. Values are expressed as generalized least squares means and standard errors for the Intent to Treat (ITT) analysis. *indicates p <0.05, ** p <0.01, *** p <0.001.
Figure 4.
Change in MADRS scores over 2 weeks by BPD subtype (I vs. II; n=15).
Adverse Events
No serious adverse events occurred during the study. Adverse events occurring during the infusion in 10% or more of the subjects receiving ketamine or placebo included feeling woozy or loopy, feeling lethargic or drowsy, cognitive impairment, fear or anxiety, nausea, dizziness, odd sensations, blurred vision, and headache. No adverse event was significantly different from placebo at 80 minutes or thereafter (Table S1 in the Supplement). Headaches, drowsiness or sedation, early morning awakening, and difficulty falling asleep were reported in ≥10% of the sample in both the ketamine and placebo phases. Dry mouth, dizziness or faintness, difficulty falling asleep, and flatulence were reported for ketamine only; irritability and muscle, bone, or joint pain were reported for placebo only. No significant changes occurred in ECG, respiratory, or laboratory values during the study.
Discussion
This double-blind, placebo-controlled, proof-of-concept study replicated our previous finding that a single intravenous infusion of an NMDA antagonist resulted in a robust and rapid (within 1 hour) antidepressant response in patients with bipolar depression. An additional—and novel—finding was that ketamine exerted rapid antisuicidal effects compared to placebo in these patients. To our knowledge, this is the first controlled report detailing such rapid antisuicidal effects (within 1 hour) associated with a single infusion of an NMDA antagonist in patients with bipolar depression.
Depressive and anxiety symptom scores improved significantly more in patients who received ketamine than in those who received placebo, and this improvement was observed as early as 40 minutes post-infusion. This difference was statistically significant across different efficacy scales—the MADRS, HDRS, self-rated BDI, and VAS-Depression—as well as the HAM-A and VAS-Anxiety scales. The fact that symptoms improved so rapidly is particularly striking given that most participants had a course of illness marked by frequent hospitalizations, multiple adequate medication trials (see Table 2), suicide attempts, comorbid anxiety disorders, unemployment, and considerable disability.
Notable similarities exist between the present study and our previous one (4). We found similar 1) rapid onset of antidepressant effects (40 minutes in both studies); 2) response rates at 40 minutes (64% vs. 56%) and Day 1 (43% vs. 44%); 3) remission rates at Day 1 (approximately 30% in both studies); and 4) duration of antidepressant effects as assessed by the MADRS and HDRS (3 days in both studies), but longer improvement in depressive symptoms as assessed by the BDI (14 days vs. 3 days).
The most intriguing finding of the present study may be that ketamine exerted measurable, rapid (onset within 40 minutes), and continued antisuicidal effects. To our knowledge, no controlled study of patients with bipolar depression has yet demonstrated this rapidity of onset, robustness of effect, and continued effect on suicidal ideation resulting from a single administration of any intervention (i.e., pharmacological, somatic device, or therapy). This difference was statistically significant on the suicide item of 3 different efficacy scales: MADRS, HDRS, and self-rated BDI. Onset occurred within 40 minutes and remained significant for 3 days. In addition, anxiety symptoms, which are a well-recognized risk factor for suicide, also improved significantly and rapidly following ketamine administration. In contrast, our previous study of ketamine in bipolar depression found no significant improvement on the MADRS suicide item; effect sizes were about half or less than in the current sample. These differences may be attributable to the variance of scores for this sub-item or to demographic and clinical characteristics.
Overall, these results further support our earlier controlled study investigating the glutamatergic system, and NMDA antagonism in particular, as putatively involved in the mechanism of rapid antidepressant response in bipolar depression (4). Preclinical studies have postulated that ketamine’s mechanism of action is initially mediated by NMDA antagonism but subsequently involves enhanced 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid (AMPA) throughput via enhanced presynaptic release of glutamate (20). In an extension of this work, a recent study found that mammalian target of rapamycin (mTOR)-dependent synapse formation underlies ketamine’s rapid antidepressant properties (21); ketamine was found to rapidly activate the mTOR pathway, leading to increased synaptic signaling proteins and increased number and function of new spine synapses in the rat prefrontal cortex. The present finding supports the concept that blocking the NMDA receptor complex with ketamine—and as a result enhancing AMPA throughput—results in the rapid onset of antidepressant effects and, furthermore, that this process is a shared therapeutic mechanism for ketamine’s rapid antidepressant effects regardless of diagnosis. Essentially, the same glutamatergic modulatory effect—NMDA antagonism—resulted in rapid antidepressant effects in two different disorders (MDD and BPD) (4, 23, 24, 29).
This study had several strengths. First, subjects were hospitalized for an average of 8 weeks before their first infusion. This allowed sufficient time to characterize the patients, conduct a prospective trial of a mood stabilizer, and document the stability of depressive and suicidal symptoms during their current episode. Second, the study was randomized and placebo-controlled. Finally, the fact that the present study replicated our previous finding that ketamine exerts antidepressant effects in patients with bipolar depression adds further credence to these results. Nevertheless, limitations also exist, and these are similar to the ones associated with our previous study (4). First, the sample size was small. In addition, these patients had a long course of illness marked by multiple past medication trials and treatment with electroconvulsive therapy (ECT). Thus, the results may not be generalizable to BPD patients with different illness and course characteristics. Whether subjects in this study met criteria for treatment-resistant bipolar depression is a matter of debate. Ambiguities in the current definition of treatment-resistance in bipolar depression have led to new proposed definitions of treatment-resistance (41). In future studies, using such criteria at study entry would be one approach to ensure the treatment-resistance status of the population under study. In addition, and in keeping with other studies (23, 24, 42, 43), we observed short-lived dissociative symptoms in patients receiving ketamine that could have compromised the study blind. Because this was a replication study conducted in tandem with our previous one, the original study design was conserved; that is, raters and patients were not required to guess treatment assignment. Thus, it is possible that the study blind may have biased patient reporting, thereby potentially confounding the results. However, the fact that this study almost precisely replicated the onset and offset of improvement in depressive symptoms after ketamine infusion and over time does add confidence to our results. Nevertheless, the finding awaits replication by an independent laboratory using an active control.
Taken together, these findings support the hypothesis that targeting the NMDA receptor complex brings about rapid antidepressant and antisuicidal effects in patients with bipolar depression. However, because the antidepressant effects of ketamine were not long-lasting for most patients, it will be important to develop alternate strategies to sustain ketamine’s rapid antidepressant effects.
Supplementary Material
Acknowledgments
Dr. Zarate had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ioline Henter provided outstanding editorial assistance.
Disclosures
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health, and Department of Health & Human Services (IRP-NIMH-NIH-DHHS), and the Brain & Behavior Research Foundation. A patent application for the use of ketamine in depression has been submitted listing Dr. Zarate among the inventors; he has assigned his rights on the patent to the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Nock MK, Hwang I, Sampson N, Kessler RC, Angermeyer M, Beautrais A, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6:e1000123. doi: 10.1371/journal.pmed.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68:1058–1064. doi: 10.1001/archgenpsychiatry.2011.113. [DOI] [PubMed] [Google Scholar]
- 3.Post RM, Leverich GS, Altshuler LL, Frye MA, Suppes T, Keck PE, et al. Differential clinical characteristics, medication usage, and treatment response of bipolar disorder in the US versus The Netherlands and Germany. Int Clin Psychopharmacol. 2011;26:96–106. doi: 10.1097/YIC.0b013e3283409419. [DOI] [PubMed] [Google Scholar]
- 4.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinton SM, Meador-Woodruff JH. Abnormalities of the NMDA Receptor and Associated Intracellular Molecules in the Thalamus in Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2004;29:1353–1362. doi: 10.1038/sj.npp.1300451. [DOI] [PubMed] [Google Scholar]
- 9.Toro C, Deakin JF. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr Res. 2005;80:323–330. doi: 10.1016/j.schres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Itokawa M, Yamada K, Iwayama-Shigeno Y, Ishitsuka Y, Detera-Wadleigh S, Yoshikawa T. Genetic analysis of a functional GRIN2A promoter (GT)n repeat in bipolar disorder pedigrees in humans. Neurosci Lett. 2003;345:53–56. doi: 10.1016/s0304-3940(03)00501-9. [DOI] [PubMed] [Google Scholar]
- 11.Martucci L, Wong AH, De Luca V, Likhodi O, Wong GW, King N, et al. N-methyl-D-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: Polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Mundo E, Tharmalingham S, Neves-Pereira M, Dalton EJ, Macciardi F, Parikh SV, et al. Evidence that the N-methyl-D-aspartate subunit 1 receptor gene (GRIN1) confers susceptibility to bipolar disorder. Mol Psychiatry. 2003;8:241–245. doi: 10.1038/sj.mp.4001218. [DOI] [PubMed] [Google Scholar]
- 13.Szczepankiewicz A, Skibinska M, Suwalska A, Hauser J, Rybakowski JK. No association of three GRIN2B polymorphisms with lithium response in bipolar patients. Pharmacol Rep. 2009;61:448–452. doi: 10.1016/s1734-1140(09)70085-4. [DOI] [PubMed] [Google Scholar]
- 14.Laje G, Paddock S, Manji HK, Rush AJ, Wilson AF, Charney DS, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry. 2007;164:1530–1538. doi: 10.1176/appi.ajp.2007.06122018. [DOI] [PubMed] [Google Scholar]
- 15.Menke A, Lucae S, Kloiber S, Horstmann S, Bettecken T, Uhr M, et al. Genetic markers within glutamate receptors associated with antidepressant treatment-emergent suicidal ideation. Am J Psychiatry. 2008;165:917–918. doi: 10.1176/appi.ajp.2008.08020274. [DOI] [PubMed] [Google Scholar]
- 16.Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675:157–164. doi: 10.1016/0006-8993(95)00057-w. [DOI] [PubMed] [Google Scholar]
- 17.Holemans S, De Paermentier F, Horton RW, Crompton MR, Katona CL, Maloteaux JM. NMDA glutamatergic receptors, labelled with [3H]MK-801, in brain samples from drug-free depressed suicides. Brain Res. 1993;616:138–143. doi: 10.1016/0006-8993(93)90202-x. [DOI] [PubMed] [Google Scholar]
- 18.Aguado L, San Antonio A, Perez L, del Valle R, Gomez J. Effects of the NMDA receptor antagonist ketamine on flavor memory: conditioned aversion, latent inhibition, and habituation of neophobia. Behav Neural Biol. 1994;61:271–281. doi: 10.1016/s0163-1047(05)80010-x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxyl-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 24.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 25.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney D, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunney BG, Bunney WE. Rapid-acting antidepressant strategies: mechanisms of action. Int J Neuropsychopharmacol. 2011 Jul 7; doi: 10.1017/S1461145711000927. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Diazgranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price RB, Nock MK, Charney D, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–529. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams AR. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 33.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16) 1j0-17. [PubMed] [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 36.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989–993. [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 38.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 39.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 40.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 41.Pacchiarotti I, Mazzarini L, Colom F, Sanchez-Moreno J, Girardi P, Kotzalidis GD, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120:429–440. doi: 10.1111/j.1600-0447.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 42.Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 43.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.