Abstract
BACKGROUND
Second-look endoscopy after initial therapeutic endoscopy for bleeding peptic ulcer disease (PUD) may decrease the risk of rebleeding; however, it is not recommended routinely. Understanding conditions under which second-look endoscopy is beneficial might be useful for clinical decision making.
METHODS
Using a decision model, literature-based probabilities, and Medicare reimbursement costs, we compared routine second-look endoscopy to no second-look endoscopy. We measured rebleeding, need for surgery, hospital mortality, and costs, and calculated the cost to avoid each outcome, expressed as the number needed to treat (NNT), along with the cost per outcome prevented.
RESULTS
In the base case, routine second-look endoscopy reduced rebleeding from 16% to 10% (NNT=16) but had no effect on other outcomes. The cost to prevent one case of rebleeding was nearly $13,000. Threshold analysis revealed a rebleeding threshold of 31% to neutralize the cost difference between routine second-look endoscopy and no routine second-look endoscopy. If routine second-look endoscopy were 100% effective in preventing rebleeding, then the rebleeding threshold for cost neutrality would be 17.5%. When rebleeding risks after the index endoscopy and second-look endoscopy were simultaneously considered, the cost per bleed prevented ranged from a cost savings of $165 when the respective risks were 25% and 5%, to a cost of nearly $33,000 when the risks were 20% and 15%.
CONCLUSION
The results suggest that routine second-look endoscopy is not indicated following therapeutic endoscopy for bleeding PUD. However, if rebleeding risk is 31% or greater, then routine second-look endoscopy reduces this risk at no additional cost.
Keywords: gastrointestinal bleeding, peptic ulcer disease, endoscopy
INTRODUCTION
A “second-look” endoscopy refers to the performance of an upper endoscopy usually within 16-24 hours of an index endoscopy that successfully treats a bleeding peptic ulcer containing high-risk stigmata such as active bleeding or a visible vessel.1 Second-look endoscopy is performed despite the absence of clinical evidence of continued bleeding or rebleeding to identify and treat persistent high-risk stigmata with the goal of preventing rebleeding and its downstream events, including comorbid complications, the need for surgery, and bleeding-related mortality.
The basis for second-look endoscopy stems from several randomized trials and meta-analyses of the trials. The clinical trials show a variable reduction in rebleeding from 0% to 24%, an inconsistent effect on the need for surgery, and no reduction in mortality.2-9 Four meta-analyses of varying subsets of the trials show a mean absolute risk reduction of 8% for rebleeding, and no effect on bleed-related surgery and mortality.10-13 On the basis of this body of evidence, recently updated guidelines state that second-look endoscopy is not routinely recommended following initial successful therapeutic endoscopy. The guideline statement reflects an overall recommendation based on cumulative evidence. However, it does not identify conditions under which second-look endoscopy should be considered or performed. Intuitively, these conditions would include a high risk of rebleeding following the index endoscopy and high effectiveness of the second-look procedure. Given the clinical heterogeneity of this issue, we sought to identify probabilistic conditions under which second-look endoscopy should at least be considered.
MATERIALS AND METHODS
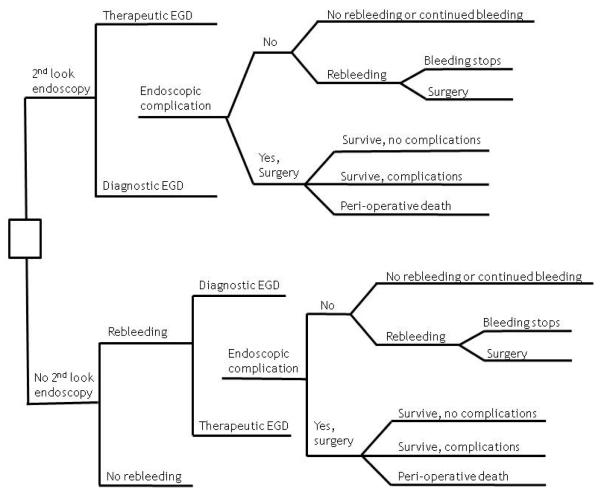
We used decision analysis to create a decision tree, assign probabilities and costs, determine the optimal strategy, and test the stability of the results through use of sensitivity analysis. Figure 1 displays the tree and clinical strategy, the only decision for which is whether to perform a second-look endoscopy. If a second-look endoscopy is performed, therapeutic methods may or may not be used, and an endoscopic complication may or may not occur (only perforation and endoscopy-induced hemorrhage were considered). If there is no complication, further ulcer bleeding may or may not occur. If rebleeding occurs, it may stop on its own or after another endoscopy, or if bleeding continues, surgical management of the bleeding occurs. If a complication occurs (only perforation and hemorrhage were considered) or if bleeding continues after a second therapeutic endoscopy, patients are managed surgically where they either die peri-operatively or survive with or without post-operative complications. On the other hand, if no second-look endoscopy is performed, patients may or may not rebleed. If rebleeding occurs, a second endoscopy, which may be diagnostic or therapeutic, is performed. The remainder of the subtree in the lower half of the figure is identical to that in that upper half. The model does not consider the effects of minor endoscopic complications, comorbid events that result from episodes of recurrent bleeding, or the adjunctive use of therapy with proton pump inhibitors, which have been shown to decrease the risk of recurrent bleeding from high-risk ulcer stigmata independently of therapeutic endoscopy.14
Figure 1.
Decision Tree for “Second-Look” Endoscopy
The study population is one with high-risk ulcer bleeding and initial successful therapeutic endoscopy with hemostasis. The perspective is that of the health care system, for which all direct costs (but no indirect costs) are considered.15 The primary outcomes are rebleeding, the need for surgery, mortality, cost, and the cost to prevent one case of rebleeding; secondary outcomes include endoscopic and surgical complications.
We used the published literature to obtain probabilities of rebleeding following initial hemostasis; reapplication of therapeutic endoscopy for non-bleeding, high-risk stigmata, and for rebleeding; rebleeding following a second therapeutic endoscopy for no bleeding and for bleeding; and major complications following therapeutic endoscopy. We assumed a base case mortality risk of 5%, and surgical survival with and without complications of 10% and 85%, respectively (Table 1). Base case costs or reimbursements were obtained from the Center for Medicare and Medicaid Services or the published literature for diagnostic and therapeutic endoscopy, surgery, and death (Table 1).
Table 1.
Base Case Probabilities & Costs
| Variable | Probability (range) | Sources |
|---|---|---|
| Rebleeding following initial hemostasis | 16% (5-30%) | 3, 4, 6, 8, 9, 11, 24-26 |
| Re-application of T-EGD (no bleeding) | 35% (20-50%) | |
| Rebleeding after 2nd T-EGD (no bleeding) | 10% (0-50%) | 4, 11 |
| Re-application of T-EGD (bleeding) | 40% (10-70%) | 27 |
| Continued bleeding after 2nd T-EGD (bleeding) | 15% (0-50%) | Expert opinion |
| Major complication after T-EGD | 1% (0-4%) | 27 |
| Major complication after D-EGD | 0.5% (0-2%) | 28 |
| Survive surgery without complications | 85% (60-100%) | Expert opinion |
| Survive surgery with complications | 10% (0-20%) | Expert opinion |
| Peri-operative mortality | 5% (0-20%) | Expert opinion |
| Cost (range) | ||
| Diagnostic (D-) EGD | $732 ($300 - $1500) | Medicine reimbursed |
| Therapeutic (T-) EGD | $868 ($400 - $1,500) | Medicine reimbursed |
| Hospital (no rebleeding) | $3,780 ($2,000 - $7,000) | 29 |
| Hospital (rebleeding) | $6,080 ($4,000 - $10,000) | 29 |
| Surgery (perf/bleed) | $3,850 ($2,000 - $8,000) | Medicine reimbursed |
| Surgical complication | $11,600 (47000-$20,000) | Medicine reimbursed |
| Death | $250,000 ($0 - $500,000) | Expert opinion |
We performed one-way sensitivity analysis on all variables, the ranges for which were determined by the authors a priori based on judgment. Clinically relevant two-way sensitivity analyses were performed as well. As part of the sensitivity analyses, we considered the difference between the risk of rebleeding after index therapeutic endoscopy and the risk of bleeding after second-look endoscopy, and did so at different levels of effectiveness of the second-look endoscopy strategy. For this analysis, we assumed an absolute risk difference of at least 5% in favor of the second-look endoscopy strategy, and as high as 20%. We reported those outcomes that favor second-look endoscopy and calculated the cost per bleed prevented at different levels of rebleeding risk and effectiveness of the second-look endoscopy strategy. The model was developed using TreeAge Pro2010 by TreeAge Software (Williamstown, MA). The primary decision analyses were performed in TreeAge Pro2010. Additional analyses were preformed in Microsoft Excel by (Microsoft, Inc., Redmond, WA).
RESULTS
Base Case and Threshold Analyses
In the base case, a second-look endoscopy reduced the risk of rebleeding after the index therapeutic endoscopy from 16% to 10%. However, all other outcomes favored forgoing a second look, which resulted in fewer complications, less surgery, lower mortality and lower costs, although the clinical magnitude of the differences for all outcomes was small (Table 2). The 6% absolute difference in rebleeding translates into a number needed to treat – in this case, the number of persons who undergo a second-look endoscopy - of 16 to prevent one case of rebleeding, and at a cost per case prevented of $12,950.
Table 2.
Base Case Results
| Outcomes | 2nd Look | No 2nd Look |
|---|---|---|
| Rebleeding | 10% | 16% |
| Surgery | 3.1% | 1.7% |
| Endoscopic complications | 0.7% | 0.2% |
| Surgical complications | 0.3% | 0.2% |
| Hospital mortality | 0.15% | 0.08% |
| Costs | $5,376 | $4,599 |
Number needed to scope to prevent one case of rebleeding = 16 Cost per case of rebleeding prevented = $12,950
A threshold analysis for rebleeding following an index endoscopy revealed that the only outcome benefiting from a second-look endoscopy was rebleeding, as long as the risk of rebleeding exceeded 10%, though the amount of benefit was potentially negligible. The cost threshold for rebleeding was 31%, meaning that rebleeding after the index endoscopy had to exceed 31% to favor doing a second look endoscopy from a cost perspective (Table 3). For surgery, surgical complications and mortality, the index rebleeding threshold had to exceed 29% to favor doing a second-look endoscopy for these three outcomes. For endoscopic complications, there was no rebleeding threshold that favored performing second-look endoscopy. For rebleeding following second-look endoscopy, which was 10% in the base case, the only outcomes that benefited from a second-look endoscopy was rebleeding as long as the risk of rebleeding following a second-look endoscopy was less than 16%; the magnitude of the benefit was potentially negligible, depending on how much less than 16% the risk was. For the second-look strategy to benefit the need for surgery, surgical complications, and hospital mortality, the risk of rebleeding after a second-look endoscopy must be 4% or lower. There were no rebleeding thresholds for endoscopic complications and cost that favored performing second-look endoscopy.
Table 3.
Sensitivity Threshold Analysis
| Rebleeding following index T-EGD 16% (range 5%-30%) |
Rebleeding following 2nd Look Endoscopy 10% (0-50%) |
|||
|---|---|---|---|---|
| Outcomes | 2nd Look | Threshold | 2nd Look | Threshold |
| Rebleeding | Yes | 10% | Yes | 16% |
| Surgery | No | 29% | No | 4% |
| Surgical complications |
No | 29% | No | 4% |
| Hospital mortality | No | 29% | No | 4% |
| Cost | No | 31% | No | ---------- |
| Endoscopic complications |
No | ---------- | No | ---------- |
Sensitivity Analysis
In sensitivity analysis, we considered simultaneously the two rebleeding risks, specifically, rebleeding after the index endoscopy and after second-look endoscopy (Table 4). When differences between the two risks were small, only rebleeding favored second-look endoscopy, with the magnitude of benefit dependent on the difference between the two rebleeding risks. As the risk for rebleeding after the index endoscopy increases, other outcomes, including the need for surgery and mortality, favor second-look endoscopy, although this also depends on the effectiveness of the second-look endoscopy.
Table 4.
Sensitivity Analysis: Risks of Rebleeding
| Absolute Difference in Risks of Rebleeding (Index – 2nd LE) | ||||
|---|---|---|---|---|
| Rebleeding after 2nd look endoscopy |
5% | 10% | 15% | 20% |
| 5% | Rebleeding $14,700 |
Rebleeding $4,790 |
Rebleeding $1,486 Surgery Mortality Complications |
All outcomes favor 2nd look ($165 ) |
| 10% | Rebleeding $16,560 |
Rebleeding $5,720 |
Rebleeding $2,107 |
Rebleeding $300 Surgery Mortality Complications |
| 15% | Rebleeding $32,840 |
Rebleeding $6,660 |
Rebleeding $2,733 |
Rebleeding $770 |
The corresponding costs per case of rebleeding prevented range from nearly $33,000 when the risk of rebleeding after second-look endoscopy is 15% and the difference between the two rebleeding risks is small to a small cost savings when the risk of rebleeding after second-look endoscopy is low and the difference between the two risks is large (Table 4).
DISCUSSION
The main finding of this analysis is that routine second-look endoscopy of initially successful treatment of high-risk acute upper GI non-variceal bleeding is unnecessary. Our results suggest that the absolute risk reduction for rebleeding is small and, in the current era of high-dose intravenous PPI therapy, may be non-existent. Further, the cost to prevent one rebleeding episode is nearly $13,000, and may be even higher depending on the true magnitude of effect. These findings support current guideline recommendations that routine second-look endoscopy is not warranted.
In sensitivity analysis, we found that two scenarios tended to favor consideration of second-look endoscopy, one of which is a high-risk for rebleeding following the index therapeutic endoscopy. In the base case, the risk of rebleeding is 16%, while second-look endoscopy reduces the risk to 10%, a modest benefit with a number needed to treat of 16. When the risk of rebleeding is 31% or higher, however, second-look endoscopy adds no cost or is cost-saving. For surgery and mortality, the corresponding rebleeding threshold is 29%.
The other scenario favoring a second-look endoscopy is a high level of effectiveness for the second-look procedure itself. With a rebleeding risk of 16% and a reduction in the risk for rebleeding following the second-look procedure to 4% or less, a second-look endoscopy is favored for all outcomes, although the absolute benefit is small.
Our findings are consistent with those of Spiegel and colleagues,17 who compared the cost-effectiveness of four strategies: 1) repeat endoscopy only for evidence of rebleeding; 2) use intravenous PPIs after initial hemostasis and repeat endoscopy only for rebleeding; 3) second-look endoscopy for everyone, and; 4) selective second-look endoscopy for high-risk patients only, with the Baylor Bleeding Score18 used to determine high-risk. In this analysis, the baseline risk of rebleeding was 18.8% following initial hemostasis, 13.2% with intravenous PPI therapy, and 11% with second-look endoscopy. The selective second-look strategy was both most effective and least costly, with a cost per case of rebleeding prevented of $7,262 and number needed to treat of 10. The intravenous PPI strategy required 50% fewer endoscopy than the other strategies, and became the dominant strategy when the risk of rebleeding was less than 9%.17
This current study has at least two important limitations that require comment. First, while we obtained probabilities from the randomized trials of routine second-look endoscopy, most of these trials pre-date the use of therapy with intravenous proton pump inhibitors. It is possible that the probability of rebleeding following initial therapeutic endoscopy from those trials is higher than the risk of rebleeding in current clinical practice. In the analysis by Spiegel and colleagues, the rebleeding risk was 13.2% and 18.8% with and without intravenous PPI, respectively. In randomized trials and meta-analyses of the trials, the risk of rebleeding in patients with endoscopically treated high-risk bleeding ranges from 5.9% to 22.8%,14, 19-22 with at least one trial showing no difference between standard and intensive regimens.19 However, in a recent randomized controlled trial from China just 3% of patients in the high-dose group rebled as compared to 16% in the standard-dose group, a difference that was statistically significant and clinically important, although the generalizability to other ethnic and racial groups is uncertain.23 In this analysis, the base case probability for risk of rebleeding was 16%, and was10% with second-look endoscopy, point estimates that are within the range of these recent studies. In sensitivity analysis, we assumed an absolute risk difference in favor of second look endoscopy of at least 5%. However, if current rebleeding risk is truly lower than those in the second-look endoscopy trials, then the lower baseline of rebleeding would reduce or possibly even eliminate any risk reduction due to second-look endoscopy.
A second limitation of this analysis is that we did not consider any additional risks or costs resulting from the effect of rebleeding on comorbidity. Since rebleeding episodes may result in new comorbid conditions (e.g., aspiration pneumonia, stroke, myocardial infarction, renal failure) or exacerbate pre-existing ones (e.g., congestive heart failure, renal insufficiency), there may be more value to preventing an episode of rebleeding than this analysis suggests. Related to this issue is the fact that we did not consider the effect of second-look endoscopy on length of hospital stay. Second-look endoscopy alone would not prolong hospital stay. If anything, it might provide reassurance of a low-risk of rebleeding, in which case earlier hospital discharge might occur, with a reduction in total cost.
There are essentially two reasons to consider performing a second-look endoscopy. One reason is when the index exam is incomplete, either because of obscuring intraluminal contents such as blood and blood clots or because of endoscopist uncertainty about the technical efficacy of the applied hemostatic techniques. These circumstances, however, may be considered peripheral, if not separate, from a true second look endoscopy, which is more properly elective and technically should not include an index endoscopy that is either diagnostically or therapeutically incomplete. The second reason is more germane to this issue: the risk of rebleeding is high enough to warrant the repeat endoscopy. Risk factors for rebleeding include a large initial bleeding episode as evidenced by shock or hypotension at baseline, a large ulcer (2 cm or larger), comorbid disease, fresh blood in the stomach, active bleeding at the time of index endoscopy, and ulcers on the high lesser curve or posterior wall of the duodenal bulb,16, 24 which are locations where therapeutic endoscopy may be technically challenging.
In conclusion, our results support the practice of forgoing routine second-look endoscopy following therapeutic endoscopy for bleeding peptic ulcer disease. However, if the risk for rebleeding after the index procedure is considered high, then a second-look endoscopy reduces this risk. In the current analysis, when the risk of rebleeding was 31% or greater, the additional procedure added no cost or was cost saving. Subsequent research should consider second-look endoscopy in patients at highest risk for rebleeding, either by clinical criteria or by scoring system, who receive either high-dose or standard-dose intravenous PPI therapy following successful hemostasis.
Acknowledgments
Supported in part by NIH grant DK 002756 (Dr. Imperiale)
Glossary
- TFI
Study concept and design
- TFI
acquisition of data
- TFI, NK
analysis and interpretation of data
- TFI
drafting of the manuscript
- NK, TFI
critical revision of the manuscript
- NK
statistical analysis
Footnotes
Presented in part at the 2010 annual, international meetings of the American College of Gastroenterology, October 20, 2010 San Antonio, TX and Society for Medical Decision Making, October 24, 2010, Toronto, Ontario, Canada
Neither Dr. Imperiale nor Dr. Kong has a conflict of interest with the topic of this manuscript
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010 Jan 19;152(2):101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 2.Lin CK, Lai KH, Lo GH. The value of second-look endoscopy after endoscopic injections for bleeding peptic ulcer. Gastroenterology. 1996;110:A177. al e. [Google Scholar]
- 3.Chiu PW, Joeng HK, Choi CL, Kwong KH, Lam SH. The effect of scheduled second endoscopy again intravenous high dose adjunctive omeprazole infusion in preventing peptic ulcer rebleeding - a prospective randomized study. Gastroenterology. 2006;130(S2):A121. [Google Scholar]
- 4.Chiu PW, Lam CY, Lee SW, et al. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003 Oct;52(10):1403–1407. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eill C. Scheduled endoscopic retreatment vs. single injuections therapy in bleeding gastroduodenal ulcers: Results of a multicenter study. Gastrointest Endosco. 1998;47:AB83. [Google Scholar]
- 6.Messmann H, Schaller P, Andus T, et al. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: a prospective, randomized controlled trial. Endoscopy. 1998 Sep;30(7):583–589. doi: 10.1055/s-2007-1001360. [DOI] [PubMed] [Google Scholar]
- 7.Rutgeerts P, Rauws E, Wara P, et al. Randomised trial of single and repeated fibrin glue compared with injection of polidocanol in treatment of bleeding peptic ulcer. Lancet. 1997 Sep 6;350(9079):692–696. doi: 10.1016/s0140-6736(97)03233-9. [DOI] [PubMed] [Google Scholar]
- 8.Saeed ZA, Cole RA, Ramirez FC, Schneider FE, Hepps KS, Graham DY. Endoscopic retreatment after successful initial hemostasis prevents ulcer rebleeding: a prospective randomized trial. Endoscopy. 1996 Mar;28(3):288–294. doi: 10.1055/s-2007-1005455. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva C, Balanzo J, Torras X, Soriano G, Sainz S, Vilardell F. Value of second-look endoscopy after injection therapy for bleeding peptic ulcer: a prospective and randomized trial. Gastrointest Endosc. 1994 Jan-Feb;40(1):34–39. doi: 10.1016/s0016-5107(94)70006-0. [DOI] [PubMed] [Google Scholar]
- 10.Barkun A, Wyse J, Romagnuolo J, Gralnek I, Bardou M. Should we be performing routine second-look endoscopy in acute peptic ulcer bleeding in 2009? A meta-analysis [Abstract] Gastroenterology. 2009:134. [Google Scholar]
- 11.Chiu P-Y, Lau T-S, Kwong K-H, Suen D-K, Kwok S-Y. Impact of programmed second endoscopy with appropriate re-treatment on peptic ulcer bleeding: A systematic review. Ann Coll Surg. 2003;7:106–115. [Google Scholar]
- 12.Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: Is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003 Jan;57(1):62–67. doi: 10.1067/mge.2003.48. [DOI] [PubMed] [Google Scholar]
- 13.Tsoi KK, Chan HC, Chiu PW, Pau CY, Lau JY, Sung JJ. Second-look endoscopy with thermal coagulation or injections for peptic ulcer bleeding: a meta-analysis. J Gastroenterol Hepatol. 2010 Jan;25(1):8–13. doi: 10.1111/j.1440-1746.2009.06129.x. [DOI] [PubMed] [Google Scholar]
- 14.Khuroo MS, Farahat KL, Kagevi IE. Treatment with proton pump inhibitors in acute non-variceal upper gastrointestinal bleeding: a meta-analysis. J Gastroenterol Hepatol. 2005 Jan;20(1):11–25. doi: 10.1111/j.1440-1746.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 15.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996 Oct 23-30;276(16):1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 16.Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008 Oct;103(10):2625–2632. doi: 10.1111/j.1572-0241.2008.02070.x. quiz 2633. [DOI] [PubMed] [Google Scholar]
- 17.Spiegel BM, Ofman JJ, Woods K, Vakil NB. Minimizing recurrent peptic ulcer hemorrhage after endoscopic hemostasis: the cost-effectiveness of competing strategies. Am J Gastroenterol. 2003 Jan;98(1):86–97. doi: 10.1111/j.1572-0241.2003.07163.x. [DOI] [PubMed] [Google Scholar]
- 18.Saeed ZA, Ramirez FC, Hepps KS, Cole RA, Graham DY. Prospective validation of the Baylor bleeding score for predicting the likelihood of rebleeding after endoscopic hemostasis of peptic ulcers. Gastrointest Endosc. 1995 Jun;41(6):561–565. doi: 10.1016/s0016-5107(95)70191-5. [DOI] [PubMed] [Google Scholar]
- 19.Andriulli A, Loperfido S, Focareta R, et al. High- versus low-dose proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding: a multicentre, randomized study. Am J Gastroenterol. 2008 Dec;103(12):3011–3018. doi: 10.1111/j.1572-0241.2008.02149.x. [DOI] [PubMed] [Google Scholar]
- 20.Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007 Dec;11(51):iii–iv. 1–164. doi: 10.3310/hta11510. [DOI] [PubMed] [Google Scholar]
- 21.Mesihovic R, Vanis N, Mehmedovic A, Gornjakovic S, Gribajcevic M. Proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding. Med Arh. 2009;63(6):323–327. [PubMed] [Google Scholar]
- 22.Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009 Apr 7;150(7):455–464. doi: 10.7326/0003-4819-150-7-200904070-00105. [DOI] [PubMed] [Google Scholar]
- 23.Chan WH, Khin LW, Chung YF, Goh YC, Ong HS, Wong WK. Randomized controlled trial of standard versus high-dose intravenous omeprazole after endoscopic therapy in high-risk patients with acute peptic ulcer bleeding. Br J Surg. 2011 May;98(5):640–644. doi: 10.1002/bjs.7420. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CL, Lin CH, Kuo CJ, et al. Predictors of rebleeding and mortality in patients with high-risk bleeding peptic ulcers. Dig Dis Sci. 2010 Sep;55(9):2577–2583. doi: 10.1007/s10620-009-1093-9. [DOI] [PubMed] [Google Scholar]
- 25.Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000 Aug 3;343(5):310–316. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 26.Lee SD, Kearney DJ. A randomized controlled trial of gastric lavage prior to endoscopy for acute upper gastrointestinal bleeding. J Clin Gastroenterol. 2004 Nov-Dec;38(10):861–865. doi: 10.1097/00004836-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999 Mar 11;340(10):751–756. doi: 10.1056/NEJM199903113401002. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995 Feb;90(2):206–210. [PubMed] [Google Scholar]
- 29.Viviane A, Barkun AN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11(1):1–3. doi: 10.1111/j.1524-4733.2007.00208.x. [DOI] [PubMed] [Google Scholar]