Abstract
The strongest serological correlate for lupus nephritis is antibody to double-stranded DNA although the mechanism by which anti-DNA antibodies initiate lupus nephritis is unresolved. Most recent reports indicate that anti-DNA must bind chromatin in the glomerular basement membrane or mesangial matrix to form glomerular deposits. Here we determined whether direct binding of anti-DNA antibody to glomerular basement membrane is critical to initiate glomerular binding of anti-DNA in experimental lupus nephritis. Mice were co-injected with IgG monoclonal antibodies or hybridomas with similar specificity for DNA and chromatin but different IgG subclass and different relative affinity for basement membrane. Only anti-DNA antibodies that bound basement membrane bound to glomeruli, activated complement, and induced proteinuria whether injected alone or co-injected with a non-basement membrane-binding anti-DNA antibody. Basement membrane-binding anti-DNA antibodies co-localized with heparan sulfate proteoglycan in glomerular basement membrane and mesangial matrix but not with chromatin. Thus, direct binding of anti-DNA antibody to antigens in the glomerular basement membrane or mesangial matrix may be critical to initiate glomerular inflammation. This may accelerate and exacerbate glomerular immune complex formation in human and murine lupus nephritis.
Introduction
The contribution of anti-DNA antibody to glomerulonephritis in mouse (1) and human (2) systemic lupus erythematosus (SLE) is well established. Although anti-double-stranded DNA (dsDNA) antibody is the best serological correlate for lupus nephritis (3, 4), the frequent lack of correlation between serum anti-dsDNA and glomerulonephritis is a long recognized conundrum in the clinical evaluation of individual SLE patients (3, 5, 6). The lack of correlation between anti-dsDNA and lupus nephritis within individual patients may be a consequence of how anti-dsDNA antibodies bind in the glomerulus and initiate glomerulonephritis (6), a process not yet fully resolved (7). Mechanisms proposed to explain glomerular deposition of anti-DNA antibody include glomerular binding of soluble immune complexes of nucleosomes and IgG anti-DNA (2, 8–10), in situ formation of immune complexes when anti-DNA antibody binds to chromatin that has bound to glomerular basement membrane (GBM) or mesangial matrix (MM) (11–17), and direct binding of anti-DNA antibody that cross-reacts with GBM or cell surface antigens (18–25). Recent morphologic studies (12–14, 16) have identified chromatin and IgG within the glomerular subendothelial and subepithelial electron dense deposits (EDS) in nephritic kidneys from lupus patients (26) and lupus-prone mice (27). The recent results were interpreted to indicate that anti-DNA antibody could form glomerular deposits only when bound to chromatin or nucleosomes (28–30).
The present experiments were designed to test the hypothesis that initial glomerular binding of anti-DNA antibody in lupus nephritis is a function of direct, cross-reactive binding to glomerular antigens, particularly in GBM or MM, and independent of DNA, nucleosomes, or chromatin. The experiments took advantage of a panel of anti-DNA monoclonal antibodies (mAbs) with similar relative affinities for DNA and chromatin but different relative affinities for basement membrane (BM) antigens in GBM and MM. Only anti-DNA mAbs that also bound BM antigens bound glomeruli in vivo and induced proteinuria. Glomerular binding of the anti-DNA mAbs was independent of DNA, nucleosomes, or chromatin. The results may explain why some anti-DNA mAbs are very effective at inducing lupus nephritis, but others are not. Similarly, the results may help to explain why SLE patients with similar serum anti-dsDNA antibody may have different susceptibility for lupus nephritis.
Results
In vitro binding of anti-DNA mAb to BM
Culture supernatants from 69 autoimmune anti-DNA mAbs from eight different (NZB × NZW)F1 mice (BWF1) were randomly selected for analysis (Table 1). Total IgG and relative affinity for binding to ssDNA, dsDNA, chromatin, and BM were quantified for each supernatant. The mAbs were stratified by relative affinity for BM into four different specificity groups (Table 1). There is a significant difference among the four specificity groups for competitive binding to ssDNA and dsDNA and direct binding to BM but not for direct binding to chromatin. There is a strong and highly significant correlation between binding to BM and binding to dsDNA and a moderate, highly significant inverse correlation between binding to BM and binding to ssDNA. Anti-DNA mAbs that bound best to dsDNA are generally the mAbs that also bound best to BM. The correlation between BM and chromatin binding, although significant, was low compared to that for BM and dsDNA. The results indicate that mAbs with high relative affinity for dsDNA are more likely to bind BM than mAbs with high relative affinity for ssDNA. The results also indicate that anti-DNA mAb binding to BM is unrelated to relative affinity for chromatin.
Table 1.
Specificity of Monoclonal Antibodies
| Groupa | Number mAbs | Competitive ELISA (ng/ml competitor)a
|
Direct ELISA (ng/ml IgG)b
|
|||
|---|---|---|---|---|---|---|
| ssDNA | dsDNA | DNA | Chromatin | BM | ||
| A | 14 | 111 ± 84d | NI | 75 ± 61 | 826 ± 904 | NB |
| B | 21 | 560 ± 351 | 5 770 ± 2 580 | 6 730 ± 6 110 | 1 810 ± 2 590 | NB |
| C | 18 | 658 ± 302 | 4 200 ± 2 580 | 7 040 ± 5 560 | 81 ± 94 | 5 510 ± 1 490 |
| D | 16 | 957 ± 469 | 1 570 ± 690 | 971 ± 1 990 | 52 ± 60 | 94 ± 780 |
Sixty-nine mAbs were stratified according to BM binding into (A) NB to BM (14 mAb), (B) NB to BM but binding to dsDNA (21 mAb), (C) BM binding with ≥ 1,000 ng/ml IgG (18 mAbs), and (D) BM binding with ≤ 1,000 ng/ml IgG (16 mAbs).
ng/ml competitor is the amount of dsDNA or ssDNA competitor required to produce 50% inhibition of mAb binding to solid phase DNA in a competitive ELISA (24). NI = no inhibition with 10,000 ng/ml competitor.
ng/ml mAb that yields 50% maximum binding in a direct ELISA. NB = no binding with ≥10,000 ng/ml mAb.
The values are means ± 95% confidence intervals. ANOVA among groups for the category of binding to: ssDNA, p= 0.025; dsDNA, p = 0.033; DNA, p = n.s.; chromatin, p = n.s.; BM, p = 3.6 x 10−8. Linear regression with BM-binding as dependent variable (R2 = 0.465, p = 4.3 x 10−8): Chromatin, B = 0.381 and β = 0.290, p = 0.00298; ssDNA, B = −0.496 and β = −.301, p = 0.0022; and dsDNA, B = 0.606 and β = 0.423, p = 0.00010 (B = slope and β = correlation coefficient, PASW Statistics18).
The correlations between mAb binding to DNA and chromatin versus their potential to bind BM were further confirmed with purified mAbs (Table 2). BM binding by purified mAbs was independent of relative binding affinity for dsDNA, chromatin, or nucleosomes since 163p.132, 452s.160, DNA3, and 3H9 mAbs bound nucleosomes and/or chromatin with high relative affinity but bound poorly or not at all to BM. MAb 452s.46 bound dsDNA with high relative affinity but did not bind BM. DNA6 mAb bound chromatin similarly to 163p.132 and DNA3 but unlike 163p.132 and DNA3, DNA6 also bound to BM. MAbs 163p.64, 163p.77, and 163p.124 had 20–650 fold higher relative affinity for BM than for nucleosomes. Binding to BM was also independent of mAb pI. These results further indicate that anti-DNA mAb binding to BM is correlated with dsDNA binding and to lesser extent chromatin binding, but is independent of both for binding to BM.
Table 2.
Monoclonal Antibody Binding to DNA, Chromatin, and Basement Membrane Antigens.
| mAba | Isotypeb | pIc | Direct Binding ELISAd
|
Competitive ELISAd
|
In vivo Activity
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| DNA | Chromatin | Nucleosome | BM | ssDNA | dsDNA | Glomerular Bindinge | Induces Proteinuriaf | |||
|
| ||||||||||
| (ng/ml IgG) | (ng/ml IgG) | (ng/ml IgG) | (μg/ml IgG) | (ng/ml ssDNA) | (ng/ml dsDNA) | |||||
| 163p.64 | 2a | 8.4 | 60 | 8.0 | 19 700 | 30 | 470 | 1 030 | Yes | Yes |
| 163p.77 | 2b | 8.5 | 20 | 30 | 11 500 | 20 | 1 420 | 700 | Yes | Yes |
| 163p.124 | 2a | 8.4 | 30 | 4.0 | 1 880 | 90 | 1 690 | 470 | Yes | Yes |
| DNA6 | 2a | 7.6 | 10 | 10 | NDd | 200 | 2 900 | 1 000 | Yes | Yes |
| DNA5 | 2a | 8.7 | 1 000 | 10 | ND | 3 380 | 1 500 | 10 800 | Yes | Yes |
| 163p.132 | 2b | 8.5 | 50 | 10 | 50 | 8 600 | 660 | NId | Nog | Nog |
| DNA3 | 2a | 6.5 | 11 000 | 10 | ND | NBd | 54 | 1 600 | No | No |
| 452s.46 | 2b | 7.6 | 10 | 200 | 2 300 | NB | 730 | 490 | No | No |
| 452s.160 | 2a | 7.3 | 70 | 90 | ND | NB | 80 | 4 400 | No | ND |
| 3H9 | 2b | 8.3 | 4 720 | 50 | ND | NB | ND | ND | Noh | Noh |
163p.64, 77, 124 and DNA6: Group D, Table 1; DNA 5 and 163p.132: Group C, Table 1; 452s.46, 160 and DNA3: Group B, Table 1.
IgG subclass of hybridoma mAb.
Isoelectric point of the respective mAb (calculated using the Swiss Institute of Bioinformatics ExPASy pI calculation tool, http://web.expasy.org/compute_pi/)
Table 1 legend. ND = not done. NB = no binding. NI = no inhibition.
Glomerular binding of mAb was determined by immunofluorescence on kidney cryosections 24 hrs after mice were injected with 1 mg purified mAb(s).
Summary of results presented in Table 3.
MAb 163p.132, IgG2b, produced minimal glomerular fluorescence and no proteinuria 5 days after injection of hybridoma cells but readily detected immunofluorescence and proteinuria 8 days after injection.
Gilkeson et al. (36). Only 2/5 mice had glomerular-bound IgG, and the glomerular disease score was not different from the negative control.
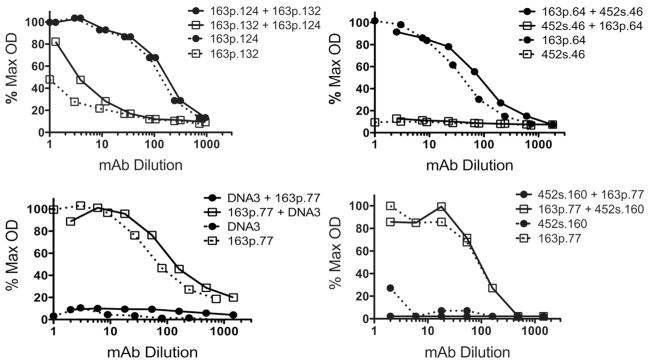
Since previous investigators had concluded that anti-dsDNA mAb binding to BM was consequential to nucleosome contamination of hybridoma supernatants and purified mAbs (10), we performed co-incubation assays to insure that differential binding of anti-dsDNA mAbs to BM was not simply a consequence of contaminating chromatin in some but not all hybridoma supernatants. When hybridoma supernatants of mAb pairs 163p.132 and 163p.124, 452s.46 and 163p.64, 163p.77 and DNA3, and 163p.77 and 452s.160 were assayed for binding to BM, only the mAb that bound to BM in the individual assays, 163p.124, 163p.64, and 163p.77, bound to BM when co-incubated with a non BM-binding mAb (Fig. 1 and Table 2). MAb 163p132 does bind BM but with 100–500-fold less relative affinity than mAbs 163p.64, 77, and 124. The results in Fig 1 corroborate the conclusion that anti-DNA mAb binding to BM is independent of dsDNA or chromatin.
Figure 1.
Supernatant mAbs that do not bind BM when assayed alone do not bind BM when combined with supernatant mAbs that do bind BM. Supernatant mAbs from the indicated hybridoma pairs were assayed by direct ELISA for BM binding. Titration curves represent serial dilution of supernatants assayed independently for IgG2a or IgG2b binding to BM: solid circles, IgG2a; open squares, IgG2b; solid lines, IgG2a and IgG2b mAbs co-incubated; and broken lines, IgG2a or IgG2b mAb incubated alone. Supernatant concentrations of mAbs: 163p.124, 12.1 μg/ml; 163p.132, 34.7 μg/ml; DNA3, 29.1 μg/ml; 163p.77, 23.5 μg/ml; 163p.64, 10.0 μg/ml; 452s.46, 6.4 μg/ml; and 452s.160, 18.7 μg/ml. Maximum OD405 = 2.600.
MAb 163p.64 was tested by direct ELISA for binding to individual components of BM, including laminin, perlecan, entactin, and agrin. The mAb bound perlecan, entactin, and agrin (59, 250, and 220 ng IgG/ml, respectively, for 50% maximum binding) but not laminin. The recombinant agrin did not include the amino-terminal extracellular matrix interaction domains (R&D Systems). Binding to collagen IV was not tested. The results indicate that a BM binding mAb may also bind to some but not all of the individual components of GBM.
In vivo glomerular binding of anti-DNA mAbs
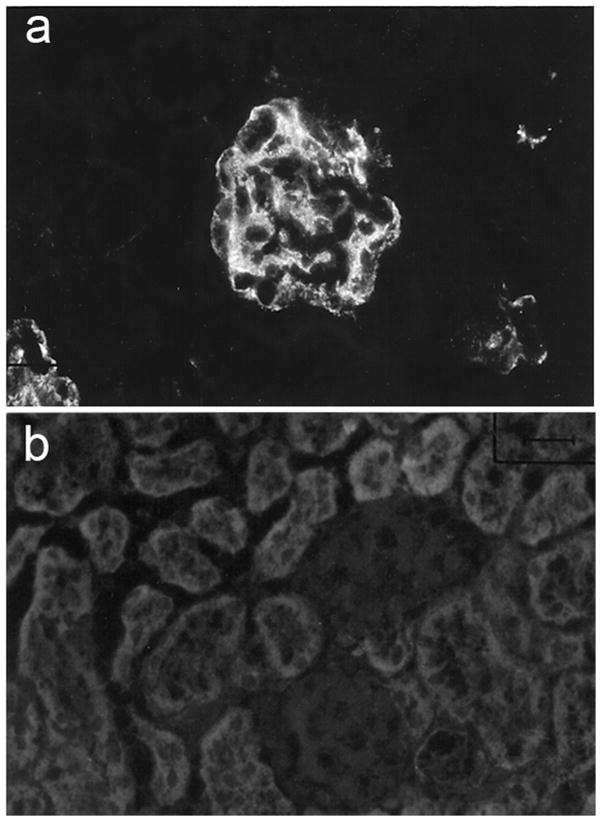
Six purified mAbs were further tested for glomerular binding when injected into non autoimmune-prone BALB/c mice alone or co-injected with a mAb with different BM binding potential and different IgG subclass. The co-injected pairs were 163p.77, IgG2b with 452s.160, IgG2a; 163p.64, IgG2a with 452s.46, IgG2b; and 163p.124, IgG2a with 163p.132, IgG2b (Table 2). The co-injection experiments were included to exclude the possibility that co-purified chromatin or nucleosomes influenced glomerular binding (10). Only mAbs that bound BM by ELISA, 163p77, 16p.64, and 163p.124, bound glomeruli in vivo when injected either alone or co-injected with a mAb of different IgG subclass (Table 2 and Fig. 2). Glomerular binding was unrelated to relative affinity of the mAbs for DNA, chromatin, or mononucleosomes or to IgG subclass.
Figure 2.
Detection of glomerular (a) IgG2b, 163p.77 but not (b) IgG2a, 452s.160 in kidney serial cryosections 24 hours after co-injecting 1 mg of each purified mAb into a BALB/c mouse. Serial cryosections had granular IgG2b but no IgG2a within MM. Mice injected with 163p.64, IgG2a and 452s.46, IgG2b (see Fig. 3a and c) and 163p.124, IgG2a and 163p.132, IgG2b had IgG2a but no IgG2b staining. Results were similar in replicate mice.
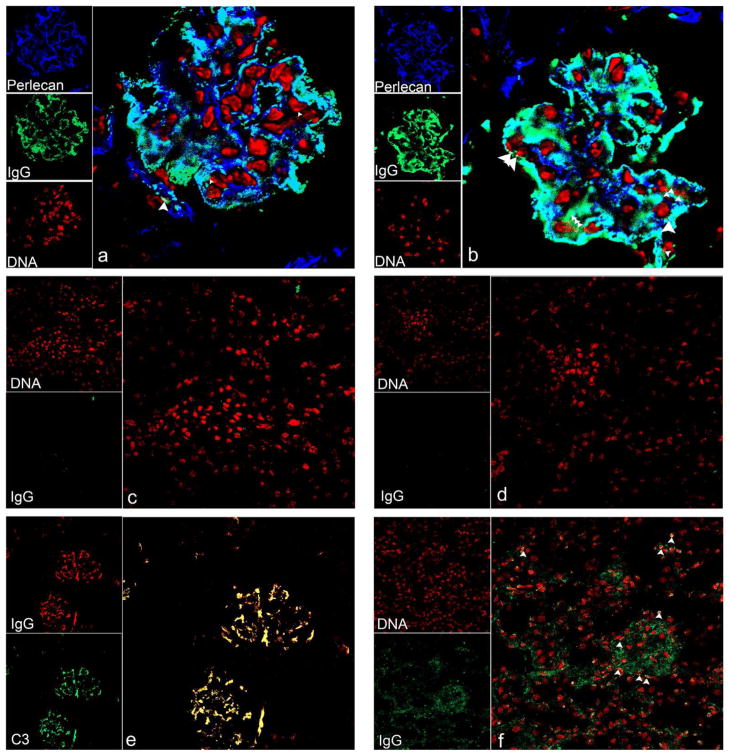
Confocal microscopy indicated that 163p.64 mAb chronically injected over a 3-month period was co-localized with heparan sulfate proteoglycan (HSPG) in GBM and MM but minimally with chromatin (Fig. 3a). Glomerular IgG was also co-localized with HSPG and minimally with chromatin in autoimmune BWF1 kidneys (Fig. 3b). As expected, there was no glomerular binding of anti-DNA mAb 452s.46 after similar 3-months chronic injection (Fig. 3c). Complement C3 was co-localized with mAb 163p.64 in glomeruli from chronically injected mice (Fig. 3e). These results indicate that BM-binding anti-DNA antibodies also bind directly to MM and GBM antigens independently of DNA, chromatin, or nucleosomes and initiate complement activation. The small regions of chromatin and IgG co-localization and perlecan, chromatin, and IgG co-localization in the kidneys from BALB/c mice chronically injected with 163p.64 mAb (Fig. 3a) were more numerous in kidneys from autoimmune BWF1 (Fig. 3b). Those regions of co-localization may be the glomerular EDS identified by electron microscopy in kidneys from autoimmune BWF1 (13) and anti-DNA mAb-injected mice (11).
Figure 3.
Confocal micrographs of kidney cryosections from an autoimmune BWF1 mouse or BALB/c mice injected with purified anti-DNA mAb. a and e) 100 μg 163p.64 mAb twice weekly for 3 months; b) uninjected BWF1, c) 100 μg 452s.46 mAb twice weekly for 3 months; d) uninjected BALB/c; and f) BALB/c with 163p.132 hybridoma-induced ascites 8 days after hybridoma injection. Images a and b show chromatin as red, perlecan in GBM and MM as dark blue, and IgG as green. Co-localization of IgG with HSPG is clearly identified as turquoise; co-localization of IgG with chromatin, yellow; and co-localization of IgG and chromatin with HSPG, white. The large white arrowheads in a and b indicate areas of IgG, chromatin, and perlecan co-localization. Small arrowheads indicate IgG and chromatin co-localization. Image e shows IgG as red and C3 as green with co-localization of 163p.64 mAb and C3 as yellow. Confocal images a and b: 512 pxels2, 180 nm/pixel (92 μm2), optical sections collected at 0.6 μm intervals; c–f: 512 pixels2, 450 nm/pixel (230 μm2), optical sections collected at 0.8 μm intervals (c–e) and 0.5 μm (f). All images are from optical sections near the center of respective z-stacks. Replicate mice yielded similar results.
Only BM-binding anti-DNA mAbs induce proteinuria in non autoimmune-prone mice
Ascites tumors were induced in non-autoimmune BALB/c mice by injecting hybridoma cells either individually or as co-injected pairs, one producing IgG2a and the other, IgG2b (Table 3). Only mice injected with hybridomas producing mAbs that bound BM, 163p.64, 77, or 124 or DNA 5 or 6, had glomerular-bound IgG of the expected IgG subclass and moderate to severe proteinuria 5 days after hybridoma injection. Mice injected with 163p.64 or 163p.124, IgG2a hybridoma cells with either 452s.46 or 163p.132, IgG2b hybridoma cells had only glomerular-bound IgG2a. Glomerular IgG binding was not IgG2 subclass dependent, nor was glomerular binding simply a correlate of circulating mAb titers. The average serum anti-DNA titer after 5 days was 25,568 (range 12,000 – 36,000) for glomerular-bound mAbs and 31,272 (range 14,000 – 41,000) for mAbs that did not bind in glomeruli. Only BM-binding mAbs initiated glomerular disease detected as proteinuria.
Table 3.
Hybridomas producing BM-binding mAb induce proteinuria.
| Hybridoma(s) Injected | mAb Isotype | Glomerular Isotypeb | Daysc | Anti-DNA Serum Titer | Proteinuria |
|---|---|---|---|---|---|
| (2a/2b)d | (mg/dl)e | ||||
| 163p.64 | 2a | 2a | 4 | 1 601/<90 | 100 |
| 2a | 5 | 11 842/<90 | 300 | ||
| 163p.77 | 2b | 2b | 5 | <90/36 000 | 100 |
| 163p.124 | 2a | 2a | 5 | 24 000/<90 | 100 |
| DNA5 | 2a | 2a | 5 | 36 000/<90 | 100 |
| DNA6 | 2a | 2a | 5 | 36 000/<90 | 100 |
| 452s.46 | 2b | None | 5 | <90/28 024 | <30 |
| DNA3 | 2a | None | 5 | 36 000/<90 | <30 |
| 163p.132 | 2b | ~2bf | 5 | <90/32 938 | <30 |
| 2b | 8 | <90/>200 000 | 100 | ||
|
| |||||
| 163p.64 | 2a | 2a | 4 | 2 578/4 546 | 100 |
| 163p.132 | 2b | 2a | 5 | 24 704/25 202 | 300 |
|
| |||||
| 163p.77 | 2b | 2b | 5 | 36 000/36 000 | 30 |
| DNA3 | 2a | ||||
|
| |||||
| 163p.124 | 2a | 2a | 5 | 24 000/24 000 | 100 |
| 163p.132 | 2b | ||||
|
| |||||
| 163p.64 | 2a | 2a | 5 | 12 001/41 470 | 100 |
| 452s.46 | 2b | ||||
Ten mice per group were injected with the indicated hybridomas on day 0 and monitored daily for proteinuria. Two mice per group were terminated daily. Results are presented from one mouse in each group. Similar results were obtained with the other mouse in each group on the respective day.
The subclass of IgG detected within kidney serial cryosections was determined by immunofluorescence from kidneys excised on the indicated days after hybridoma injection.
The number of days after injection of hybridoma cells.
Serum IgG2a and IgG2b anti-DNA titers were determined on the indicated days after hybridoma injection.
Proteinuria measured on the indicated days after hybridoma injection.
Weak immunofluorescence only slightly above background. Two mice were separately injected with 163p.132 in a later experiment. Sera and kidneys were collected and proteinuria was measured 8 days after hybridoma injection. See Fig. 3f.
Gilkeson et al. (31) observed that mice injected with163p.77 and 163p.132 hybridoma cells developed glomerular IgG deposits and proteinuria after the injected mice developed pronounced ascites. The results with 163p.77 are similar to those in Fig. 2 and Table 3. We extended the time before euthanasia of mice injected with 163p.132 from 5 days to 8 days and observed similar results to those of Gilkeson et al. After 8 days mice injected with 163p.132 cells had glomerular IgG deposits (Fig. 3f) and moderate proteinuria (Table 3). The difference between 163p.132 injected mice at 5 and 8 days is likely a consequence of much higher mAb serum titer after 8 days. MAb 163p.132 does bind to BM but with 300-fold less relative affinity than mAb 163p.64 (Table 2). Alternatively 163p.132 mAb deposition after 8 days may have been due to circulating immune complexes. There was co-localization of 162p.132 mAb with DNA (yellow pixels in Fig. 3f) although most of the glomerular 163p.132 IgG was not co-localized with DNA.
Discussion
The present results demonstrate that some but not all anti-DNA mAbs bind directly to BM antigens and that direct binding of anti-dsDNA antibody to GBM or MM is critical for the initiation of experimental lupus nephritis. Glomerular binding of IgG and complement and the initiation of glomerular disease, identified as proteinuria, were independent of mAb binding to DNA or chromatin whether the mAbs were injected or produced in situ. MAb binding to GBM and MM was correlated with relative affinity for dsDNA but independent of binding to DNA or chromatin. Only anti-dsDNA mAbs that bound BM antigens bound to GBM and MM in vivo. These results and conclusion are consistent with previous reports that anti-dsDNA antibodies may initiate glomerulonephritis after binding directly to glomerular antigens (18–24). The results and conclusion contrast with results (10–14, 16, 17, 32) interpreted to indicate that anti-DNA antibodies can only bind to GBM or MM as immune complexes of anti-DNA antibody and nucleosomes or by binding to chromatin already bound to GBM or MM (28–30). The results from co-injection of mice with a hybridoma producing a BM-binding anti-DNA mAb with a hybridoma producing a non BM-binding anti-DNA mAb are difficult to reconcile with the previous interpretation. MAbs produced by the co-injected hybridomas had similar relative affinity for DNA, nucleosomes, or chromatin, but only the mAbs that bound BM also bound glomeruli in vivo. The results cannot be explained by potential absence of circulating nucleosomes or chromatin in non autoimmune-prone BALB/c mice. Circulating or glomerular-bound chromatin or nucleosomes, including that released from necrotic or apoptotic hybridomas, would have been equally accessible to the two mAbs.
The present results may explain why autoimmune female BWF1 transgenic for VH of the 3H9 anti-DNA mAb (33, 34) do not develop nephritis (35). 3H9 mAb binds DNA and chromatin (36) but does not bind BM. Autoimmune, 3H9 VH transgenic BWF1 had similar serum IgG2a/b anti-DNA titers as non-transgenic BWF1 of similar age but did not develop proteinuria even after 1 year of age. Similar outcome was reported for D42 VH (37) and 3–32 μ (38) transgenic BWF1. Non-transgenic, female BWF1 invariably produce anti-DNA autoantibody and develop glomerulonephritis with proteinuria by 10 months of age (1). BALB/c mice injected with the 3H9 hybridoma had relatively low glomerular immunofluorescence and disease scores compared with mice injected with 163p.77 or 163p.132 hybridomas (31). The majority of anti-DNA hybridomas from VH3H9 transgenic BWF1 had VH3H9 H chains (39). Likely those mAbs could not bind BM and could not initiate disease.
Essentially three experimental systems have described nucleosome-dependent glomerular binding of anti-DNA antibodies. Schmiedeke et al. (32) and Termaat et al. (17) allowed soluble DNA to bind to histones after the histones were perfused into kidneys or added to isolated glomeruli or GBM. Anti-DNA mAb bound to the immobilized DNA but not to GBM, histone-coated GBM, or DNA added to GBM. Although interesting, the experiments do not accurately reflect the physical chemical properties of intact nucleosomes, nor how nucleosomes or chromatin may interact with GBM or MM. Kramers et al. (10) reported that purified anti-DNA mAbs perfused into kidneys may only bind in glomeruli as immune complexes with histones or nucleosomes, presenting as example mAb 32. Nucleosomes in the immune complexes were presumed to promote binding to GBM through histone-dependent charge interaction. Nucleosomes in physiological saline have a net negative charge with more exposed acidic than basic regions (40, 41). The basic termini of H2B and H3 that protrude from the octamer cores through the DNA superhelix bind with the acidic patches on the octamer surface of consecutive nucleosomes and with linker DNA to organize the nucleosomes into chromatin (40, 42). Nucleosomal organization into chromatin precludes surface availability of positive charge contributed by histones (41). The net charge of the GBM lamina rara interna and externa initially accessible to chromatin or nucleosomes is anionic (43, 44) and unlikely to promote binding. Although nucleosomes bound isolated collagen IV, laminin (15), and agrin (16) on laboratory sensor chips, radiolabeled nucleosomes (45, 46) were rapidly cleared from blood into the liver with insignificant localization to kidneys unless nucleosome injections were preceded by injection of soluble histones (45). DNA-anti-DNA immune complexes were likewise rapidly cleared from the circulation (47, 48). Perfusion into the renal artery (10) would bypass initial circulation to the liver. An alternative explanation for why mAb 32-nucleosome immune complexes bound GBM, but mAb alone did not, might be that the mAb 32 in nucleosome immune complexes had increased relative avidity for GBM. The mAb 32-nucleosome immune complexes were created at a 15:1 molar ratio of mAb to mononucleosome (10). Multiple unbound antibody combining sites in mAb 32-nucleosome immune complexes prepared in antibody excess may have created higher avidity of the complexes for GBM than mAb 32 alone. The DNA, nucleosome, and BM binding characteristics of mAb 32 were similar to those for mAb 163p.132 in the present study. MAb 163p.132 bound glomeruli only after reaching a serum concentration of ~10 mg/ml. MAbs 163p.64 and 163p.77 that bind with high relative affinity to BM, both bound glomeruli at serum concentrations of ≤720 μg/ml. MAb 163p.132 binds BM but with low relative affinity. Alternatively, the additional 3 days of 163p.132 hybridoma growth from 5 to 8 days may have produced sufficient chromatin or nucleosomes from dying cells to produce immune complexes, likely in mAb excess. There was more glomerular co-localization of DNA with 163p.132 mAb than with the BM-binding 163p.64 mAb.
GBM-associated EDS in kidneys from nephritic BWF1 (13), nephritic lupus patients (12), and BALB/c mice chronically injected with an anti-DNA mAb (11) contained both chromatin and IgG. The EDS chromatin was presumed to have originated from mesangial cells undergoing apoptosis (13). The released chromatin was presumed to bind GBM and present target antigens to chromatin-binding antibody. Caspace 3-positive mesangial cells were detected in kidneys from nephritic but not pre-nephritic BWF1 (13), and chromatin was never detected in EDS that did not also contain IgG (11, 13). Direct binding of nucleosomes or chromatin to GBM was not tested. If chromatin binding to GBM determines when and where anti-DNA antibody binds GBM to initiate EDS, mAbs with similar relative affinity for chromatin should have similar potential to initiate nephritis, which our results show not to be true. Nucleosomes do not bind GBM as discussed above. The recent morphologic studies have elegantly refined our understanding of glomerular EDS in nephritic kidneys (12–14, 16, 49) but fail to clarify how EDS are initiated in lupus nephritis.
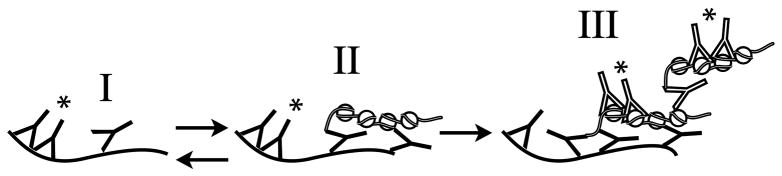
Earlier experiments determined that small lattice immune complexes prepared with a cationized antibody were retained within glomerular subendothelial EDS but persisted only when they were able to form larger lattice immune complexes (50). Glomerular mAb deposition in the present study was independent of mAb pI or immune complexes. Immune complex deposition in GBM can also be initiated by GBM-binding antibody that also binds circulating free antigen to produce immune complexes and EDS (51). Our results are most consistent with this latter mechanism to explain how anti-DNA antibody can initiate glomerular EDS (illustrated in Fig. 4). The initial event toward glomerular IgG binding and initiation of EDS is direct binding of anti-DNA antibody to GBM or MM (Fig. 4I). Complement activation and the ensuing inflammation could provide a source for locally released oligonucleosomes. If the locally released oligonucleosomes are bound by GBM or MM-bound antibody (Fig. 4II) (51), both BM-binding and non BM-binding anti-DNA antibodies could bind the progressively accumulating complex and induce more complement activation, inflammation, and oligonucleosome release (Fig. 4III). Reduced glomerular DNase I would contribute to and accelerate stage III (Fig. 4) (52). The progressive accumulation of immune complexes would eventually produce chronic inflammation and lupus nephritis. BM-binding and non BM-binding anti-DNA mAbs were not co-localized in mice 5 days after co-injection with respective hybridomas. There may have been insufficient circulating oligonucleosomes or chromatin from the ascites tumors to generate the glomerular complexes depicted in Fig. 4II. Similarly, 3H9 transgenic BWF1 may fail to develop glomerulonephritis not only because the transgenic anti-DNA antibody cannot bind glomerular antigens, but also because locally released oligonucleosomes are unavailable to create large lattice immune complexes.
Figure 4.
Hypothetical mechanism for the initiation of lupus nephritis by BM-binding anti-dsDNA antibody. The stage I to II transition is likely to be reversible (62). The stage II to III transition associated with the progressive accumulation of antibody and chromatin into immune complexes will eventually reach a threshold for which the immune complex deposition is no longer reversible. This stage would yield chronic inflammation and lupus nephritis. EDS (11, 12, 27) are predicted to be formed by the stage II into III transition.
 , GBM or MM;
, GBM or MM;
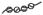 , chromatin;
, chromatin;
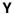 , BM-binding anti-dsDNA;
, BM-binding anti-dsDNA;
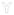 , non BM-binding anti-dsDNA;
, non BM-binding anti-dsDNA;
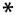 , activated complement.
, activated complement.
Anti-DNA antibodies that bind directly to glomerular endothelial, mesangial, or other cell surface antigens can function similarly to anti-DNA antibodies that bind GBM or MM(19–21, 23, 53–55). Cell-bound anti-DNA antibodies can initiate inflammation by directly altering cell function, inducing apoptosis or necrosis, or interrupting cell-cell or cell-matrix interactions (6, 56). Oligonucleosomes released from apoptotic or necrotic cells as a consequence of the induced inflammation can form large lattice immune complexes locally (50) that persist as subendothelial EDS (34).
Our results do not exclude the potential for GBM binding of circulating nucleosome-antibody immune complexes (10) or antibody binding to GBM chromatin, but they do indicate that neither is necessary for BM-binding anti-DNA antibody to bind GBM or MM.
We did not directly test whether injected anti-DNA mAbs would bind differently in nephritic or pre-nephritic BWF1 kidneys compared to BALB/c mouse kidneys. Confocal images of IgG co-localization with GBM and MM in kidneys from 9 month-old BWF1 were similar to those from BALB/c mice chronically injected with BM-binding anti-DNA mAb.
The present results provide additional insight to explain why lupus patients with similar serum antibody to dsDNA or nucleosomes can have different antibody-dependent disease outcomes (6).
Methods
Mice
BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN) and maintained within the UTHSC Laboratory Animal Care Unit. All experimental protocols were approved by IACUC.
Antibodies and antigens
The generation, DNA specificity, and V-region sequences for the mAbs used in these studies have been described (34, 57–59). All hybridomas were derived from autoimmune (NZB × NZW)F1 mice (BWF1) except 3H9 (34) provided by Drs. M. Weigert and M. Radic (Chicago, IL and Memphis, TN). Matrigel® (BM) (BD Biosciences, Bedford, MA) is a soluble basement membrane matrix of laminin, collagen IV, HSPG, and entactin (nidogen 1). Only high molecular weight bands corresponding to laminin, collagen IV, entactin, and HSPG were detected after high sensitivity staining of an SDS-PAGE of 12.5 μg of Matrigel®. DNA, ssDNA, and dsDNA were prepared as described (58). Chromatin and mononucleosomes were isolated from mouse liver or cultured P3x63-Ag8.653 cells as described (60). Perlecan (HSPG2) and heparan sulfate were purchased from Sigma-Aldrich (St. Louis, MO), and recombinant human nidogen (entactin) and C-terminal recombinant rat agrin, from R&D Systems, (Minneapolis, MN). Agrin is a heparin sulfate proteoglycan in GBM (61). Biotinylated goat anti-mouse IgG, IgG2a, and IgG2b; FITC-goat anti-mouse IgG; and FITC-streptavidin were purchased from Southern Biotechnology (Birmingham, AL); alkaline phosphatase-streptavidin, from Jackson Immunoresearch Laboratories (West Grove, PA); biotinylated rat anti-perlecan mAb (clone A7L6), from Lab Vision (Thermo Fisher Scientific, Fremont, CA); Alexa 546-strepatavidin and TO-PRO3® DNA dye, from Molecular Probes (Invitrogen, Carlsbad, CA); and anti-C3-FITC, from BD Bioscience.
MAb isolation
MAbs were isolated from hybridoma supernatants by affinity chromatography on protein G-Sepharose 4B (Invitrogen) essentially as described (10). MAbs were eluted with glycine-HCl, pH 2.8 and immediately neutralized. SDS-PAGE of eluted mAbs stained with a high sensitivity Coomassie (Biorad, Hercules, CA) yielded bands corresponding only to immunoglobulin H and L polypeptides. DNA was not detected in purified mAbs by ethidium bromide staining after agarose electrophoresis but was detected in the high salt eluate.
ELISA for DNA, chromatin, nucleosome, and BM binding
Direct and competitive ELISAs for DNA binding were performed as described (59). ELISAs for mAb binding to chromatin, nucleosomes, BM, and the BM constituents HSPG, heparan sulfate, and entactin were performed identically to the direct DNA ELISA. Plates (Immulon I, Thermo-Fisher) were coated with DNA, chromatin or mononucleosomes at 1 μg/well DNA; 1/250 dilution of Matrigel®, ~5 μg/well, estimated as 2.8 μg/well laminin, 1.5 μg/well collagen IV, 0.4 μg/well entactin, and 0.25 μg/well HSPG (BD Bioscience assay); or 0.2 μg/well of purified BM proteins. Bound IgG from serially diluted supernatant, purified mAb, or serum antibody were detected as described (59). A biotinylated rat anti-laminin mAb (clone A5) (Neomarkers) was used as a positive control for the anti-BM ELISA. Statistical analyses were performed with PASW Statistics 18 (SPSS Statistics, IBM, Armonk, NY).
In vivo glomerular binding of anti-DNA mAb and measurement of proteinuria
BALB/c mice, eight-to-twelve weeks old, were injected once intravenously with 1 mg of a single, purified mAb or 1 mg each of two purified mAbs, one IgG2a, the other IgG2b. Twenty-four hours later injected mice were euthanized and their kidneys removed and snap frozen in OCT embedding medium (Tissue-Tek, Miles Laboratories, Elkhart, IN). Serial one μm cryosections were fluorescently stained with biotinylated goat anti-mouse IgG2a or IgG2b and FITC-streptavidin. In separate experiments, mice were chronically injected with 100 μg per intraperitoneal (ip) injection of a single mAb twice weekly for 3 months or injected ip with hybridoma cells 5–7 days after ip injection with 0.5 ml pristane (Sigma). The hybridoma injection consisted of 107 cells from one hybridoma or 107 cells each from two hybridomas, one producing IgG2a and the other, IgG2b. Kidneys were removed and embedded for cryosection after 3 months chronic injection of purified mAb or 1–5 days after hybridoma injection. Serial cryosections from the same kidney were stained for detection of mouse IgG2a or IgG2b. For confocal microscopy 4–12 μm cryosections were stained with TO-PRO3 for DNA, goat anti-mouse IgG-FITC, and rat anti-perlecan and streptavidin Alexa 546 or anti-C3-FITC and biotinylated goat anti-mouse IgG and streptavidin-Alexa 546. Confocal images were collected with a Zeiss LSM510 confocal microscope (Carl Zeiss Microimaging, Thornwood, NY). Proteinuria was measured with Ames Uristix (Miles) according to manufacturer’s instructions.
Acknowledgments
The research was supported by NIH NIAID grant AI26833, NIH NCRR grant RR301812, and a grant from the UTHSC Center of Excellence for Diseases of Connective Tissue.
The authors wish to acknowledge Ryle Holder for technical assistance in mAb purification, Tim Higgins for help in figure preparations, Dr. Michael Madaio for help in interpreting glomerular immunofluorescence, and the Microbiology, Immunology, and Biochemistry Confocal Microscope Facility for assistance with confocal microscopy. We also acknowledge Drs. David Isenberg, Marc Monestier, Marko Radic, and Ole Petter Rekvig for critical commentary on the manuscript.
Abbreviations
- BWF1
(NZB × NZW)F1 mice
- dsDNA
native, double-stranded DNA
- ssDNA
denatured, single-stranded DNA
- BM
basement membrane
- GBM
glomerular basement membrane
- mAb
monoclonal antibody
- MM
glomerular mesangial matrix
- EDS
electron dense substance, electron dense region, electron dense deposit
- HSPG
heparan sulfate proteoglycan
Footnotes
Disclosure
The authors declare no commercial financial support or competing financial interests.
Contributor Information
Meera R. Krishnan, Email: kmeera@bellsouth.net.
Congmiao Wang, Email: congmiao@yahoo.com.
References
- 1.Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126:607–24. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isenberg DA. Autoantibodies: markers of disease or pathogenic? Ann N Y Acad Sci. 1997;823:256–62. doi: 10.1111/j.1749-6632.1997.tb48398.x. [DOI] [PubMed] [Google Scholar]
- 4.Manson JJ, Ma A, Rogers P, et al. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res Ther. 2009;11:R154. doi: 10.1186/ar2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serological features. Am J Med. 1979;66:210–5. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 6.Madaio MP. The role of autoantibodies in the pathogenesis of lupus nephritis. Semin Nephrol. 1999;19:48–56. [PubMed] [Google Scholar]
- 7.Isenberg DA, Manson JJ, Ehrenstein MR, et al. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology (Oxford) 2007;46:1052–6. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 8.Dixon FJ, Oldstone MB, Tonietti G. Pathogenesis of immune complex glomerulonephritis of new zealand mice. J Exp Med. 1971;134:65–71. [PMC free article] [PubMed] [Google Scholar]
- 9.Morioka T, Woitas R, Fujigaki Y, et al. Histone mediates glomerular deposition of small size DNA anti-DNA complex. Kidney Int. 1994;45:991–7. doi: 10.1038/ki.1994.134. [DOI] [PubMed] [Google Scholar]
- 10.Kramers C, Hylkema MN, van Bruggen MC, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J Clin Invest. 1994;94:568–77. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenton KA, Tommeras B, Marion TN, et al. Pure anti-dsDNA mAbs need chromatin structures to promote glomerular mesangial deposits in BALB/c mice. Autoimmunity. 2010;43:179–88. doi: 10.3109/08916930903305633. [DOI] [PubMed] [Google Scholar]
- 12.Kalaaji M, Fenton KA, Mortensen ES, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71:664–72. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 13.Kalaaji M, Mortensen E, Jorgensen L, et al. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168:1779–92. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mjelle JE, Kalaaji M, Rekvig OP. Exposure of chromatin and not high affinity for dsDNA determines the nephritogenic impact of anti-dsDNA antibodies in (NZBxNZW)F1 mice. Autoimmunity. 2009;42:104–11. doi: 10.1080/08916930802375729. [DOI] [PubMed] [Google Scholar]
- 15.Mjelle JE, Rekvig OP, Fenton KA. Nucleosomes possess a high affinity for glomerular laminin and collagen IV and bind nephritogenic antibodies in murine lupus-like nephritis. Ann Rheum Dis. 2007;66:1661–8. doi: 10.1136/ard.2007.070482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mjelle JE, Rekvig OP, Van Der Vlag J, et al. Nephritogenic antibodies bind in glomeruli through interaction with exposed chromatin fragments and not with renal cross-reactive antigens. Autoimmunity. 2011;44:373–83. doi: 10.3109/08916934.2010.541170. [DOI] [PubMed] [Google Scholar]
- 17.Termaat RM, Assmann KJ, Dijkman HB, et al. Anti-DNA antibodies can bind to the glomerulus via two distinct mechanisms. Kidney Int. 1992;42:1363–71. doi: 10.1038/ki.1992.428. [DOI] [PubMed] [Google Scholar]
- 18.Faaber P, Rijke TP, van de Putte LB, et al. Cross-reactivity of human and murine anti-DNA antibodies with heparan sulfate. The major glycosaminoglycan in glomerular basement membranes. J Clin Invest. 1986;77:1824–30. doi: 10.1172/JCI112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madaio MP, Carlson J, Cataldo J, et al. Murine monoclonal anti-DNA antibodies bind directly to glomerular antigens and form immune deposits. J Immunol. 1987;138:2883–9. [PubMed] [Google Scholar]
- 20.Raz E, Brezis M, Rosenmann E, et al. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142:3076–82. [PubMed] [Google Scholar]
- 21.Raz E, Ben-Bassat H, Davidi T, et al. Cross-reactions of anti-DNA autoantibodies with cell surface proteins. Eur J Immunol. 1993;23:383–90. doi: 10.1002/eji.1830230213. [DOI] [PubMed] [Google Scholar]
- 22.Vlahakos DV, Foster MH, Adams S, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 23.D’Andrea DM, Coupaye-Gerard B, Kleyman TR, et al. Lupus autoantibodies interact directly with distinct glomerular and vascular cell surface antigens. Kidney Int. 1996;49:1214–21. doi: 10.1038/ki.1996.175. [DOI] [PubMed] [Google Scholar]
- 24.Sabbaga J, Line SRP, Potocnjak P, et al. A murine nephritogenic monoclonal anti-DNA autoantibody binds directly to mouse laminin, the major non-collagenous protein component of the glomerular basement membrane. Eur J Immunol. 1989;19:137–43. doi: 10.1002/eji.1830190122. [DOI] [PubMed] [Google Scholar]
- 25.Mostoslavsky G, Fischel R, Yachimovich N, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol. 2001;31:1221–7. doi: 10.1002/1521-4141(200104)31:4<1221::aid-immu1221>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Farquhar MG, Vernier RL, Good RA. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med. 1957;106:649–60. doi: 10.1084/jem.106.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Channing AA, Kasuga T, Horowitz RE, et al. An ultrastructural study of spontaneous lupus nephritis in the NZB-BL-NZW mouse. Am J Pathol. 1965;47:677–94. [PMC free article] [PubMed] [Google Scholar]
- 28.Fenton KA, Rekvig OP. A central role of nucleosomes in lupus nephritis. Ann N Y Acad Sci. 2007;1108:104–13. doi: 10.1196/annals.1422.012. [DOI] [PubMed] [Google Scholar]
- 29.van Bavel CC, Fenton KA, Rekvig OP, et al. Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1892–9. doi: 10.1002/art.23626. [DOI] [PubMed] [Google Scholar]
- 30.van Bavel CC, van der Vlag J, Berden JH. Glomerular binding of anti-dsDNA autoantibodies: the dispute resolved? Kidney Int. 2007;71:600–1. doi: 10.1038/sj.ki.5002126. [DOI] [PubMed] [Google Scholar]
- 31.Gilkeson GS, Bernstein K, Pippen AM, et al. The influence of variable-region somatic mutations on the specificity and pathogenicity of murine monoclonal anti-DNA antibodies. Clin Immunol Immunopathol. 1995;76:59–67. doi: 10.1006/clin.1995.1088. [DOI] [PubMed] [Google Scholar]
- 32.Schmiedeke TM, Stockl FW, Weber R, et al. Histones have high affinity for the glomerular basement membrane. Relevance for immune complex formation in lupus nephritis. J Exp Med. 1989;169:1879–94. doi: 10.1084/jem.169.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Nagy Z, Prak EL, et al. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–55. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 34.Shlomchik MJ, Aucoin AH, Pisetsky DS, et al. The structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84:9150–4. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steeves MA, Marion TN. Tolerance to DNA in (NZB x NZW)F1 mice that inherit an anti-DNA V(H) as a conventional micro H chain transgene but not as a V(H) knock-in transgene. J Immunol. 2004;172:6568–77. doi: 10.4049/jimmunol.172.11.6568. [DOI] [PubMed] [Google Scholar]
- 36.Neeli I, Richardson MM, Khan SN, et al. Divergent members of a single autoreactive B cell clone retain specificity for apoptotic blebs. Mol Immunol. 2007;44:1914–21. doi: 10.1016/j.molimm.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedmann D, Yachimovich N, Mostoslavsky G, et al. Production of high affinity autoantibodies in autoimmune New Zealand Black/New Zealand white F1 mice targeted with an anti-DNA heavy chain. J Immunol. 1999;162:4406–16. [PubMed] [Google Scholar]
- 38.Wellmann U, Letz M, Schneider A, et al. An Ig mu-heavy chain transgene inhibits systemic lupus erythematosus immunopathology in autoimmune (NZB x NZW)F1 mice. Int Immunol. 2001;13:1461–9. doi: 10.1093/intimm/13.12.1461. [DOI] [PubMed] [Google Scholar]
- 39.Steeves MA. Tolerance and Autoimmunity in (NZB x NZW)F1 Mice Transgenic for Anti- DNA Antibody. Memphis: The University of Tennessee Health Science Center; 2005. [Google Scholar]
- 40.Luger K, Mader AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 41.Materese CK, Savelyev A, Papoian GA. Counterion atmosphere and hydration patterns near a nucleosome core particle. J Am Chem Soc. 2009;131:15005–13. doi: 10.1021/ja905376q. [DOI] [PubMed] [Google Scholar]
- 42.Schalch T, Duda S, Sargent DF, et al. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436:138–41. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- 43.Kanwar YS, Farquhar MG. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979;81:137–53. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caulfield JP, Farquhar MG. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976;73:1646–50. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gauthier VJ, Tyler LN, Mannik M. Blood clearance kinetics and liver uptake of mononucleosomes in mice. J Immunol. 1996;156:1151–6. [PubMed] [Google Scholar]
- 46.Rumore P, Muralidhar B, Lin M, et al. Haemodialysis as a model for studying endogenous plasma DNA: oligonucleosome-like structure and clearance. Clin Exp Immunol. 1992;90:56–62. doi: 10.1111/j.1365-2249.1992.tb05831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben Chetrit E, Dunsky EH, Wollner S, et al. In vivo clearance and tissue uptake of an anti-DNA monoclonal antibody and its complexes with DNA. Clin Exp Immunol. 1985;60:159–68. [PMC free article] [PubMed] [Google Scholar]
- 48.Emlen W, Mannik M. Clearance of circulating DNA-anti-DNA immune complexes in mice. J Exp Med. 1982;155:1210–5. doi: 10.1084/jem.155.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenton K, Fismen S, Hedberg A, et al. Anti-dsDNA antibodies promote initiation, and acquired loss of renal Dnase1 promotes progression of lupus nephritis in autoimmune (NZBxNZW)F1 mice. PLoS One. 2009;4:e8474. doi: 10.1371/journal.pone.0008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gauthier VJ, Mannik M, Striker GE. Effect of cationized antibodies in performed immune complexes on deposition and persistence in renal glomeruli. J Exp Med. 1982;156:766–77. doi: 10.1084/jem.156.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agodoa LY, Gauthier VJ, Mannik M. Antibody localization in the glomerular basement membrane may precede in situ immune deposit formation in rat glomeruli. J Immunol. 1985;134:880–4. [PubMed] [Google Scholar]
- 52.Seredkina N, Zykova SN, Rekvig OP. Progression of murine lupus nephritis is linked to acquired renal Dnase1 deficiency and not to up-regulated apoptosis. Am J Pathol. 2009;175:97–106. doi: 10.2353/ajpath.2009.080943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lafer EM, Rauch J, Andrzejewski C, Jr, et al. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. J Exp Med. 1981;153:897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qing X, Pitashny M, Thomas DB, et al. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121:61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qing X, Zavadil J, Crosby MB, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- 56.Madaio MP. Lupus autoantibodies 101: one size does not fit all; however, specificity influences pathogenicity. Clin Exp Immunol. 2003;131:396–7. doi: 10.1046/j.1365-2249.2003.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnan MR, Marion TN. Structural similarity of antibody variable regions from immune and autoimmune anti-DNA antibodies. J Immunol. 1993;150:4948–57. [PubMed] [Google Scholar]
- 58.Marion TN, Lawton ARd, Kearney JF, et al. Anti-DNA autoantibodies in (NZB X NZW)F1 mice are clonally heterogeneous, but the majority share a common idiotype. J Immunol. 1982;128:668–74. [PubMed] [Google Scholar]
- 59.Tillman DM, Jou NT, Hill RJ, et al. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB x NZW)F1 mice. J Exp Med. 1992;176:761–79. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rekvig OP, Hannestad K. The specificity of human autoantibodies that react with both cell nuclei and plasma membranes: the nuclear antigen is present on core mononucleosomes. J Immunol. 1979;123:2673–81. [PubMed] [Google Scholar]
- 61.Raats CJ, Bakker MA, Hoch W, et al. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem. 1998;273:17832–8. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier VJ, Mannik M. Only the initial binding of cationic immune complexes to glomerular anionic sites is mediated by charge-charge interactions. J Immunol. 1986;136:3266–71. [PubMed] [Google Scholar]