Abstract
Nicotine administration alters various forms of hippocampus-dependent learning and memory. Increasing work has found that the dorsal and ventral hippocampus differentially contribute to multiple behaviors. Thus, the present study examined whether the effects of nicotine in the dorsal and ventral hippocampus have distinct influences on contextual fear learning in male C57BL/6J mice. Direct infusion of nicotine into the dorsal hippocampus resulted in an enhancement of contextual fear learning, whereas nicotine infused into the ventral hippocampus resulted in deficits. Nicotine infusions into the ventral hippocampus did not alter hippocampus-independent cued fear conditioning or time spent in the open arm of the elevated plus maze, a measure of anxiety, suggesting the effects are due to alterations in contextual learning and not other general processes. Finally, results from using direct infusions of MLA, a low-affinity α7 nicotinic acetylcholine receptor (nAChR) antagonist, in conjunction with systemic nicotine, provide evidence that α7-nAChRs in the ventral hippocampus mediate the detrimental effect of ventral hippocampal nicotine on contextual fear learning. These results suggest that with systemic nicotine administration, competition exists between the dorsal and ventral hippocampus for behavioral control over contextual learning.
Keywords: Acetylcholine, learning, nicotine, low-affinity nAChRs, mouse
Introduction
Nicotinic acetylcholine receptors (nAChRs) are known to play a modulatory role in cognitive function in both rodents and humans (Heishman et al., 2010; Kenney and Gould, 2008a; Placzek et al., 2009). In particular, hippocampus-dependent learning and memory is particularly susceptible to modulation by nicotine administration (Kenney and Gould, 2008a). However, in recent years it has become apparent that the hippocampus is not a unitary structure and that the contribution of the hippocampus to behavioral and cognitive tasks varies along the septal-temporal (dorsal-ventral) axis (Bannerman et al., 2004; Fanselow and Dong, 2010; Moser and Moser, 1998). The dorsal and ventral poles of the hippocampus differ with respect to their afferent anatomical connections to the entorhinal cortex (Amaral and Witter, 1989; Dolorfo and Amaral, 1998; van Groen et al., 2003), their efferent connections to other cortical and subcortical structures (van Groen and Wyss, 1990), gene expression (Christensen et al., 2010; Leonardo et al., 2006), glutamate receptor subunit expression (Pandis et al., 2006), and response to nicotine (Abdulla et al., 1996; Singer et al., 2004). These findings suggest that there may be variation along the dorsal-ventral axis of the hippocampus in the effects of nicotine on learning.
Fear conditioning has proven to be a useful task for the analysis of drug effects on learning and memory due to its rapid acquisition and clearly defined acquisition, consolidation, and retrieval phases. Training in fear conditioning consists of placing a rodent into a novel environment and presenting an auditory cue that signals the onset of a foot-shock. During training, rodents form two conditioned stimulus (CS) – unconditioned stimulus (US) associations: 1) a hippocampus-independent cue-shock association and 2) a hippocampus-dependent context-shock association (Kim and Fanselow, 1992; Logue et al., 1997; Phillips and LeDoux, 1992). The strength of these CS-US associations is then assessed by placing rodents back into the training chamber (contextual fear conditioning) or into a novel chamber while presenting the same auditory cue as during training (cued fear conditioning), and measuring freezing, a species typical fear response characterized by a lack of movement (Blanchard and Blanchard, 1969). Both the dorsal and ventral hippocampus contribute to the processing of the multimodal information involved in contextual memory formation; pharmacological blockade of NMDA receptors and protein synthesis, and lesions of the dorsal or ventral hippocampus result in contextual fear memory deficits (Barrientos et al., 2002; Bast et al., 2003; Kim and Fanselow, 1992; Maren and Holt, 2004; Rudy et al., 2002; Rudy and Matus-Amat, 2005; Zhang et al., 2001). These findings suggest that the dorsal and ventral hippocampus both play a role in contextual information processing. However, recent work using more focal hippocampal subregion specific lesions suggests that the dorsal and ventral hippocampus may differ in their specific contribution to contextual fear memory formation and retention (Hunsaker and Kesner, 2008).
Prior work has found that infusion of nicotine into the hippocampus enhances contextual fear memories (Davis et al., 2007), however, this study made no attempt to determine if the dorsal and ventral hippocampus differentially contribute to the effects of nicotine on this task.. Nicotine administration has been found to upregulate high-affinity nAChR binding in the dorsal but not ventral hippocampus (Abdulla et al., 1996) and systemic nicotine administration differentially regulates neurotransmitter pre-cursor and metabolites in dorsal and ventral hippocampus (Singer et al., 2004). Furthermore, recent work from our laboratory found differential effects of nicotine infusion into the prefrontal cortex, dorsal and ventral hippocampus on trace fear conditioning (Raybuck and Gould, 2010). Taken together, these results suggest that the effects of nicotine on contextual learning could differ along the dorsal-ventral axis. At the molecular level, learning in the presence of nicotine results in an increase in c-jun N-terminal kinase 1 (JNK1) phosphorylation in the dorsal but not ventral hippocampus, an event thought to be necessary for the consolidation of nicotine-enhanced contextual fear memories (Kenney et al., 2010a). Given the results demonstrating functional differences between the dorsal and ventral hippocampus, the ability of nicotine to consistently alter hippocampus-dependent learning (Kenney and Gould, 2008a; MacLeod et al., 2006; Tian et al., 2011), and the importance of hippocampal nAChRs in a variety of cognitive diseases (Picciotto and Zoli, 2008; Woodruff-Pak and Gould, 2002), we sought to examine how the dorsal and ventral hippocampus differ in their contribution to effects of nicotine on hippocampus-dependent contextual learning and the underlying nAChRs involved in these effects.
Methods
Subjects
Subjects were male C57BL/6J mice aged 8–12 weeks of age. Mice were group housed (2–4 per cage) prior to surgery and singly housed following surgery with ad libitum access to food and water. Mice were maintained on a 12 hr light/dark cycle (lights on at 07:00). All procedures were approved by the Temple University Institutional Animal Care and Use Committee and were performed in accordance with NIH guidelines.
Fear Conditioning
Training and testing in contextual fear conditioning took place in four identical conditioning chambers (17.8 × 19.0 × 38.1 cm) housed in sound-attenuating boxes (Med Associates, St. Albans, VT). Each chamber consisted of Plexiglas walls in the front and back and stainless steel side walls. Chamber floors were comprised of metal rods (1.6 mm diameter) spaced 0.6 cm apart and connected to a shock scrambler and generator (Med Associates). Ventilation fans were mounted on the back of each box to provide background noise and air exchange. Speakers were mounted on the left wall for acoustic stimuli presentation. All stimuli administration was controlled via a personal computer running MED-PC software.
Testing for cued fear conditioning took place in four different chambers (20.3 × 22.9 × 17.8 cm) located in a different room than used for training. The chambers consisted of Plexiglas front and back walls, metal side walls, and a white opaque plastic floor. Speakers were mounted above chambers for acoustic stimuli presentation and fans were mounted on one side wall to provide background noise and air exchange. Vanilla extract was added to paper towels that were placed under each chamber to further distinguish the chambers from those used for training.
Mice were trained in contextual fear conditioning as previously described (Gould and Wehner, 1999). Briefly, mice were placed in the chambers and after a 120 sec baseline period two co-terminating CS (30 sec, 85 dB white noise) – US (2 sec, 0.57 mA footshock) presentations separated by a 120 sec inter-trial interval occurred. The training session ended with a 30 sec stimuli-free period. To test for contextual fear conditioning, 24 hrs after training mice were placed back into the training chambers and freezing behavior, defined as the lack of all movement aside from respiration (Blanchard and Blanchard, 1969), was assessed over 5 min. To test for cued fear conditioning 24 hrs after training, mice were placed in the altered chambers and generalized freezing was scored for 3 min in the absence of any CS followed by the presentation of the CS for 3 min at which time freezing in response to the cue was scored. All chambers were cleaned with 70% ethanol before and after all behavioral procedures.
Elevated Plus Maze
The elevated plus maze was made of a wood base and gray Plexiglas floors and walls. The maze was raised 62.6 cm off the ground and had two opposing open arms (7.6 × 30.6 cm), two opposing closed arms (7.6 × 30.6 × 15.5 cm) and a center area (7.6 × 7.6 cm). Following drug infusion, mice were placed in the center of the plus maze facing a closed arm and their behavior was recorded via a video camera over 5 min. Mice were then scored offline with the assistance of a custom made computer program. An entry into an arm was defined as all four paws crossing into the arm or center area.
Surgery
Mice were anesthetized using isoflurane gas (5% induction, 2–3% maintenance) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Lambda and bregma were aligned in the same horizontal plane and two stainless steel guide cannulae (Plastics One, Roanoke, VA) were inserted and fixed to the skull with dental cement. Dummy cannulae (Plastics One) were inserted into the guide cannulae to prevent clogging prior to infusions. Coordinates were determined using the atlas of Paxinos and Franklin (2001); dorsal hippocampus: −1.7 mm posterior to bregma, ± 1.5 mm mediolateral and −2.3 mm ventral to skull surface and ventral hippocampus: −2.8 mm posterior to bregma, ±3.0 mm mediolateral and −4.0 mm ventral to skull surface. To minimize post-operative pain Ketoprofen (2.0 mg/kg) was administered subcutaneously following surgery. Animals were allowed at least 5 days to recover before behavioral procedures were initiated.
Drugs and Infusion
All drugs were obtained from Sigma (St. Louis, MO). Nicotine hydrogen tartrate salt (reported as freebase: 0.09 mg/kg i.p, or 0.18 and 0.35 μg infusion per side), di-hydro-β-erythroidine (DHβE; 18 μg per side) and methyllycaconitine (MLA; 27 μg per side) were dissolved in physiological saline. For experiments that required systemic drug administration, nicotine was administered intraperitoneally at an injection volume of 0.01 mL/g body weight approximately 5 min prior to training. For direct drug infusions, mice were gently restrained and dummy cannulae were removed and replaced with 22 gauge infusion cannulae attached to PE50 polyethylene tubing (Plastics One) and a 10 μL Hamilton syringe (Reno, NV). Drugs were infused at a rate of 0.50 μL/min and at an injection volume of 0.50 μL per side. Drug administration was controlled by a microinfusion pump (KD Scientific, New Hope, PA). Infusion cannulae were left in place for 1 min following infusion to allow drug to diffuse away from cannula tip. Nicotine was infused immediately prior to training and/or testing and MLA or DHβE was infused 15 min prior to training and/or testing. Drug doses and infusion times were based on prior studies from our laboratory (Davis et al., 2007).
Statistical Analyses
Data were analyzed using one-way ANOVAs followed by Tukey HSD post-hoc tests or independent samples t-tests where appropriate.
Results
Nicotine Infusion into the Ventral Hippocampus
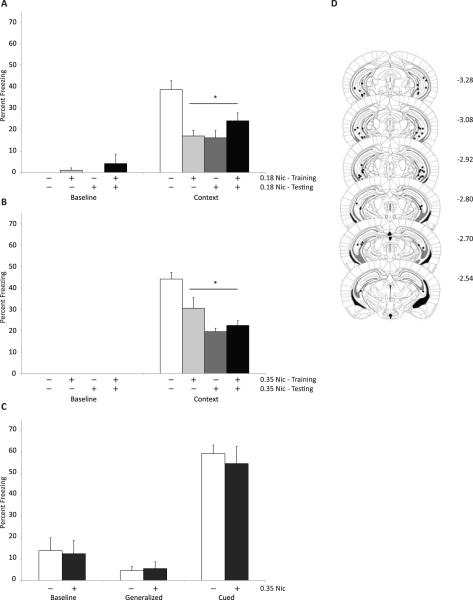
Given the differences between the ventral and dorsal poles of the hippocampus, we sought to determine if nicotine infused into the ventral hippocampus had similar effects as nicotine infused into the dorsal hippocampus. Towards this end, saline or nicotine (0.18 or 0.35 μg per side) was infused bilaterally into the ventral hippocampus prior to training, testing, or training and testing (Figure 1A & B). A one-way ANOVA revealed that there were no effects of nicotine infusion into the ventral hippocampus on baseline (F(3,27) < 1) but a main effect of infusion on contextual freezing at both the 0.18 μg (F(3,27) = 10.06, p < 0.001) and 0.35 μg (F(3,27) = 13.01, p < 0.001) doses of nicotine. Post-hoc tests revealed that nicotine infused prior to training, testing, or training and testing at both the 0.18 and 0.35 μg per side doses produced deficits in contextual fear conditioning (p's < 0.05).
Figure 1.
Infusions of nicotine into the ventral hippocampus result in deficits specifically in contextual fear conditioning. A) Infusion of 0.18 ug per side of nicotine prior to training, testing, or training and testing results in deficits in contextual fear conditioning and no alteration in baseline freezing. B) Infusions of 0.35 ug per side of nicotine prior to training, testing, or training and testing results in deficits in contextual fear conditioning and no alteration in baseline freezing. C) Infusions of 0.35 ug per side of nicotine prior to training and testing did not alter baseline, generalized or cued freezing. D) Representative depiction of placements for infusion sites into the ventral hippocampus. Depictions of the hippocampus are modified from Paxinos and Franlkin (2001). * - p < 0.05 compared to saline infused mice. Data represents mean + SEM. White bars – animals administered saline; light grey bars – animals administered nicotine prior to training only; dark grey bars – animals administered nicotine prior to testing only; black bars – animals administered nicotine prior to training and testing.
The deficit in contextual fear conditioning associated with nicotine infused into the ventral hippocampus may be due to alterations in processes not directly related to contextual fear learning such as changes in locomotor activity or anxiety. To determine if the effect of nicotine in the ventral hippocampus may be altering processes not solely involved in contextual fear learning, we examined the effects of ventral hippocampal nicotine on cued fear conditioning, a hippocampus independent form of the task (Figure 1C). Nicotine (0.35 μg per side) infused into the ventral hippocampus at both training and testing had no effect on baseline (t(10) < 1), generalized (t(10) < 1) or cued fear conditioning (t(10) < 1) suggesting that ventral hippocampal nicotine is not altering general processes. To further determine if nicotine in the ventral hippocampus could alter anxiety or locomotor activity, we examined the effects of ventral hippocampal nicotine on activity in the elevated plus maze (Table 1). Ventral hippocampal nicotine did not alter the amount of time mice spent in the open arm (t(14) = 1.35, p = 0.20), closed arm (t(14) < 1), or center area of the elevated plus maze (t(14) = 1.14, p = 0.27). Additionally, as an indirect measure of locomotor activity, the number of times mice moved from an arm of the elevated plus maze to the center area was recorded. Ventral hippocampal nicotine had no effect on arm changes in the elevated plus maze (t(14) < 1). Thus, the deficit in contextual fear conditioning due to nicotine infused into the ventral hippocampus is specific to altering contextual information processing and does not alter other general processes such as anxiety or locomotor activity.
Table 1.
Effect of 0.35 μg nicotine in the ventral hippocampus on the elevated plus maze
| Treatment | Open Arm (s) | Closed Arm (s) | Center (s) | Arm Changes |
|---|---|---|---|---|
| Saline | 92.8 ± 30.2 | 148.4 ± 31.5 | 59.2 ± 5.0 | 32.9 ± 6.9 |
| Nicotine | 51.1 ± 13.2 | 180.3 ± 19.5 | 68.9 ± 7.6 | 35.4 ± 6.7 |
Data presented as mean ± SEM.
Nicotine Infusion into the Dorsal Hippocampus
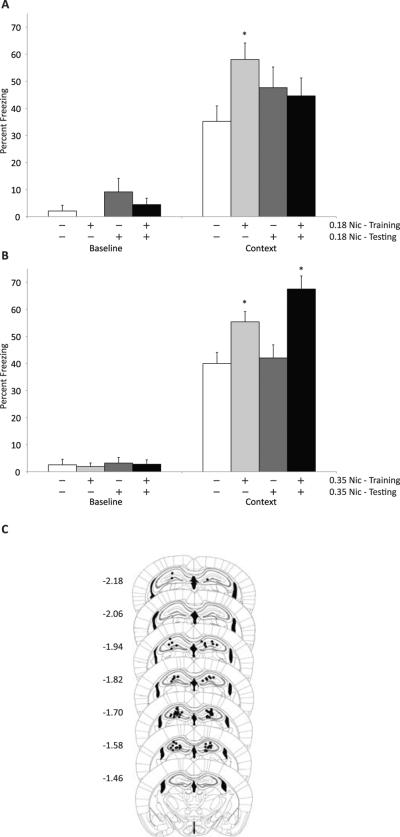
Previous studies have found that systemic nicotine must be administered prior to both training and testing to enhance contextual fear conditioning and that administration prior to training alone or testing along is insufficient for enhancement (Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney and Gould, 2008b). The fact that nicotine infused into the ventral hippocampus results in a deficit in contextual fear conditioning suggests that when nicotine is given systemically its detrimental effect in the ventral hippocampus might oppose the enhancing effect of nicotine in the dorsal hippocampus. Therefore, to determine if the effect of nicotine in the dorsal hippocampus at training or testing alone would be sufficient to enhance contextual fear conditioning, two doses of nicotine were infused directly into the dorsal hippocampus prior to training only, testing only, or training and testing (Figure 2). A one-way ANOVA revealed that there was a strong trend towards an effect at the 0.18 μg per side dose of nicotine (F(3,47) = 2.50, p = 0.071) and a significant effect at the 0.35 μg per side dose of nicotine (F(3,48) = 7.50, p < 0.001) on contextual fear conditioning and no effect on baseline freezing at either the 0.18 or 0.35 μg dose (F(3,47) = 2.10, p = 0.11 and F(3,48) < 1, respectively). Tukey post-hoc tests revealed that the 0.18 μg per side dose of nicotine administered prior to training only enhanced contextual fear conditioning (p < 0.05) and the 0.35 μg per side dose of nicotine enhanced contextual fear conditioning when given prior to training only and prior to training and testing (p's < 0.05).
Figure 2.
Infusions of nicotine into the dorsal hippocampus result in the enhancement of contextual fear conditioning. A) Infusion of 0.18 ug per side of nicotine prior to training only results in an enhancement of contextual fear conditioning and did not alter baseline freezing. B) Infusion of 0.35 ug per side of nicotine prior to training only or training and testing results in an enhancement of contextual fear conditioning and did not alter baseline freezing. C) Representative depiction of placements for infusion sites into the dorsal hippocampus. Depictions of the hippocampus are modified from Paxinos and Franlkin (2001). * - p < 0.05 compared to saline infused mice. Data represents mean + SEM. White bars – animals administered saline; light grey bars – animals administered nicotine prior to training only; dark grey bars – animals administered nicotine prior to testing only; black bars – animals administered nicotine prior to training and testing.
Nicotinic Receptor Antagonists in the Ventral Hippocampus and Systemic Nicotine
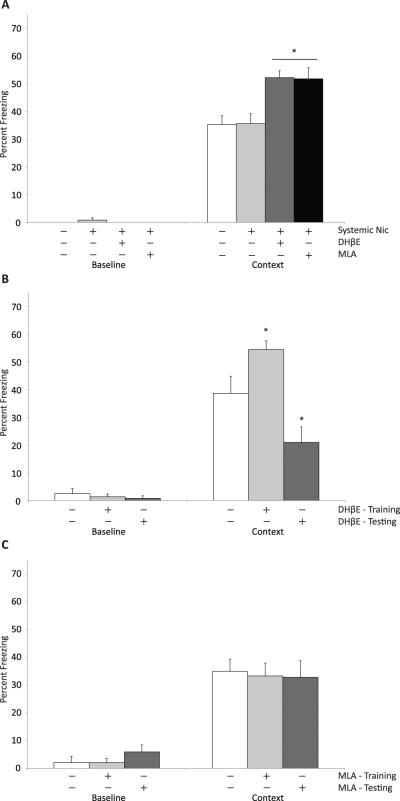
Dorsal hippocampal nicotine infusion resulted in the enhancement of contextual fear conditioning whereas nicotine in the ventral hippocampus resulted in a deficit. This suggests that when nicotine is given systemically prior to only training in contextual fear conditioning, the effect of nicotine in the ventral hippocampus may compete with the effect of nicotine in the dorsal hippocampus resulting in no enhancement of learning. To directly test this possibility a high-affinity nAChR antagonist (DHβE) or a low-affinity α7-nAChR antagonist (MLA) was infused into the ventral hippocampus prior to the systemic administration of nicotine before training in contextual fear conditioning (Figure 3A). A one-way ANOVA revealed a main effect of treatment on contextual fear conditioning (F(3,43) = 8.56, p < 0.001) and no effect on baseline freezing (F(3,43) =1.25, p = 0.30). Post-hoc tests revealed that mice infused with DHβE or MLA into the ventral hippocampus along with systemic nicotine demonstrated an enhancement of contextual fear conditioning (p's < 0.05). In agreement with prior work, there was no enhancement of contextual fear conditioning in mice given systemic nicotine prior to training only (Gould and Higgins, 2003; Gould and Wehner, 1999).
Figure 3.
Inhibition of nAChRs in the ventral hippocampus during systemic nicotine administration results in an enhancement of contextual fear conditioning. A) Administration of DHβE (18 ug per side) or MLA (27 ug per side) into the ventral hippocampus during systemic nicotine administration at training results in the enhancement of contextual fear conditioning and no effect on baseline freezing. B) Infusions of DHβE (18 ug per side) into the ventral hippocampus at training results in an enhancement of contextual fear conditioning whereas infusion of DHβE prior to testing only results in a deficit. DHβE infusions did not alter baseline freezing. C) Infusion of MLA (27 ug per side) into the ventral hippocampus at training only or testing only did not alter contextual fear conditioning or baseline freezing. * - p < 0.05 compared to saline infused/injected mice. Data represents mean + SEM.
The administration of systemic nicotine in conjunction with the infusion of either DHβE or MLA in ventral hippocampus prior to training alone resulted in an enhancement of contextual fear conditioning. This enhancing effect could be due to either antagonist administration alone or to the ability of the antagonists to block the detrimental effect of nicotine in the ventral hippocampus. To adjudicate between these two possibilities, DHβE or MLA was infused into the ventral hippocampus prior to training or testing only (Figure 3B & C, respectively). Planned contrasts revealed that mice infused with DHβE into the ventral hippocampus prior to training demonstrated enhancement of contextual fear conditioning (t(21) = 2.42, p = 0.025) whereas mice infused with DHβE prior to testing demonstrated a deficit (t(21) = 2.80, p = 0.012). This suggests that the enhancing effect observed when nicotine was administered systemically and DHβE was infused into the ventral hippocampus prior to training was likely due in part to an enhancing effect of DHβE on contextual fear conditioning. In contrast, there was no effect of MLA infusions prior to training or testing alone on contextual fear conditioning (t(14) < 1 and t(13) < 1, respectively). This suggests that the enhancement of contextual fear conditioning that was observed when systemic nicotine and ventral hippocampal MLA were administered prior to training was a result of MLA blocking the detrimental effect of nicotine in the ventral hippocampus; thus, inhibition of low-affinity α7-nAChRs in the ventral hippocampus allowed expression of the enhancing effect of systemic nicotine on contextual fear conditioning.
Discussion
Nicotine in the dorsal and ventral hippocampus has opposing effects on contextual fear conditioning. Infusion of nicotine into the dorsal hippocampus enhanced contextual fear conditioning whereas nicotine infusion into the ventral hippocampus resulted in a deficit. Additionally, the disruptive effects of nicotine in the ventral hippocampus may be mediated by α7-nAChRs since an infusion of a low-affinity α7-nAChR antagonist into the ventral hippocampus paired with systemic nicotine administered at training only, a condition that typically does not produce enhancement (Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney and Gould, 2008b), resulted in an enhancement of contextual fear conditioning. These findings not only aid in understanding the effects of nicotine on hippocampus-dependent learning and understanding hippocampal function but may also help explain why the dose-response curve for the effects of nicotine on contextual fear conditioning is an inverted U (Gould and Higgins, 2003) and why systemic nicotine has to be given at both training and testing to see enhancement (Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney and Gould, 2008b) but direct infusion into dorsal hippocampus on training day alone is sufficient to enhance contextual fear conditioning.
Neuronal nAChRs are pentameric ligand gated ion channels that are comprised of either only α-subunits or a combination of α and β subunits (Millar and Gotti, 2009). Neuronal nAChRs can be broadly characterized into two groups based on their affinity for nicotine, the low-affinity homomeric α7-containing nAChRs and the high-affinity heteromeric α and β-subunit containing receptors. The α4β2* nAChRs (*indicates may contain a variety of other subunits) make up the majority of high-affinity nAChRs in the central nervous system (Picciotto et al., 2001). Low- and high-affinity nAChRs differ with respect to their functional properties, such as rates of desensitization and relative ion permeability, as well as their distribution throughout the brain (Alkondon and Albuquerque, 2004; Picciotto, 2003; Seguela et al., 1993; Sudweeks and Yakel, 2000; Wada et al., 1989; Whiteaker et al., 1999) and the behaviors they mediate (Davis and Gould, 2007; Hoyle et al., 2006; Picciotto et al., 1998; Walters et al., 2006; Wehner et al., 2004). There is also considerable variability in the expression of nAChR subtypes along the dorsal-ventral axis of the hippocampus (Huang and Winzer-Serhan, 2006). Most striking is that there is much higher expression of α7-nAChRs in the ventral versus the dorsal hippocampus (Huang and Winzer-Serhan, 2006). This may explain, in part, why in the present study α7-nAChRs in ventral hippocampus were found to play an important role in modulating contextual fear conditioning, whereas previous work found no clear contribution of α7-nAChRs in the dorsal hippocampus for the enhancing effect of nicotine on contextual fear conditioning (Davis et al., 2007). The faster rate of desensitization and greater calcium permeability of α7-nAChRs, as compared to high-affinity nAChRs, in conjunction with differences in cellular localization, likely plays an important role in the different effects of nicotine in the dorsal versus ventral hippocampus. Such differences could result in differential activation of cell signaling cascades involved in learning thereby resulting in divergent effects on plasticity. For example, JNK1 has been implicated in the enhancement of contextual fear conditioning by nicotine and it was found that acquiring fear conditioning in the presence of nicotine results in an increase in JNK1 phosphorylation in the dorsal, but not ventral, hippocampus (Kenney et al., 2010a).
In addition to the differential effects of nicotine in the dorsal and ventral hippocampus, in the present study we found that infusion of a high-affinity nAChR antagonist, DHβE, into the ventral hippocampus results in either an enhancement or a deficit in contextual fear conditioning depending on whether it was infused prior to training or testing, respectively, whereas previous work found no effect of DHβE infusion into the dorsal hippocampus (Davis et al., 2007). Thus, there may also be considerable differences in the localization and/or subunit composition of high-affinity nAChRs along the dorsal-ventral axis. It may be the case that high-affinity nAChRs in the dorsal and ventral hippocampus are present at different cellular or subcellular locations or that they contain a different combination of α and β subunits thereby conferring varying functional properties (Albuquerque et al., 2009; Millar and Gotti, 2009).
The behavioral effects of nicotine often demonstrate a tight inverse U-shaped dose-response curve (Picciotto, 2003) and the effects of nicotine on contextual fear conditioning are no different (Gould and Higgins, 2003). The narrow dose-response curve for many of the behavioral effects of nicotine is thought to be due to the combinatorial effects of nAChR activation on various excitatory and inhibitory neurons throughout the brain (Picciotto, 2003). The findings from the present study suggest that the inverted U shape dose-response curve for the effects of nicotine on contextual fear conditioning may be a result of competition between nAChRs in the dorsal and ventral hippocampus. It may be the case that certain conditions exist in which nicotine adequately stimulates high-affinity nAChRs in the dorsal hippocampus important for enhancing contextual learning while minimizing the contribution of low-affinity nAChRs in the ventral hippocampus that work in opposition. The dorsal hippocampus appears to make a greater contribution to the effects on contextual fear conditioning when systemic nicotine is given at both training and testing as it has consistently been found that the drug must be administered prior to both time points for systemic nicotine to enhance hippocampus-dependent contextual or spatial learning (Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney et al., 2011; Kenney and Gould, 2008b). This is not a state-dependent effect as state-dependency would predict that a deficit in learning would occur if training occurred in the presence of nicotine but testing did not (Overton, 1991), an effect not seen in either the present or previous work (Bevins et al., 2007; Gould, 2003; Gould and Higgins, 2003; Kenney et al., 2011; Kenney and Gould, 2008b). The findings from the present study may provide insight into why nicotine must be given at training and testing to see enhancement of hippocampus-dependent learning (Gould and Higgins, 2003; Gould and Wehner, 1999; Kenney et al., 2011; Kenney and Gould, 2008b). The nAChRs in the dorsal and ventral hippocampus may differentially respond to a second administration of nicotine depending on the dose, perhaps due to the differential expression of nAChR subunits in the dorsal and ventral hippocampus (Huang and Winzer-Serhan, 2006). Administering nicotine at both training and testing could lead to greater contribution of the high-affinity nAChRs in the dorsal hippocampus and/or a decreased contribution of low-affinity nAChRs in the ventral hippocampus to the behavioral outcome. In support of this interpretation, we have previously found that administration of a high-affinity nAChR agonist at training day only results in the enhancement of contextual fear conditioning (Kenney et al., 2010b) suggesting that in the absence of low-affinity nAChR stimulation, systemic high-affinity nAChRs activation at training alone is sufficient to enhance contextual learning.
In addition to differences in nAChRs in the dorsal and ventral hippocampus, other intrinsic properties of neurons in the dorsal and ventral hippocampus may contribute to the differential effects of nicotine within these two structures. The dorsal and ventral hippocampus have different interconnections with the rest of the brain (see Fanselow and Dong, 2010 for review) that likely underlie any distinct contributions to contextual learning. The dorsal hippocampus both receives afferents and sends efferents to various cortical areas known to be important for the processing of visuospatial information whereas the most prominent ventral hippocampal afferents are from the olfactory and gustatory areas and efferents project to the amygdala and hypothalamus (Amaral and Lavenex, 2007; Cenquizca and Swanson, 2007; van Groen and Wyss, 1990). As would be expected based on the anatomical data, lesions of the dorsal CA1 region lead to deficits in spatial learning and spatial and visual temporal processing whereas lesions of the ventral CA1 region lead to deficits in olfactory temporal processing, but not spatial learning (Bannerman et al., 2002; Hunsaker et al., 2008; Kesner et al., 2010; Moser et al., 1993; Moser and Moser, 1998; Pothuizen et al., 2004; Zhang et al., 2004). Nonetheless, the ventral hippocampus has been found to make important mnemonic contributions to contextual learning (Rudy and Matus-Amat, 2005), although it is not yet clear what the specific or unique contributions may be. In addition to the connectivity of the dorsal and ventral hippocampus, there are also differences in the intrinsic properties of pyramidal cells in the two regions. CA1 pyramidal cells in the ventral hippocampus have lower levels of excitability than those in the dorsal hippocampus (Maggio and Segal, 2009). Furthermore, long-term potentiation (LTP), a cellular model of plasticity, in the ventral CA3-CA1 pathway is of lower magnitude than in the dorsal CA3-CA1 pathway and involves different mechanisms (Maggio and Segal, 2007; Maruki et al., 2001; Papatheodoropoulos and Kostopoulos, 2000). Thus, the interconnections of the dorsal and ventral hippocampus with other neural areas important for processing various aspects of contextual fear memory and differences in the intrinsic properties of pyramidal cells in these structures likely contribute to the opposing effects of nicotine in the dorsal and ventral hippocampus on contextual fear learning.
The dorsal and ventral poles of the hippocampus have been found to make distinct contributions to anxiety and locomotor activity. Lesions of the ventral hippocampus are consistently anxiolytic whereas lesions of the dorsal hippocampus tend to be without effect on measures of anxiety (Bannerman et al., 2002; Bannerman et al., 2003; Kjelstrup et al., 2002; McHugh et al., 2004). Ventral hippocampal manipulations have also been found to alter locomotor activity (Bast et al., 2001; Yoon and Otto, 2007), but this finding is less consistent than changes in anxiety (Bannerman et al., 2003; Bannerman et al., 1999). In the present study, however, it is unlikely that the deficits in contextual fear conditioning due to nicotine infusion into the ventral hippocampus were strictly due to alterations in anxiety or locomotor activity. If this were the case, then one would expect that nicotine in the ventral hippocampus would also result in deficits in cued fear conditioning and alterations in time spent in the open arms of the elevated plus maze behavior, neither of which were observed. Thus, the detrimental effect of nicotine on contextual fear conditioning when infused into the ventral hippocampus most likely reflect changes in contextual learning and not alterations in anxiety or locomotion.
Overall, the present study found that nAChRs in the dorsal and ventral hippocampus differentially modulate contextual fear learning. Combined with previous work, our findings provide evidence for a model in which the effects of nicotine at high-affinity nAChRs in the dorsal hippocampus act in opposition to the effects of nicotine at low-affinity nAChRs in the ventral hippocampus to alter contextual fear learning. Thus, manipulations that result in greater nAChR activation in the ventral hippocampus may shift the mnemonic effect of nicotine from facilitative to detrimental whereas manipulations that increase the contribution of the dorsal hippocampus may result in facilitation of learning. The findings of the current study are of broad importance given that alterations in hippocampal nAChR function have been implicated in various neuropathologies such as Alzheimer's disease, Parkinson's disease, and addiction (Picciotto and Zoli, 2008; Woodruff-Pak and Gould, 2002). Interestingly, patients with Alzheimer's disease initially show atrophy in the anterior (ventral) hippocampus while the posterior (dorsal) hippocampus is largely spared (Raji et al., 2009; Whitwell et al., 2007). This suggests that understanding differences in nAChRs across the dorsal/ventral axis may aid in developing therapeutics that could potentially target nAChRs in the anterior hippocampus. Thus, the present study suggests that a deeper understanding of these diseases, and the role of nAChRs in their etiology and pathology, requires an examination of how nAChR function varies along the dorsal-ventral axis of the hippocampus.
Acknowledgments
The authors would like to acknowledge grant support from the National Institute of Drug Abuse and the National Cancer Institute (DA01749, DA024787, TJG; CA143187, PI Caryn Lerman). JWK and JDR were supported by a NIDA training grant (DA07237). All behavioral procedures were performed in accordance with NIH guidelines for the use of laboratory animals.
Grant Information
National Institute of Drug Abuse and the National Cancer Institute: DA01749, DA024787, TJG; DA07237, JWK & JDR; CA143187, PI Caryn Lerman
Footnotes
The authors declare that they have no conflicts of interest.
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology (Berl) 1996;124(4):323–31. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–20. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. Oxford University Press; New York: 2007. pp. 37–114. [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116(5):884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139(1–2):197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113(6):1170–88. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134(1–2):299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139(1):39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13(6):657–75. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal tracking task with rats. Behav Brain Res. 2007;177(1):134–41. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969;68(1):129–35. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56(1):1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T, Bisgaard CF, Nielsen HB, Wiborg O. Transcriptome differentiation along the dorsoventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience. 2010;170(3):731–41. doi: 10.1016/j.neuroscience.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190(3):343–52. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27(40):10870–7. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol. 1998;398(1):25–48. [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003;38(2):124–32. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80(2):147–57. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102(1–2):31–9. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189(2):211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain Res. 2006;1113(1):94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal and ventral CA1 for the temporal processing of spatial locations, visual objects, and odors. Behav Neurosci. 2008;122(3):643–50. doi: 10.1037/0735-7044.122.3.643. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89(1):61–9. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Adoff MD, Wilkinson DS, Gould TJ. The effects of acute, chronic, and withdrawal from chronic nicotine on novel and spatial object recognition in male C57BL/6J mice. Psychopharmacology (Berl) 2011;217(3):353–65. doi: 10.1007/s00213-011-2283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Florian C, Portugal GS, Abel T, Gould TJ. Involvement of Hippocampal Jun-N Terminal Kinase Pathway in the Enhancement of Learning and Memory by Nicotine. Neuropsychopharmacology. 2010a;35(2):483–92. doi: 10.1038/npp.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008a;38(1):101–21. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008b;122(5):1158–65. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Wilkinson DS, Gould TJ. The enhancement of contextual fear conditioning by ABT-418. Behav Pharmacol. 2010b;21(3):246–9. doi: 10.1097/FBP.0b013e32833a5b9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Ziegler W. The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiol Learn Mem. 2010;93(1):111–6. doi: 10.1016/j.nlm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99(16):10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo ED, Richardson-Jones JW, Sibille E, Kottman A, Hen R. Molecular heterogeneity along the dorsal-ventral axis of the murine hippocampal CA1 field: a microarray analysis of gene expression. Neuroscience. 2006;137(1):177–86. doi: 10.1016/j.neuroscience.2005.08.082. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111(1):104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Potter AS, Simoni MK, Bucci DJ. Nicotine administration enhances conditioned inhibition in rats. Eur J Pharmacol. 2006;551(1–3):76–9. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Segal M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus. 2007;17(1):10–25. doi: 10.1002/hipo.20237. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J Neurosci. 2009;29(9):2857–66. doi: 10.1523/JNEUROSCI.4399-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav Neurosci. 2004;118(1):97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Nomura M, Yamauchi T. Differences in paired-pulse facilitation and long-term potentiation between dorsal and ventral CA1 regions in anesthetized rats. Hippocampus. 2001;11(6):655–61. doi: 10.1002/hipo.1080. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118(1):63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13(9):3916–25. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Overton DA. Historical context of state dependent learning and discriminative drug effects. Behav Pharmacol. 1991;2(4 And 5):253–264. [PubMed] [Google Scholar]
- Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140(1):163–75. doi: 10.1016/j.neuroscience.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. Decreased ability of rat temporal hippocampal CA1 region to produce long-term potentiation. Neurosci Lett. 2000;279(3):177–80. doi: 10.1016/s0304-3940(99)01002-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Edition n. Academic Press; San Diego, CA: 2001. translator. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;24(9):493–9. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92(2–3):89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta Pharmacol Sin. 2009;30(6):752–60. doi: 10.1038/aps.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19(3):705–12. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73(22):1899–905. doi: 10.1212/WNL.0b013e3181c3f293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem. 2010;94(3):353–63. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–8. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav Neurosci. 2005;119(1):154–63. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29(9):1779–92. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J Physiol. 2000;527(Pt 3):515–28. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Huang F, Li P, Li Z, Zhou S, Deng H, Yang Y. Nicotine enhances contextual fear memory reconsolidation in rats. Neurosci Lett. 2011;487(3):368–71. doi: 10.1016/j.neulet.2010.10.058. [DOI] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13(1):133–49. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990;302(3):515–28. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284(2):314–35. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184(3–4):339–44. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129(1):11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Davies AR, Marks MJ, Blagbrough IS, Potter BV, Wolstenholme AJ, Collins AC, Wonnacott S. An autoradiographic study of the distribution of binding sites for the novel alpha7-selective nicotinic radioligand [3H]-methyllycaconitine in the mouse brain. Eur J Neurosci. 1999;11(8):2689–96. doi: 10.1046/j.1460-9568.1999.00685.x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130(Pt 7):1777–86. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Gould TJ. Neuronal Nicotinic Acetylcholine Receptors: Involvement in Alzheimer's Disease and Schizophrenia. Behavioral and Cognitive Neuroscience Reviews. 2002;1(1):5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87(4):464–75. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126(1–2):159–74. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Pothuizen HH, Feldon J, Rawlins JN. Dissociation of function within the hippocampus: effects of dorsal, ventral and complete excitotoxic hippocampal lesions on spatial navigation. Neuroscience. 2004;127(2):289–300. doi: 10.1016/j.neuroscience.2004.05.007. [DOI] [PubMed] [Google Scholar]