Abstract
Metastatic melanoma is one of the most deadly and evasive types of cancer. On average, cancer patients with metastatic melanoma survive only 6–9 months after diagnosis. Epidemiological and animal studies suggest that obesity increases the metastatic ability of malignant melanoma, though the mechanism is not known. In the present studies, we assessed the ability of 3T3L1 adipocytes to modulate B16BL6 melanoma cell invasion and the Epithelial-to-Mesenchymal Transition (EMT). For this purpose, we induced the differentiation of 3T3L1 fibroblasts to adipocytes. Then, we collected the cell culture media from both fibroblasts and adipocytes and determined their effect on the invasive ability and EMT gene expression of B16BL6 melanoma cells. Results show that adipocyte media increased that ability of B16BL6 cells to invade. The higher invasive ability of B16BL6 melanoma cells was associated with increased expression of EMT genes such as Snai1, MMP9, Twist, and Vimentin. Additionally, the expression of the cell-to-cell adhesion protein E-cadherin and the metastasis suppressor gene Kiss1 were down-regulated in these B16BL6 cells. Also, adipocytes had high levels of the pro-inflammatory cytokine Interleukin 6 (IL-6). Treatment of B16BL6 cells with IL-6 elicited similar effects as the adipocyte media; IL-6 promoted the invasive ability of B16BL6 melanoma cells, increased the expression of Snai1, and decreased Kiss1 expression. IL-6 neutralization, however, did not have a visible effect on adipocyte media-induced invasion and snai1 staining. In summary, adipocytes may increase the invasive ability of B16BL6 melanoma cells by promoting EMT and decreasing the expression of genes such as E-cadherin and Kiss1.
Keywords: 3T3L1 adipocytes, B16BL6, EMT, Snai1, Kiss1
Introduction
Malignant melanoma accounts for about 4% of all skin cancer cases but is responsible for 75% of skin cancer-related deaths, which is approximately 9,000 deaths per year, according to the American Cancer Society [1]. Current treatments for malignant melanoma are not very effective; as a result, metastatic melanoma patients typically survive for only about 6–9 months after diagnosis [2]. Studies suggest that obesity increases the ability of malignant melanoma to metastasize [3, 4].
Approximately 60% of the U.S. population is considered overweight or obese [5]. In obesity, fat cells produce inflammatory factors such as the cytokine Interleukin 6 (IL-6), which can lead to the development of a low-grade inflammation environment [6]. This inflammation environment has been hypothesized to increases cancer risk [7]. Studies show that obesity increases the risk of melanoma. Dennis et al. showed that obesity increased the risk of developing subcutaneous melanoma [8]; furthermore, Samanic at al. demonstrated that obesity increased the risk of developing malignant melanoma [3]. In animal studies, Mori et al. showed that obesity promoted B16BL6 melanoma pulmonary metastasis [4]. The mechanism by which obesity exacerbates melanoma metastasis, however, is not completely known.
Our hypothesis is that the microenvironment surrounding the melanoma cells plays an important role in obesity’s ability to promote metastasis. Obesity may cause a pro-cancerous microenvironment by providing growth factors and inflammatory factors that can increase metastasis [9]. Some of the cells that comprise the cancer microenvironment include cancer-associated fibroblasts (FACs), macrophages (TAMs), and adipocytes [10–12]. These cancer-associated cells are capable of secreting factors that can impact cancer metastasis [13, 14].
Our present objective is to determine if adipocytes promote melanoma metastasis. For this purpose, we differentiated 3T3L1 fibroblasts into adipocytes, and then investigated the effect of 3T3L1 fibroblast and adipocyte media on B16BL6 melanoma cell invasiveness and the Epithelial-to-Mesenchymal Transition (EMT). EMT is a critical cellular program for the initiation of the metastatic cascade [15]. Cancer cells with a mesenchymal phenotype have a higher propensity to metastasize than those with an epithelial phenotype [16]. Increased expression of the transcription factors Snai1 and Twist are associated with increased EMT and increased ability of cancer cells to migrate, invade, and metastasize [17, 18]. Furthermore, the aberrant expression of Snai1 and Twist can lead to a significant loss of cell-to-cell adhesion proteins such as E-cadherin [19]. The mesenchymal phenotype is also associated with high expression of Matrix Metalloproteases (MMPs) such as MMP9 that can degrade the extracellular matrix (ECM) and allow the cancer cells to metastasize [20]. Thus, inflammatory cytokines such as IL-6 may promote EMT and increase the metastatic ability of melanoma cells.
Furthermore, increased EMT has been associated with decreased expression of various metastasis suppressors [21]. Metastasis suppressor genes encode proteins that have the ability to inhibit the establishment of metastases [22]. Metastasis suppressor genes such as Kiss1 can inhibit the metastatic ability of melanomas [23]. Thus, it is feasible that adipocytes secrete factors that promote the metastatic ability of melanomas by increasing the expression of pro-metastatic EMT genes and by decreasing the expression of metastasis suppressors such as Kiss1.
In the present study, we show that adipocyte media increased B16BL6 cell migration and invasion. Increased motility and invasion are hallmarks of metastasis and rely on the induction of EMT [24]. We further show that adipocyte media increased the expression of EMT-associated genes such as Snai1, Twist, MMP9, and Vimentin while the expression of E-cadherin and Kiss1 were decreased in B16BL6 cells by exposure to the adipocyte media. Lastly, we show that the cytokine IL-6, which was increased in adipocytes, could also promote B16BL6 cell invasion, increase Snai1 expression, and decrease Kiss1 expression to levels comparable to the adipocyte media. Thus, results suggest that adipocytes may increases the metastatic ability of melanoma cells via inflammatory factors, such as IL-6.
Methods and Procedures
Cancer Cells and Cell Culture Reagents
B16BL6 melanoma cells were kindly provided by Dr. Isaiah J. Fidler, University of Texas MD Anderson Cancer Center, Houston TX. They were maintained in high glucose Dulbecco’s modified minimum essential medium (DMEM) (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (Invitrogen) and 1% antibiotic-antimycotic solution (CellGro, Manassas, VA), and grown at 37°C in a humidified atmosphere of 5% CO2. For cell culture studies, B16BL6 cells were treated with 5% adipocyte media, 5% fibroblast media, or control DMEM with no phenol red (Invitrogen). Purified IL-6 was purchased and reconstituted in PBS containing 0.1% BSA to a concentration of 10 ug/mL and stored in −80°C until used (R&D Systems, Minneapolis, MN). Cells were treated with 1 ng/mL IL-6 in control DMEM for the IL-6 treatments at the various time points.
Differentiation of 3T3L1 Fibroblasts to Adipocytes
To test the effect of 3T3L1 adipocytes on B16BL6 cell invasion and EMT gene expression, 3T3L1 fibroblasts were purchased from the American Type Culture Collection (ATCC, Chicago, IL; no. CL-173). They were maintained in DMEM supplemented with 10% FBS until they were differentiated into adipocytes as described previously [25]. Adipocyte differentiation was confirmed through visual observation of lipid droplets as well as Oil Red O staining as previously described [26]. For collection of fibroblast or adipocyte media, 3T3L1 cells were split into two equal plates. For fibroblast-conditioned media, cells were allowed to proliferate until the plate was 70% confluent. Cells were rinsed twice with PBS, and DMEM was subsequently added. After 24 h, the media was collected, centrifuged at 10,000 rpm for 10 min at 4°C, and the supernatant was collected and stored at −80°C (Fibroblast Media). The other plate was differentiated into adipocytes accordingly, and after full differentiation (day 14), adipocytes were rinsed twice with PBS, and DMEM was subsequently added. After 24 h, the media was collected, centrifuged at 10,000 rpm for 10 min at 4°C, and then the supernatant was collected and stored at −80°C (Adipocyte Media). These conditioned media were then used to determine their effect on B16BL6 melanoma migration and invasive ability as well as their ability to influence the EMT phenotype.
IL-6 Neutralization of Adipocyte Media
Adipocyte media was thawed on ice before being incubated with 2 ug/mL of IL-6 antibodies (Ambion/Applied Biosystems, Austin, TX) on a rotary shaker for 4 h at 4°C. 100 uL of Protein A/G agarose slurry (Pierce Thermo Scientific, Waltham, MA) was added and subsequently incubated overnight at 4°C. Then, the media was centrifuged at 10,000 rpm for 5 min at 4°C to remove the beads. The supernatant was collected and stored at −80°C. This media was referred to as IL6-neutralized adipocyte media throughout this manuscript. A decrease in IL-6 expression was confirmed with western blot analysis before the media was used for subsequent assays.
Wounding Assay
To determine the effect of adipocyte media on B16BL6 cell migration, we performed the wounding assay. Cells were plated to 100% confluency and a scratch was drawn in the middle of the plate. The decrease in gap distance was measured over time and quantified; the larger the gap distance that remained after a given time point, the less the cells have migrated. Briefly, B16BL6 cells were grown on a 24-well plate until they were confluent. Then, cells were grown in FBS-free DMEM overnight. The next day, wells were washed twice with PBS and a scratch was drawn on each well using a p200 pipette tip. After two more washes with PBS to get rid of cell debris, the following cell culture media was added: 5% adipocyte media, 5% fibroblast media or control DMEM. Each well was photographed at the time of treatment (0 h) and after 16 h of incubation (16 h) at 40X. The difference in gap distance was measured (16 h time point — 0 h time point) to quantify cell motility. Each experiment was repeated three times with each group having 3 wells per experiment.
Invasion Assay
The effect of adipocyte media on B16BL6 cell invasion was determined by the Boyden chamber invasion assay. In this assay, the top chamber was previously coated with BD Matrigel™ that is composed of various extracellular matrix proteins such as laminin and collagen (BD Biosciences, Franklin Lakes, NJ). Cells were FBS-starved overnight, then rinsed with PBS before being trypsinized and counted by hemocytometry. 100,000 cancer cells were placed in the top chamber in serum-free DMEM with 0.1% Bovine Serum Albumin (BSA). The lower chamber was filled with DMEM (control), 5% adipocyte media, 5% IL-6 neutralized adipocyte media, 5% fibroblast media, or DMEM containing 1 ng/mL IL-6. Cells were allowed to invade from the top side towards the bottom side of each chamber for 28 h. Cells that invaded to the bottom side were fixed and stained using Siemens Diff-Quick Stain Set (Siemens, Malvern, PA). Stained cells were visualized and quantified by microscopy. To determine the average number of cells that migrated for each well, we counted 3 random fields in each well at 200X; each treatment had three individual wells. Three separate experiments were carried out.
Quantitative Real-Time PCR (qRT-PCR)
To determine the effect of adipocyte media on the expression of several genes associated with EMT, qRT-PCR was performed. Total RNA was collected from cells that were FBS-starved overnight and treated with control media, 5% fibroblast media, 5% adipocyte media, or 1 ng/mL IL-6 for the stated amount of time. RNA was extracted using an RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). Reverse transcription was performed with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), using 1 μg of RNA for each reaction. Primers for 18S, Snai1, MMP9, Twist, Vimentin, E-cadherin, and Kiss1 were purchased from Integrated DNA Technologies (IDT, Coralville, IA) (Table 1). qRT-PCR was performed with a SYBR GreenER qPCR kit (Invitrogen) in a Mastercycler ep Realplex Real-time PCR thermocycler (Eppendorf North America, Hauppauge, NY). The relative expression levels of target genes were normalized to the housekeeping 18S rRNA. Amplification specificity was confirmed by melting curve analysis. Each gene was measured in quadruplicate, and the average ΔCt was taken from the 4 wells before fold change was calculated using the ΔΔCt method. At least three separate experiments were carried out.
Table 1.
Primer sequences used for qRT-PCR
| Gene | Primer Sequence (5′ to 3′) | NT |
|---|---|---|
| 18S | GCATGGCCGTTCTTAGTTGGTGGA | 24 |
| TCTCGGGTGGCTGAACGCCA | 20 | |
| Snai1 | CACCTCCAGACCCACTCAGAT | 21 |
| CCTGAGTGGGGTGGGAGCTTCC | 22 | |
| MMP9 | GCCCACCGTCCTTTCTTGTTGGA | 23 |
| GGGAGAGGTGGTTTAGCCGGTG | 22 | |
| Twist | CCACGCTGCCCTCGGACAAG | 20 |
| CCAGGCCCCCTCCATCCTCC | 20 | |
| Vimentin | CCAGAGACCCCAGCGCTCCT | 20 |
| GCCGGAGCCACCGAACATCC | 20 | |
| E-cadherin | TTGAGGAGTTGAATGCTGAC | 20 |
| AGCTCGAACTTTCCAAGCAG | 20 | |
| Kiss1 | GCAAGCCTGGGTCTGCAGGG | 20 |
| CGACTGCGGGAGGCACACAG | 20 |
Western Blot Analysis
To determine the protein expression of IL-6 in 3T3L1 fibroblasts and 3T3L1 adipocytes, Western blot analysis was carried out on whole cell lysates. 3T3L1 fibroblasts and differentiated 3T3L1 adipocytes were washed twice with PBS before being lysed in 300 uL RIPA buffer (Thermo Scientific) supplemented with a protease inhibitor cocktail (Roche, South San Francisco, CA). After 45 min incubation on ice, cells were centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was collected and protein concentration was measured using the Bradford Assay. 100ug of protein was loaded and run on an SDS-PAGE gel at 100–150 V for 1 h. The gel was subsequently transferred to a PVDF membrane, blocked in 5% milk, washed numerous times in TBST, and blotted with appropriate antibodies and HRP-conjugated secondary antibodies before incubation with ECL and film exposure. Membranes were probed using primary antibody for IL-6 (Abcam, Cambrigde, UK; no.ab6672) and β-actin (Cell Signaling, Danvars, MA, no. 4967 L) and respective secondary antibodies (Cell Signaling, no. 7074; and Santa Cruz Biotechnology Inc., Santa Cruz, CA, no. 2768).
We also measured the protein expression of IL-6 Receptor α and Snai1 in B16BL6 cells as a response to the various treatments. For this purpose, B16BL6 cells were grown to about 70% confluency in 10 cm3 plates. Cells were rinsed with PBS and then FBS-starved overnight. Then, cells were treated with control DMEM, 5% adipocyte media, 5% fibroblast media, or 1 ng/mL IL-6 in DMEM. After the various time-point treatments, cells were rinsed twice with PBS, trypsinized, and centrifuged at 10,000 rpm for 5 min at 4°C. The supernatant was discarded, and the pellets were stored in −80°C overnight. Total protein was collected and quantified as described above. For western blotting, 100 ug of protein samples were loaded in each well, ran, and transferred to a PMSF membrane before being probed for IL-6 Receptor α (Santa Cruz Biotechnology Inc., IL-6Rα (H-300), no. 13947), Snai1 (Abcam, no. 63371), and β-actin.
Flow Cytometry
B16BL6 cells were grown to 70% confluency in 6 cm3 plates. Cells were rinsed with PBS and FBS-starved overnight. Then, cells were treated with control DMEM, 5% adipocyte media, or 1 ng/mL IL-6 in DMEM. After the various time point treatments, cells were rinsed with PBS, trypsinized, and centrifuged at 10,000 rpm for 5 min at 4°C. The supernatant was decanted and the pellet was subsequently vortexed thoroughly to make a single cell suspension. Cells were resuspended in 1 mL cold PBS. Cells were subsequently added drop-wise into 2 mL of cold 100% ethanol while vortexing and stored at −20°C for up to 1 week. Then, 2 mL of this solution containing the cells were centrifuged at 1,600 rpm for 4 min at 4°C. Cells were washed twice with PBS, then permeabilized with 0.1% triton-X100/PBS and treated with antibodies against Snai1 or Kiss1 (Santa Cruz Biotechnology Inc., no. sc-18134) and appropriate fluorescently-conjugated secondary antibodies (Cell Signaling, no. 4412; and Abcam, no. ab6949) as recommended by the manufacturer before being subjected to flow cytometry. Mean fluorescent intensity was used as a measure of protein expression.
Immunofluorescence Microscopy
Wax pencils were used to mark a closed circle on microscope coverslips. B16BL6 cells were plated into the circles and allowed to attach for 24 h before being FBS-starved overnight. They were subsequently treated with 5% adipocyte media, 5% IL-6 neutralized adipocyte media, 1 ng/mL IL-6, or control DMEM for 24 h. Cells were washed twice with PBS and fixed in 4% formalin/PBS for 10 min. Then, cells were subsequently washed twice with PBS before being stored in PBS at 4°C overnight. The next day, cells were permeabilized with 0.1% Triton-X100/PBS and neutralized with 100 uM glycine/PBS, before being treated with antibodies against Snai1 or Kiss1 and appropriate fluorescently-conjugated secondary antibodies (Cell Signaling and Abcam) as recommended by the manufacturer. After three washes with 0.2% Tween20 in PBS, cells were counterstained with two drops of DAPI/antifade (Millipore) according to the manufacturer’s instructions for detection of cellular nuclei. After 15 min of incubation, coverslips were placed onto microscope slides and sealed with nail polish, and images were taken with the Zeiss Axiovert 200 M fluorescent microscope at UT Austin’s ICMB Core Facility. Images of control and experimental cells were acquired under identical exposure conditions for comparative analysis.
Statistical Analysis
Experiments were analyzed for significance using the independent Student’s t-test or One-way ANOVA in SPSS (PAWS version 18). P-values ≤ 0.05 were considered significant, and all data is represented as the mean ± SEM where appropriate.
Results
Adipocyte Media Promotes B16BL6 Cell Motility and Invasion
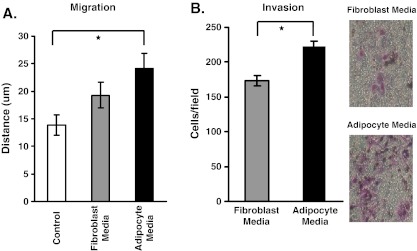
We determined the effect of adipocyte media on B16BL6 cell motility and cell invasion. Results show that adipocyte media promoted the migration and invasion of B16BL6 cells to a higher degree than DMEM alone or fibroblast media (Fig. 1a,b).
Fig. 1.
Adipocyte media increases B16BL6 cell invasion. B16BL6 cells were exposed to 5% adipocyte media, 5% fibroblast media, or control DMEM for the wounding and invasion assay. a In the wounding assay, B16BL6 cells were exposed to their respective media for 16 h, and distance migrated was calculated. b In the invasion assay, B16BL6 cells were exposed to their respective media for 28 h, and number of cells that invaded was determined. The data are representative of three separate experiments, presented as ± SEM (*p < 0.05)
Adipocyte Media Affects EMT Gene Expression in B16BL6 Cells
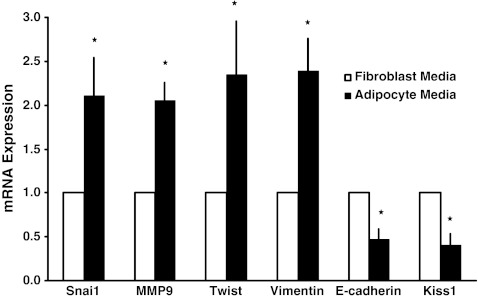
To better understand the mechanism by which adipocyte media affects the invasive phenotype of B16BL6 cells, we determined the effect of adipocyte media on the expression of genes associated with the Epithelial-to-Mesenchymal Transition (EMT). Cancer cells with a more mesenchymal phenotype metastasize better than those that have an epithelial phenotype [16]. The mesenchymal phenotype is associated with the expression of transcription factors such as Snai1 and Twist, MMPs such as MMP9, and the intermediate filament protein Vimentin [21, 27]. Results show that adipocyte media increased the expression of Snai1, Twist, MMP9, and Vimentin in B16BL6 cells (Fig. 2). Adipocyte media also decreased the expression of the cell-to-cell adhesion marker E-cadherin in B16BL6 cells (Fig. 2). EMT has been associated with a decreased expression of various metastasis suppressor genes, including Kiss1 [21]. Our results are consistent with this finding, as we show that the expression of Kiss1 is reduced by adipocyte media in B16BL6 cells (Fig. 2).
Fig. 2.
Effects of adipocyte media on EMT gene expression in B16BL6 cells. Cells were treated with 5% adipocyte media or 5% control fibroblast media for 24 h. Adipocyte media increased Snai1, Matrix Metalloprotease 9 (MMP9), Twist, and Vimentin mRNA levels. Conversely, adipocyte media decreased the expression of E-cadherin and the metastasis suppressor Kiss1. Relative mRNA fold change was normalized to 18S rRNA and expressed as fold change over untreated controls. The data are representative of at least three separate experiments, presented as ± SEM (*p < 0.05)
IL-6 Treatment Increases B16BL6 Cell Invasion
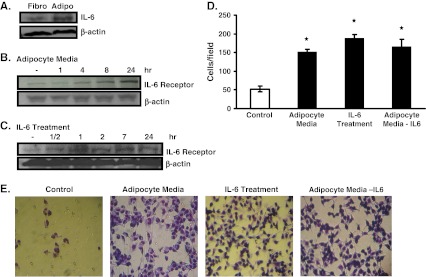
We measured the expression of the inflammation marker IL-6 in both 3T3L1 fibroblasts and 3T3L1 adipocytes. The protein expression of IL-6 is higher in 3T3L1 adipocytes than in 3T3L1 fibroblasts (Fig. 3a). Thus, it is feasible that adipocytes promote the aggressiveness and EMT phenotype in B16BL6 melanoma cells via IL-6. In fact, we show that adipocyte media increased the expression of the IL-6 Receptor α (IL-6Rα) in B16BL6 melanoma cells in a time-dependent manner (Fig. 3b). In addition, we show that direct treatment of B16BL6 melanoma cells with IL-6 also increased the expression of IL-6Rα (Fig. 3c). We next determined the ability of IL-6 to affect the invasive ability of B16BL6 melanoma cells. Results show that IL-6 treatment increased cell invasion to levels comparable to that induced by adipocyte media (Fig. 3d,e). To determine the role of IL-6 found in the cell culture media, we neutralized it using antibodies against IL-6, then determined the ability of this IL6-neutralized adipocyte media on its ability to stimulate B16BL6 invasion. Results show that neutralization of IL-6 did not alter the ability of the adipocyte media to affect the invasive ability of B16BL6 cells (Fig. 3d,e). Thus, it is feasible that IL-6 may not be the only contributing factor found in the adipocyte media that can affect the invasiveness of B16BL6 cells. Future studies that fully inhibit the IL-6/IL-6Receptor signaling pathway are necessary, however, to completely rule out the importance of IL-6 in the ability of adipocyte media to affect the aggressiveness of B16BL6.
Fig. 3.
IL-6 treatment increased B16BL6 cell invasion. a IL-6 protein expression was increased in 3T3L1 adipocytes compared to 3T3L1 fibroblasts as observed by Western blot analysis. b B16BL6 cells were exposed to 5% adipocyte media for the given time period, and IL-6Rα levels were measured by Western blot analysis. c B16BL6 cells were exposed to 1 ng/mL IL-6 for the given time period, and IL-6Rα levels were measured by Western blot analysis. d B16BL6 cells were exposed to 1 ng/mL IL-6, 5% adipocyte media, 5% IL-6 neutralized adipocyte media, or control DMEM for 28 h in the invasion assay. The average number of cells that invaded when exposed to each treatment was calculated and quantified. e Representative pictures of fields used to quantify cell invasion in d). The data are representative of at least three separate experiments and presented as ± SEM where appropriate (*p < 0.05)
IL-6 Affects the Expression of Snai1 and Kiss1 in B16BL6 Cells
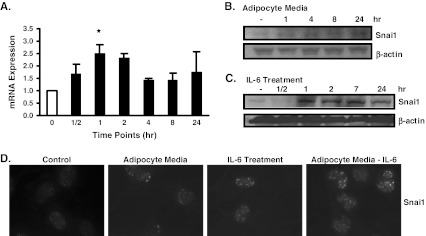
To determine if the effects of IL-6 on the expression of IL-6Rα correlated with changes in the expression of Snai1 and Kiss1, we measured both their mRNA and protein levels in B16BL6 cells. Results show that Snai1 mRNA and protein levels were increased by IL-6 treatment (Fig. 4a,c). This effect of IL-6 on Snai1 was also observed with adipocyte media, which increased the protein expression of Snai1 in a time-dependent manner (Fig. 4b). In contrast, fibroblast media did not affect the expression of Snai1 in B16BL6 cells (data not shown). We determined that adipocyte media and IL-6 treatment affected Snai1 nuclear levels. For this purpose, we used immunofluorescence microscopy. Results show that Snai1 was increased in the nucleus of B16BL6 cells as a result of adipocyte media and IL-6 treatment (Fig. 4d). However, IL-6 neutralized adipocyte media also increased Snai1 nuclear staining in B16BL6 cells (Fig. 4d). Thus, it is feasible that other factors in the adipocyte media affect Snai1 expression. All together, our results support the notion that IL-6 can increase Snai1 expression as previously observed in other cancers [28, 29].
Fig. 4.
IL-6 treatment increased Snai1 expression in B16BL6 cells. B16BL6 cells were treated with 1 ng/mL IL-6 for the stated time periods or as indicated. a mRNA levels of Snai1 were increased at the time points, where the highest and significant change was observed at the 1 h time point b B16BL6 cells were treated with 5% adipocyte media for 24 h. Snai1 protein levels were increased in a time dependent manner c Similarly, Snai1 protein levels were increased in a time dependent manner after IL-6 treatment d Nuclear staining of Snai1 was increased in B16BL6 cells treated with IL-6, 5% adipocyte media, or 5% IL-6 neutralized adipocyte media compared to control DMEM as observed by Immunofluorescence microscopy. The data is representative of at least three separate experiments and presented as ± SEM where appropriate (*p < 0.05)
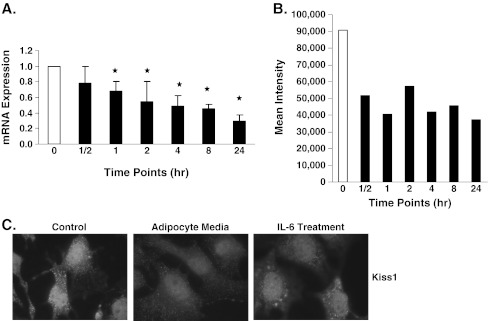
Because we show that both adipocyte media and IL-6 treatment increased the ability of B16BL6 cells to invade, we rationalized that these treatments may affect the expression of metastasis suppressor genes. We chose to determine the effect of IL-6 treatment on the expression of Kiss1 because it has been shown to be decreased during the EMT [21]. Results show that IL-6 treatment decreased Kiss1 mRNA and protein expression in a time-dependent manner (Fig. 5a,b). Immunofluorescence microscopy experiments confirmed these results and showed that both IL-6 and adipocyte media treatment decrease Kiss1 expression at the protein level (Fig. 5c). Thus, results suggest that both IL-6 and adipocytes affect EMT.
Fig. 5.
IL-6 treatment decreased Kiss1 expression in B16BL6 cells. B16BL6 cells were treated with 1 ng/mL IL-6 for the stated time periods or as indicated. a mRNA levels of Kiss1 were decreased in a time-dependent manner. b Kiss1 protein expression was decreased after IL-6 treatment as observed by Flow Cytometry. c Kiss1 expression was decreased in B16BL6 cells treated with 5% adipocyte media and IL-6 for 24 h compared to control DMEM treatment as observed by Immunofluorescence microscopy. The data are representative of at least three separate experiments and presented as ± SEM where appropriate (*p < 0.05)
Discussion
Overall, our findings show that adipocytes increase the malignancy of B16BL6 melanoma cells. We show that adipocyte media increased the ability of B16BL6 cells to migrate and invade. These effects of adipocyte media on the malignant phenotype of melanoma cells correlated with changes in EMT gene expression. We show that the expression of Snai1, Twist, MMP9, Vimentin, E-cadherin, and Kiss1 are modulated by adipocyte media. Additionally, we show that adipocytes express high levels of the cytokine IL-6. Moreover, treatment of B16BL6 with IL-6 elicited effects similar to those of adipocyte media on the ability of melanoma cells to invade. IL-6 treatment also increased the expression of Snai1 and decreased the expression of Kiss1 in B16BL6 cells. However, neutralization of IL-6 in the adipocyte media did not alter the effects of adipocyte media on the invasive phenotype of B16BL6 cells and on the expression of Snai1.
Evidence by others suggests that the EMT plays an important role in the metastatic ability of cancer cells [15]. In particular, cancer cells that have acquired a mesenchymal phenotype metastasize more than those with an epithelial phenotype [16]. The expression of Snai1 and Twist is highly correlated with the mesenchymal phenotype [24]. In fact, others have shown that the mesenchymal phenotype and metastatic ability of melanomas are increased with Snai1 and Twist overexpression [19, 21, 30]. This is pertinent to melanomas because those with an epithelial phenotype are less aggressive than those with a mesenchymal phenotype [31]. As cancer cells transition towards a mesenchymal phenotype, they often lose the expression of cell adhesion proteins that keep the cells anchored together such as E-cadherin [32]. In some cancers, both Snai1 and Twist are thought to decrease the expression of E-cadherin [33, 34]. We show that adipocyte media increased the expression of Snai1 and Twist, and correspondingly decreased the expression of E-cadherin. Moreover, adipocyte media increased the expression of MMP9. Others have shown that Snai1 can increase the expression of MMP9, a protein that can degrade the extracellular matrix [35]. The degradation of the extracellular matrix may allow cancer cells to escape into the blood or invade secondary tissues [36]. Therefore, adipocytes may promote EMT by affecting the expression of Snai1 and Twist, which subsequently affect E-cadherin and MMP9 expression levels, thus allowing melanoma cells to invade and establish metastases.
Interestingly, EMT has been associated with decreased expression of the metastasis suppressor gene Kiss1 [21]. Kiss1 was originally discovered for its ability to suppress the metastatic ability of two human melanoma cell lines [37]. Studies suggest that one anti-metastatic function of Kiss1 may be to decrease the expression of Matrix Metalloproteases (MMPs), such as MMP9 [38]. This would contribute to the inhibition of B16BL6 cell invasion. Others also show that Kiss1 is down-regulated in cancer cells that acquire a mesenchymal phenotype [21]. We found that treatment of B16BL6 melanoma cells with adipocyte media led to decreased expression of Kiss1. Thus, adipocytes may simultaneously induce the expression of EMT genes and down-regulate the expression of Kiss1, which allows the melanoma cells to become more aggressive.
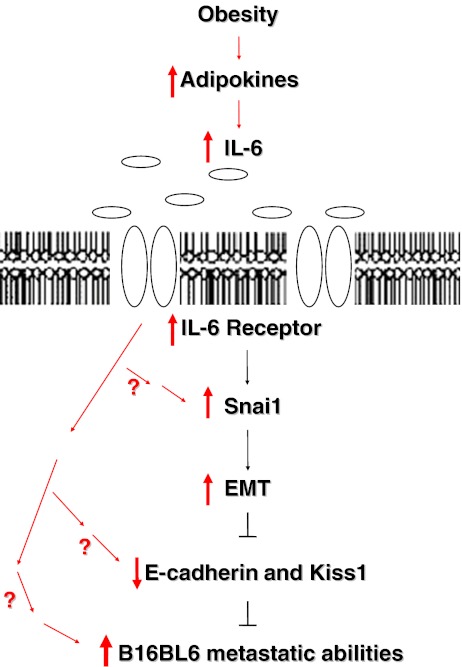
It is feasible that adipocytes affect the malignant phenotype of melanomas via multiple factors, including the Transforming Growth Factor (TGF-β). Others have shown that TGF-β plays a crucial role in EMT [39–41]. Interestingly, TGF-β is produced by 3T3L1 adipocytes [42, 43]; moreover, it is highly expressed in the adipose tissue of obese individuals [43, 44]. Thus, it is possible that the TGF-β in the adipocyte media was also partly responsible for the increase in cell invasion and Snai1 expression as others have already confirmed a positive relationship between TGF-β and Snai1 expression as well as cell invasiveness [40, 45]. This may be part of the reason why IL-6 neutralization did not have an observable effect in our study. Interestingly, TGF-β and IL-6 are not only produced cooperatively during inflammation, but it has been shown that the production of IL-6 is dependent on TGF-β signaling in the tumorigenicity of certain cancers [46]. Thus, though TGF- β may be an important factor, because we show that 3T3L1 adipocytes express high levels of IL-6 and show that IL-6 alone could increase B16BL6 cell invasiveness, increase Snai1, and decrease Kiss1 expression, we have not completely ruled out the importance of IL-6 in the ability of adipocytes to affect EMT and the malignant phenotype of melanomas. IL-6 may be sufficient to increase cell invasion and snai1 expression, but it may not be the only contributing factor found in the adipocyte media that could be affecting the invasiveness of B16BL6 cells. In summary, as Fig. 6 depicts, our results suggest that adipocytes may affect the malignancy of melanoma cells by promoting EMT and subsequently decreasing the expression of the metastasis suppressor Kiss1 partly through the IL-6 signaling pathway.
Fig. 6.
Proposed mechanism by which adipocyte media increases the metastatic ability of B16BL6 melanoma cells. Obesity is characterized by excess number of fat cells, which secrete high levels of factors such as IL-6. These factors may increase EMT by up-regulating the expression of Snai1, MMP9, Twist, and Vimentin, thereby increasing the invasive ability of B16BL6 melanoma cells. Moreover, this pro-cancerous environment may simultaneously decrease the expression of cell-to-cell adhesion proteins such as E-cadherin and the metastasis suppressor Kiss1, further increasing the metastatic phenotype of melanoma cells
Acknowledgement
This work was supported by the American Cancer Society grant ACS RSG CNE-113703 and by grants from the National Institutes of Health: National Cancer Institute grant NCI 1K22CA127519-01A1 and National Institute of Environmental Health Sciences Center grants ES09145 and ES007784.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures (2009) Atlanta, Georgia 2009
- 2.Klimek VM, Wolchok JD, Chapman PB, et al. Systemic chemotherapy. Clin Plast Surg. 2000;27(3):451–61. [PubMed] [Google Scholar]
- 3.Samanic C, Chow W, Gridley G, et al. Relation of body mass index to cancer risk in 362,552 Swedish men. Canc Causes Contr. 2006;17(7):901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 4.Mori A, Sakurai H, Choo M, et al. Severe pulmonary metastasis in obese and diabetic mice. Int J Canc. 2006;119(12):2760–7. doi: 10.1002/ijc.22248. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and prevention. Overweight and Obesity. 2009
- 6.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Percik R, Stumvoll M. Obesity and cancer. Exp Clin Endocrinol Diabetes. 2009;117(10):563–6. doi: 10.1055/s-0029-1241870. [DOI] [PubMed] [Google Scholar]
- 8.Dennis L, Lowe J, Lynch C, et al. Cutaneous melanoma and obesity in the Agricultural Health Study. Ann Epidemiol. 2008;18(3):214–21. doi: 10.1016/j.annepidem.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10(4):369–73. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 10.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 2010;15:166–79. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stout RD, Watkins SK, Suttles J. Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol. 2009;86(5):1105–9. doi: 10.1189/jlb.0209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123(2):97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010;37(Suppl 2):S2–14. doi: 10.1053/j.seminoncol.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol. 2010;222(1):1–15. doi: 10.1002/path.2727. [DOI] [PubMed] [Google Scholar]
- 15.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 16.Bonnomet A, Brysse A, Tachsidis A, et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010;15(2):261–73. doi: 10.1007/s10911-010-9174-0. [DOI] [PubMed] [Google Scholar]
- 17.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Canc. 2007;7(6):415–28. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 18.Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Przybylo JA, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail’s pace. Int J Biochem Cell Biol. 2007;39(6):1082–8. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Dissanayake SK, Wade M, Johnson CE, et al. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–71. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steeg P. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Canc. 2003;3(1):55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 23.Stafford L, Vaidya K, Welch D. Metastasis suppressors genes in cancer. Int J Biochem Cell Biol. 2008;40(5):874–91. doi: 10.1016/j.biocel.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Thiery J, Acloque H, Huang R, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Sadowski HB, Wheeler TT, Young DA. Gene expression during 3T3-L1 adipocyte differentiation. Characterization of initial responses to the inducing agents and changes during commitment to differentiation. J Biol Chem. 1992;267(7):4722–31. [PubMed] [Google Scholar]
- 26.Wu Z, Xie Y, Morrison RF, et al. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998;101(1):22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansieau S, Morel AP, Hinkal G, et al. TWISTing an embryonic transcription factor into an oncoprotein. Oncogene. 2010;29(22):3173–84. doi: 10.1038/onc.2010.92. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan N, Sasser A, Axel A, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–7. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Deng J, Rychahou P, et al. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Canc Cell. 2009;15(5):416–28. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuphal S, Palm HG, Poser I, et al. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005;15(4):305–13. doi: 10.1097/00008390-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Alonso SR, Tracey L, Ortiz P, et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Canc Res. 2007;67(7):3450–60. doi: 10.1158/0008-5472.CAN-06-3481. [DOI] [PubMed] [Google Scholar]
- 32.Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101(4):816–29. doi: 10.1002/jcb.21215. [DOI] [PubMed] [Google Scholar]
- 33.Pećina-Slaus N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Canc Cell Int. 2003;3(1):17. doi: 10.1186/1475-2867-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesuna F, Diest P, Chen JH, et al. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367(2):235–41. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordà M, Olmeda D, Vinyals A, et al. Upregulation of MMP-9 in MDCK epithelial cell line in response to expression of the Snail transcription factor. J Cell Sci. 2005;118(Pt 15):3371–85. doi: 10.1242/jcs.02465. [DOI] [PubMed] [Google Scholar]
- 36.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Miele M, Hicks D, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 38.Yan C, Wang H, Boyd D. KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha -induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276(2):1164–72. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]
- 39.Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15(2):169–90. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–23. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bortell R, Owen TA, Ignotz R, et al. TGF beta 1 prevents the down-regulation of type I procollagen, fibronectin, and TGF beta 1 gene expression associated with 3T3-L1 pre-adipocyte differentiation. J Cell Biochem. 1994;54(2):256–63. doi: 10.1002/jcb.240540214. [DOI] [PubMed] [Google Scholar]
- 43.Samad F, Yamamoto K, Pandey M, et al. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3(1):37–48. [PMC free article] [PubMed] [Google Scholar]
- 44.Samad F, Uysal KT, Wiesbrock SM, et al. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc Natl Acad Sci USA. 1999;96(12):6902–7. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu H, Hu Z, Wen J, et al. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim Biophys Sin (Shanghai) 2009;41(8):648–56. doi: 10.1093/abbs/gmp053. [DOI] [PubMed] [Google Scholar]
- 46.Yao Z, Fenoglio S, Gao DC, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA. 2010;107(35):15535–40. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]