Abstract
Depending on the cell type and tissue environment, epithelial and mesenchymal cell phenotypes are not static and can be highly dynamic. Epithelial-mesenchymal transitions (EMTs) and reverse EMTs provide flexibility during embryogenesis. While EMTs are a critical normal process during development and wound healing, properties of the EMT have been implicated in human pathology, particularly cancer metastasis. A normal undamaged epithelium does not typically exhibit features of an EMT. However, particularly under the influence of the surrounding microenvironment, cancer cells may reactivate developmental phenotypes out of context in the adult. This reactivation, such as the EMT, can facilitate tumor cell invasion and metastasis, and therefore is a major mechanism of tumor progression. Conversely, cellular senescence, which is associated with aging, is a process by which cells enter a state of permanent cell cycle arrest, thereby constituting a potent tumor suppressive mechanism. However, accumulating evidence shows that senescent cells can have deleterious effects on the tissue microenvironment. The most significant of these effects is the acquisition of a senescence-associated secretory phenotype (SASP) that turns senescent fibroblasts into pro-inflammatory cells having the ability to promote tumor progression, in part by inducing an EMT in nearby epithelial cells. Here, we summarize the potential impacts of SASP factors, particularly interleukins, on tissue microenvironments and their ability to stimulate tumor progression through induction of an EMT.
Keywords: Aging, Senescence, Cytokines, Interleukins, Proliferation, Invasion, Migration
Importance of the EMT during Development and Cancer
Cells of epithelial and mesenchymal origins play important roles during embryonic development by contributing to the formation and function of developing organs [1]. Cells exhibiting a mesenchymal phenotype are highly motile, and provide support and structure to the epithelial cells, particularly through the production of an extracellular matrix (ECM). Epithelial cells form cell-cell linkages that are necessary for organ function, and act as a barrier that delimits the internal organ environment from the external environment. Nevertheless, epithelial and mesenchymal cells can, under certain conditions, switch their phenotypes. This process is called an epithelial-mesenchymal transition (EMT) [2].
During the EMT, the cell undergoes several phenotypic changes. Throughout the process, alterations in cell-cell adhesion and cell shape must occur in order for the epithelial cell to gain mesenchymal properties. During development, the expression of different cadherins is highly dynamic, and associated with distinct stages of morphogenesis, establishment and/or maintenance of different tissues [3]. Interestingly, alterations in cadherin expression or function occur frequently during carcinogenesis [4]. For example, the replacement of a cell surface protein that promotes epithelial connections to neighboring cells and the basement membrane by N-cadherin provides more transient adhesive properties, thereby preparing the cell for the acquisition of motile mesenchymal phenotypes, and thus conferring a more migratory and invasive character [5, 6].
The cells of an epithelial tissue are closely joined together. This tight packing enables the epithelium to function as a barrier against mechanical injury, microbes and fluid loss. Thus, loss of epithelial morphology can drastically affect the efficiency of the epithelial barrier. A significant characteristic of the EMT is an increase in cellular motility. This increase is due in part to rearrangements in the actin cytoskeleton [7], and the replacement of cytokeratin intermediate filaments by vimentin intermediate filaments, which transforms the cell from a cuboidal to a spindle shape [2]. Several inducers of EMTs have been found, and there is evidence that EMTs are important during the progression of tumor cells to metastatic stages. There is also increasing evidence that the tissue microenvironment plays a key role during induction of the EMT. The next section summarizes these findings.
EMT Triggered by the Microenvironment
Stromal tissue surrounding tumor epithelial cells was seen for many years as inert, providing only physical support. In recent years, however, accumulated evidence indicates that the tumor microenvironment is a key component of both cancer initiation and tumor progression. The invasion of epithelial cells into and through the stroma requires the EMT, and it is now clear that the stroma, particularly stromal fibroblasts, play an important role in this process. Indeed, a specific subset of stromal cells, termed carcinoma-associated fibroblasts (CAFs), secrete factors that promote tumorigenesis, angiogenesis and the EMT [8]. CAFs were initially found in the stroma of prostate carcinomas, and were shown later to secrete pro-inflammatory cytokines due to activation of the NF-κB transcription factor [9, 10]. Although the origin of CAFs remains unknown, CAFs do not show any chromosomal aberration (a hallmark of cancer cells), and therefore it is likely that they do not correspond to cancerous epithelial cells that previously underwent an EMT [11].
Chemotherapy and radiotherapy are important treatments for cancer. Both treatments damage DNA, which can generate a secretory phenotype in untransformed cells in the tumor microenvironment [12]. It was recently shown that the secretory phenotype of untransformed cells after DNA damaging chemotherapy could create a chemo-resistant niche for the remaining tumor cells [13]. Specifically, Timp-1, and, highly relevant to the secretory phenotype of senescent cells that we will describe further, IL-6, were acutely secreted by thymic endothelial cells in response to DNA damage. This secretion created a niche that eventually promoted the survival of residual tumor cells and served as a reservoir for tumor relapse.
The SASP as an Inducer of Tumor Cell Aggressiveness
Cellular senescence was first identified as a process that limits the proliferation of cells in culture [14]. These early experiments showed that cultured human fibroblasts gradually lost proliferative capacity until all cells ceased division. Much of this growth arrest is now known to occur because most normal human cells do not express telomerase. Consequently, with each cell cycle, telomeres shorten and eventually fail, generating a persistent DNA damage signal that permanently arrests growth [15]. Subsequent studies showed that non-telomeric DNA damage, and many other stressors, can also induce senescence [16]. Interestingly, among these various stressors, there is now evidence supporting a key role of oncogene-induced senescence as a potent antitumor barrier [17].
Indeed, cellular senescence is recognized as a crucial tumor suppressor mechanism and barrier to malignant progression [16, 18, 19]. The hallmark of senescent cells is an essentially irreversible p53- and p16INK4A/pRb-dependent cell cycle arrest. Senescent cells also secrete many biologically active proteins, a phenotype termed the senescence-associated secretory phenotype (SASP) [20, 21]. Strikingly, functional loss of p53 markedly amplified and accelerated the development of the SASP [21], suggesting a cell-nonautonomous mechanism by which p53 was able to restrain the development of age-related diseases (such as cancer) by altering the tissue microenvironment.
Overall, it is thought that the senescence response might be beneficial or deleterious, depending on the age of the organism [22]. To understand this apparent paradox, the SASP may be particularly important. Senescent cells increase with age in many mammalian tissues and are found at sites of age-related pathologies [23–28]. The SASP includes many inflammatory cytokines, and inflammation is thought to drive aging and many age-related diseases [29]. Indeed, some SASP factors, when chronically present, can disrupt normal tissue structure and differentiation [30]. An exhaustive list of these SASP factors has recently been published by our group [31], and the major factors are described below.
Cellular senescence is accompanied by a striking increase in the secreted levels of more than 40 factors involved in intercellular signaling [20, 21, 32]. These factors can affect surrounding cells by activating cell surface receptors and corresponding signal transduction pathways, potentially leading to multiple pathologies, including cancer. Many SASP factors can be divided into two categories: soluble signaling factors (interleukins, chemokines and growth factors) and secreted proteases. SASP proteases can shed membrane-associated proteins, resulting in soluble versions of membrane-bound receptors, cleave signaling molecules, and degrade the ECM. These activities provide potent mechanisms by which senescent cells might modify the tissue microenvironment.
Senescent, but not non-senescent, human and mouse fibroblasts can promote the progression of pre-malignant or malignant epithelial cells in mouse xenografts [21, 33–35]. In the following sections, we describe behavioral changes cells can undergo when residing in the proximity of senescent cells (see Fig. 1). We also discuss how the senescent tissue microenvironment might facilitate several aspects of tumor initiation and progression, particularly through induction of an EMT.
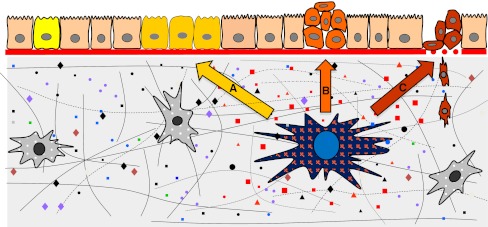
Fig. 1.
a A senescent fibroblast (blue nucleus) in the stroma secretes various factors that stimulate pre-neoplastic epithelial cells (yellow cells) to proliferate. b Further, the SASP stimulates transformed cells to undergo an EMT (orange cells). c Finally, the SASP promotes the invasion of mesenchymal-like cells (red cells) through the surrounding stroma
Effects of the SASP on Epithelial Cell Proliferation and Differentiation
Human SASPs can disrupt epithelial organization and promote the growth of pre-malignant epithelial cells in culture [21, 30, 33, 34, 36]. To determine whether mouse SASPs have similar biological activities, we compared the effects of mouse and human fibroblasts on the growth of pre-malignant or malignant mammary epithelial cells [21, 35]. In contrast to non-senescent cells, senescent human fibroblasts significantly stimulated epithelial cell proliferation. Mouse fibroblasts behaved similarly, but only when they were induced to senesce in 3%, not 20%, O2 [21, 35]. Similar results were obtained using indirect co-cultures, in which either mouse or human epithelial cells were cultured with conditioned medium (CM) from fibroblasts. These findings support the idea that biological activities of the mouse and human SASPs are conserved.
To better understand the conserved pro-oncogenic activities of SASPs, we tested specific factors for ability to stimulate the growth of pre-malignant or malignant epithelial cells. IL-6, IL-8 and CXCL-1 (human GROα) are among the most highly and consistently secreted human SASP factors [21]. Although neither the IL-6 nor IL-8 in CM from senescent fibroblasts stimulated malignant epithelial cell proliferation, CXCL-1, an epithelial cell growth factor [37], was potent in this regard. These findings identified CXCL-1 as a key conserved SASP factor responsible for stimulating the growth of pre-malignant and malignant epithelial cells [21, 35].
Senescent human and mouse fibroblasts disrupt the differentiation of mammary epithelial cells, suppressing the expression of differentiation markers [30, 38]. This activity is due in large measure to the secretion of MMP-3 by senescent cells. Furthermore, weakly tumorigenic pancreatic [39] and mammary [21] epithelial cells undergo morphologic changes in culture resembling an EMT in the presence of CM from senescent cells. The effect on mammary epithelial cells was attributable to IL-6 and IL-8 [21], as well as HGF, uPAR and MMPs [30], which are all capable of disrupting epithelial cell clusters and stimulating de-differentiation in culture and in vivo [40–42].
Strikingly, no angiostatic factors (e.g., endostatin) have been reported among SASP constituents. By contrast, the SASP contains many pro-angiogenic factors (IL-8, MCP-1 and -2, GROs, PGE2, VEGF, EGF, CSFs, u-/t-PA, MMPs, FN and laminin) [43]. Because senescent stromal cells secrete MCPs, CSFs, MIPs, GROs and CXCLs, which in turn recruit inflammatory immune cells that also secrete pro-angiogenic factors (VEGFs, IL-8 and MMPs), the senescence response may activate a positive feedback loop that stimulates angiogenesis. Thus, senescent cells are well poised to support the differentiation of a new vasculature around and within a progressing tumor.
Effects of the SASP on Cell Migration and Invasion
Senescent cells secrete an array of chemokines that can create a gradient to promote cell migration and invasion. In pancreatic cancer, HGF, and to a lesser degree bFGF, promoted cancer cell invasion in culture, and could potentially drive cancer dissemination in vivo [39, 44]. In breast cancer, the high levels of IL-6 and IL−8 secreted by senescent fibroblasts enhanced the invasiveness of a panel of cancer cell lines in cell culture [21, 45, 46]. Further, and consistent with a SASP-induced EMT, CM from senescent, but not non-senescent, cells stimulated pre-malignant and malignant cancer cells to invade a basement membrane [21].
The secretion of MMP-2 and MMP-3 by senescent cells could also promote the invasion of multiple epithelial cell types [30, 34, 38, 47, 48]. Other proteases, such as uPA and its regulator (PAI1), are likewise implicated in cancer cell invasion. These results support the idea that paracrine activities of the SASP can promote malignant phenotypes in nearby pre-malignant or malignant cells, and identify new SASP activities: the ability to induce an EMT and invasion of a basement membrane (see Fig. 1). As discussed below, the EMT is an important phenotypic switch that enables cancer cells to migrate and invade [49].
In cell culture models, endothelial cells are induced to migrate by factors secreted by senescent fibroblasts. This is in part due to VEGF secretion [50] and chemokine gradients set up by senescent cells [51]. Neo-angiogenesis, which is dependent on endothelial cell motility and invasion, is enhanced in xenograft models containing senescent fibroblasts [50]. Further, IL-1, which is a SASP component, is known to activate the endothelium and consequently increases the adhesiveness of cancer cells to blood vessel walls [52]. Thus, senescent cells might promote extravasation of cancer cells to secondary metastatic sites. However, the effects of senescent cells on angiogenesis might be cell-type dependent. For example, senescent keratinocytes oversecrete maspin, which acts as a dominant inhibitor of endothelial cell migration and invasion [53].
Senescent fibroblasts may promote leukocyte recruitment, since they chronically release chemokines [54]. In p53-deficient RAS-driven tumors induced to senesce by reestablishing p53 function [55], innate immune cells were shown to migrate into the vicinity of the senescent tumor area. CSF-1, CXCL-1, or MCP-1 and ICAM-1 transcripts were found to be higher in these senescent tumor masses, and may be responsible for the immune response. For example, neutrophils express CXCR-1, CXCR-2, and CXCR-4 to sense their microenvironment and invade tissues, eosinophils use the broad spectrum receptor CCR-3 to fulfill their function, monocytes use CCR-1, CCR-2, CCR-5, CXCR-4 and CX3CR1 to extravasate and enter peripheral sites where they differentiate, natural killer cells express CCR−2, CCR-5, CXCR-4, CX3CR1 and XCR1, and immature myeloid dendritic cells display CCR-1, CCR-2, CCR-5, CCR-6 and CXCR-4, which facilitate their transport, migration and function [54, 56–58].
The SASP Modulates the Expression of Markers of the EMT
As described above, the EMT confers invasive and metastatic properties on epithelial cells, and is an important step that presages the conversion of carcinomas in situ to potentially fatal invasive cancers [59, 60]. The fibroblast SASP has been shown to induce a classic EMT in two non-aggressive human breast cancer cell lines [21]. Secreted factors from senescent, but not non-senescent, fibroblasts caused dose-dependent epithelial cell scattering, a mesenchymal characteristic. Moreover, immunostaining showed that CM from non-senescent cells preserved surface-associated β-catenin and E-cadherin and strong cytokeratin 8/18 expression, and Western analysis showed that CM from non-senescent cells preserved low expression of vimentin. These are epithelial characteristics, frequently retained by non-aggressive cells [59, 60].
By contrast, CM from senescent cells markedly decreased overall and cell surface β-catenin and E-cadherin, and reduced cytokeratin expression [21], consistent with a mesenchymal transition. Further, CM from senescent cells down-regulated the tight junction protein claudin-1, leaving the remaining protein localized primarily to the nucleus, a hallmark of an EMT and feature of metastatic but not primary tumors [61]. Finally, CM from senescent cells increased vimentin expression, another mesenchymal marker and hallmark of an EMT [21, 60]. Therefore, addition of CM from senescent cells could trigger most of the phenotypic changes associated with the EMT and described at the beginning of this review, i.e., alterations in the expression of cell-cell adhesion molecules and proteins of the cytoskeleton.
Conclusion
Cancer is a multi-step disease in which cells acquire increasingly malignant phenotypes. These phenotypes are acquired in part by somatic mutations, which derange normal controls over cell proliferation, survival, invasion and other processes important for malignant tumorigenesis [62]. In addition, there is increasing evidence that the tissue microenvironment is an important determinant of whether and how malignancies develop [63, 64]. Normal tissue environments tend to suppress malignant phenotypes, whereas abnormal tissue environments such at those caused by inflammation can promote cancer progression.
Cellular senescence is a tumor suppressive mechanism that permanently arrests cells at risk for malignant transformation. However, recent studies show that, despite the beneficial effects of cellular senescence, senescent cells can also exert harmful effects on the tissue microenvironment. Indeed, the SASP stimulates the growth of pre-malignant and malignant epithelial cells in culture and in mouse xenographs, and the stimulatory activity resides in the secretion of various factors, such as GROα, IL-6 and IL-8.
Most insoluble components of the ECM are enzymatic targets of secreted proteases. Therefore, the senescence-associated changes in proteolytic activities could affect the physical properties of the tissue structure. In particular, the accumulation of senescent cells could lessen the supportive role of the ECM, globally diminishing tissue tension and elasticity. In addition, the relaxed tissue structure and higher levels of MMPs might help tumor cells migrate and invade the ECM, thus enabling metastasis. In fact, the panel of proteases secreted by senescent cells extensively overlaps with those found in malignant tumors.
In summary, senescence-induced alterations in secreted interleukins, chemokines, growth factors and proteases tend to establish the SASP as pro-tumorigenic. Particularly, the SASP can induce an EMT in relatively non-aggressive carcinoma cells, and stimulate their invasion through a basement membrane (see Fig. 1). In the context of epithelial cancer, the EMT provides a mechanism for tumor cells to leave the primary tumor and invade the local tissue and blood vessels, setting the stage for metastatic spread. Importantly, cells exhibiting an EMT have been specifically localized to the periphery of the tumor, where they are exposed to cytokines and an extracellular milieu that promotes EMTs [65]. Senescent cells may be an important source of these factors.
Acknowledgments
The authors wish to thank Dr. Andrew P. Smith for editing the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48(5–6):365–375. doi: 10.1387/ijdb.041794hp. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 5.Prasad CP, et al. Expression analysis of E-cadherin, Slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer. 2009;9:325. doi: 10.1186/1471-2407-9-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logullo AF, et al. Concomitant expression of epithelial-mesenchymal transition biomarkers in breast ductal carcinoma: association with progression. Oncol Rep. 2010;23(2):313–320. [PubMed] [Google Scholar]
- 7.Mori M, et al. Zyxin mediates actin fiber reorganization in epithelial-mesenchymal transition and contributes to endocardial morphogenesis. Mol Biol Cell. 2009;20(13):3115–3124. doi: 10.1091/mbc.E09-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 9.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erez N, et al. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 11.Qiu W, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40(5):650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143(3):355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 15.d’Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 16.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Rev Molec Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 17.Gorgoulis VG, Halazonetis TD. Oncogene-induced senescence: the bright and dark side of the response. Curr Opin Cell Biol. 2010;22(6):816–827. doi: 10.1016/j.ceb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Prieur A, Peeper DS. Cellular senescence in vivo: a barrier to tumorigenesis. Curr Opin Cell Biol. 2008;20:150–155. doi: 10.1016/j.ceb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Dimri GP. What has senescence got to do with cancer? Cancer Cell. 2005;7:505–512. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppe JP, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Dimri GP, et al. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeyapalan JC, et al. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paradis V, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 26.Erusalimsky JD, Kurz DJ. Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol. 2005;40(8–9):634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85:106–110. doi: 10.2106/00004623-200300002-00014. [DOI] [PubMed] [Google Scholar]
- 28.Roberts S, et al. Senescence in human intervertebral discs. Eur Spine J. 2006;15:312–316. doi: 10.1007/s00586-006-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 30.Parrinello S, et al. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118(Pt 3):485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freund A, et al. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10(3):228–230. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krtolica A, et al. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 35.Coppe JP, et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5(2):e9188. doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bavik C, et al. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006;66:794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, et al. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 38.Tsai KK, et al. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res. 2005;65:6734–6744. doi: 10.1158/0008-5472.CAN-05-0703. [DOI] [PubMed] [Google Scholar]
- 39.Ohuchida K, et al. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64(9):3215–3222. doi: 10.1158/0008-5472.CAN-03-2464. [DOI] [PubMed] [Google Scholar]
- 40.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9(8):2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paumelle R, et al. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene. 2002;21(15):2309–2319. doi: 10.1038/sj.onc.1205297. [DOI] [PubMed] [Google Scholar]
- 42.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 43.Tonini T, Rossi F, Claudio PP. Molecular basis of angiogenesis and cancer. Oncogene. 2003;22(42):6549–6556. doi: 10.1038/sj.onc.1206816. [DOI] [PubMed] [Google Scholar]
- 44.Birchmeier C, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 45.Yuan A, et al. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 46.Badache A, Hynes NE. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 2001;61:383–391. [PubMed] [Google Scholar]
- 47.Camphausen K, et al. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res. 2001;61(5):2207–2211. [PubMed] [Google Scholar]
- 48.Qian LW, et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res. 2002;8(4):1223–1227. [PubMed] [Google Scholar]
- 49.Coppe JP, et al. A role for fibroblasts in mediating the effects of tobacco-induced epithelial cell growth and invasion. Mol Cancer Res. 2008;6(7):1085–1098. doi: 10.1158/1541-7786.MCR-08-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coppe JP, et al. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281(40):29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 51.Strieter RM, et al. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42(6):768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Orr FW, Wang HH. Tumor cell interactions with the microvasculature: a rate-limiting step in metastasis. Surg Oncol Clin N Am. 2001;10(2):357–381. [PubMed] [Google Scholar]
- 53.Nickoloff BJ, et al. Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res. 2004;64(9):2956–2961. doi: 10.1158/0008-5472.CAN-03-2388. [DOI] [PubMed] [Google Scholar]
- 54.Mantovani A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14(3):147–148. doi: 10.1016/j.semcancer.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–650. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Homey B, Muller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2(3):175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 57.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16(1):38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Kokkinos MI, et al. Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 61.Dhawan P, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765–1776. doi: 10.1172/JCI24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 63.Bissell MJ, Radisky D. Putting tumours in context. Nature Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brabletz T, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179(1–2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]