Abstract
Hyperactive inflammatory responses following cancer initiation have led to cancer being described as a ‘wound that never heals’. These inflammatory responses elicit signals via NFκB leading to IL-6 production, and IL-6 in turn has been shown to induce epithelial to mesenchymal transition in breast cancer cells in vitro, implicating a role for this cytokine in cancer cell invasion. We previously have shown that conditioned medium derived from cancer-associated fibroblasts induced an Epithelial to Mesenchymal transition (EMT) in PMC42-LA breast cancer cells and we have now identify IL-6 as present in this medium. We further show that IL-6 is expressed approximately 100 fold higher in a cancer-associated fibroblast line compared to normal fibroblasts. Comparison of mouse-specific (stroma) and human-specific (tumor) IL-6 mRNA expression from MCF-7, MDA MB 468 and MDA MB 231 xenografts also indicated the stroma rather than tumor as a significantly higher source of IL-6 expression. Mast cells (MCs) feature in inflammatory cancer-associated stroma, and activated MCs secrete IL-6. We observed a higher MC index (average number of mast cells per xenograft section/average tumor size) in MDA MB 468 compared to MDA MB 231 xenografts, where all MC were observed to be active (degranulating). This higher MC index correlated with greater mouse-specific IL-6 expression in the MDA MB 468 xenografts, implicating MC as an important source of stromal IL-6. Furthermore, immunohistochemistry on these xenografts for pSTAT3, which lies downstream of the IL-6 receptor indicated frequent correlations between pSTAT3 and mast cell positive cells. Analysis of publically available databases for IL-6 expression in patient tissue revealed higher IL-6 in laser capture microdissected stroma compared to adjacent tissue epithelium from patients with inflammatory breast cancer (IBC) and invasive non-inflammatory breast cancer (non-IBC) and we show that IL-6 expression was significantly higher in Basal versus Luminal molecular/phenotypic groupings of breast cancer cell lines. Finally, we discuss how afferent and efferent IL-6 pathways may participate in a positive feedback cycle to dictate tumor progression.
Keywords: IL-6, Inflammation, Mast cells, EMT, Breast cancer, Stroma
Introduction
Chronic irritation, which typically elicits a perpetual inflammatory response resulting in fibrosis, has been associated with cancer causation. This generic response to tissue damage, as exemplified in renal fibrosis in response to urinary tract obstruction, involves the induction of tumor necrosis factor alpha (TNFα) and interleukin-1 (IL-1) which in turn induce NFκB, the master regulator of inflammatory responses (reviewed in [1]). At this key inflammatory-neoplastic interface, NFκB recruits several cytokines (such as IL-6), which further recruit inflammatory cells such as tumor-associated macrophages (TAMs), tumor-associated mast cells (TAMCs) and cancer-associated fibroblasts (CAFs). These, in turn, produce more NFκB and HIFlα and release more cytokines creating a microenvironment conducive for tumor growth [2]. NFκB also transcriptionally upregulates potent epithelial to mesenchymal transition (EMT) inducers such as Snail1 and Zeb1 [3, 4] and given the central role EMT plays in tumor metastasis (reviewed in [5]), NFκB could be thought of as a bridge connecting the inflammatory response, tumor growth and tumor metastasis.
Cancer-subversion of inflammatory responses has been implicated in several states of irritation, including Helicobacter pylori-mediated gastric ulcers (stomach cancer), Barrett’s esophagus (esophageal cancer), obesity-related hepatic inflammation (liver cancer), and Crohn’s disease (colon cancer), all of which feature detrimental inflammatory responses involving IL-6 [6–9]. Hyperactive inflammatory responses following cancer initiation is also a significant issue, and cancer has been described as a ‘wound that never heals’ [10].
IL-6 belongs to a family of cytokines which signal via a gp130 receptor-JAK-STAT3 pathway. Other family members which share this pathway of activation include interleukin 11 (IL-11), leukaemia inhibitory factor (LIF), Oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1) and cardiotrophin-like cytokine (CLC) [11, 12]. Due to its production and secretion by infiltrating inflammatory cells, IL-6 plays a central role in the progression of immune-related cancers such as B lymphomas, diffuse large-cell lymphoma and multiple myeloma (reviewed in [13]). It is less well known, however, that IL-6 is expressed in keratinocytes and endothelial and dermal dendritic cells of the skin, and is upregulated in psoriasis [14].
In order to gain a clearer understanding of the role of IL-6 in the tumor microenvironment, we sought to investigate the expression of IL-6 in various sources of cancer-associated stroma. We also investigated the occurrence of a lesser-known IL-6 stromal contributor, the mast cell, in human breast cancer cell xenografts, and finally compared these findings with IL-6 expression from publicly available microarray datasets of human breast cancer cell lines and human breast tumors.
Materials and Methods
Collection of Conditioned Media and ChemiArray
This was used as described in the ChemiArray Human Antibody Array kit (Millipore, Billerica, Massachusetts, USA) protocol. Briefly, membranes were blocked for 30 mins at RT, following incubation with conditioned medium from NMFs and CAFs overnight at 2–8°C. Arrays were washed and incubated with Biotin-conjugated anti-cytokine primary antibody for 2 h at RT. Arrays were washed and incubated with HRP-conjugated Streptavidin for 2 h at RT, following which membranes were washed and detection buffers added prior to exposing arrays using a LAS-3000 Fuji Film intelligent dark box (Fuji Photo Film Co., Ltd., Tokyo, Japan). The illuminated arrays were detected and the image captured using Image Reader LAS-3000 software, and relative dot densities determined with the Multi-Gauge V2.3 program (both programs from Fuji Photo Film Co., Ltd., Tokyo, Japan). Conditioned medium was collected as described previously [15].
Cell lines and Mammary Fibroblasts
Primary human normal mammary fibroblasts (NMF) and breast cancer-associated fibroblasts (CAF) were previously described [15, 16]. Additional NMF populations (B6RA1H and F127H) were obtained from Dr. Geoff Lindeman, Walter and Eliza Hall Institute, Melbourne, Australia. MCF-7, MDA MB 468 and MDA MB 231 were originally obtained from the ATCC (Manassas, Virginia, USA), and maintained in 37°C, 5% CO2 in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, Massachusetts, USA) containing 18 mM HEPES (4- [2-hydroxyethyl]-1-piperazineethanesulfonicacid) and 10% FCS (fetal calf serum, Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Quantitative Real-time PCR
All RNA preparations were extracted and purified via column chromatography (RNeasy kit, Qiagen, Doncaster, Victoria, Australia) as recommended by the manufacturer. The concentration and purity of RNAs was determined using spectrophotometry (Nanodrop ND-1000 spectrophotometer, Thermo Fisher Scientific, Scoresby, Victoria, Australia). 100 ng of RNA was subjected to cDNA synthesis with gene-specific priming using thermoscript II reverse transcriptase (Invitrogen Australia Pty Limited, Mulgrave, Victoria, Australia) according to manufacturer’s instructions, using 10nM dNTPs and the RNA degradation inhibitor RNAsin (Promega, Alexandria, New South Wales, Australia). Quantitative determination of RNA levels of various genes was performed in triplicate using SYBR green (Applied Biosystems, Mulgrave, Victoria, Australia). The following pairs of primers (sense/antisense) were used (synthesized by Geneworks, Hindmarsh, South Australia, Australia): Human specific L32 (housekeeping gene), 5′- CAGGGTTCGTAGAAGATTCAAGGG –3′/5′- GATCGCTCACAATGTTTCCTCCAAG –3′; with the reverse primer used as the RT primer in gene-specific priming (cDNA synthesis); human specific IL-6: 5′-CTCACCTCTTCAGAACGAATTGACAAACAA A-3′/5′- GGTACTCTAGGTATACCTCA AACTCCAAAA -3′ with RT primer CTGGCTTGTTCCTCACTACTCTCAAA; mouse specific L32, 5′- GATCCTGATGCCCAACATCGGTTACA-3′/5′- GCACCTCCGGCTCCTTGATA-3′ with reverse primer used as RT primer; mouse specific IL-6: 5′- GGACCAAGACCATCCAATTC ATCTTGAAA-3′/5′- GACCACAGTGAGGAATGTCCACAAA-3′ with RT primer CCCAACATTC ATATTGTCAGTTCTTCGTAGA. RT-PCR and data collection were performed on an Applied Biosystems 5700 or 7300 analyser (Applied Biosystems, Mulgrave, Victoria, Australia). All quantitations were normalized to expression of mRNA for the human ribosomal protein L32.
Generation of Human Breast Cancer Cell Line Xenografts
Tumour xenografts of the MCF-7, MDA MB 468 and MDA MB 231 cell lines were produced into 6 week old mice as described previously [17]. These xenografts were part of studies approved by the St. Vincent’s Hospital Animal Ethics Committee.
Quantification of Mast Cells in Xenograft Sections and Immunostaining for STAT3 and p-STAT3
Mammary fat pad tumors excised from euthanized mice were fixed in 10% neutral buffered formalin (Fisher Scientific, Scoresby, Victoria, Australia) and embedded in paraffin. 5 μM thick sections were cut, deparaffinized and stained with toluidine blue for 2 min (5 ml of a 1% w/v toluidine blue [Sigma-Aldrich Pty. Ltd., Castle Hill, New South Wales, Australia] in 70% ethanol solution mixed with 45 ml 1% sodium chloride immediately prior to use). Slides were rinsed in distilled water, dehydrated in ethanol, cleared in xylene and mounted. Average mast cell numbers were determined from single toluidine blue stained sections of MDA MB 468 (n = 5) and MDA MB 231 (n = 5) xenografts and then divided by the average tumor size in mm to give the mast cell index (MCI). Cut and deparaffinized sections were immunostained for STAT3, p-STAT3 and a rabbit IgG isotype control. Both STAT3 primary antibodies were purchased from Cell Signaling Technology (#4904 and #9131, diluted 1:100) and the isotype control from DAKO (X0903) and incubated overnight at 4°. Sections were then incubated at room temperature for 1 h in an anti-rabbit biotinylated secondary antibody (DAKO, E0431, diluted 1:200) and then incubated for 30 min with HRP-conjugated streptavidin (DAKO, P0397, diluted 1:400). 3,3-Diaminobenzidine was used as chromogen.
Statistical Analyses of IL-6 Expression Data
Statistical analyses for human versus mouse IL-6 expression and mast cell indices were performed using GraphPad Prism (GraphPad Software, California, USA). Statistical analyses of human breast cancer and human breast cancer cell line datasets were performed using Partek Incorporated Next Generation Sequencing and Microarray Software, St Louis, Missouri, USA.
Results and Discussion
Cancer-associated Fibroblasts as a Potential Source of IL-6 within the Tumor Microenvironment
If cancer is the ‘wound that never heals’ then cancer-associated stroma can be considered the scar. This is as far as the analogy goes, because far from being the end-state non-functional bridging tissue which forms across skin wounds, cancer-associated stroma secretes a plethora of factors such as IL-6 which promote the growth and invasion of the underlying tumor (reviewed in [18]). Increased concentration (>4 pg/ml) of IL-6 in the serum of breast cancer patients is an indicator of overall poor prognosis, predicting liver metastases and pleural effusion, and lack of response to chemo-endocrine therapy [19–21]. IL-6 serum levels were increased in 27% of breast cancer patients [22] and a correlation in breast cancer patients between highly elevated IL-6 serum levels and tumor progression has been reported [23]. IL-6 induced significant migration of MDA-MB-231 human breast carcinoma cells, but not of MCF-7 or T-47D breast carcinoma cell lines [24], suggesting that it could potentiate the invasive phenotype. This work led to the key discovery by Sullivan and colleagues that IL-6 could induce EMT in ER alpha positive MCF-7 breast cancer cell lines cultured in three-dimensions (3D) [25]. In this study, transient expression of IL-6 dramatically reduced E-cadherin protein by 24 h, and constitutive expression of IL-6 led to an EMT in which Snail, Twist, vimentin and N-cadherin were induced, along with the development of more invasive tumors in mice. Conversely, Twist overexpression in MCF-7 caused an EMT along with high IL-6 expression and STAT3 activation [25]. This finding illustrated that not only could IL-6 induce an EMT, but EMT itself could result in production of IL-6. Thus, the work by Sullivan and colleagues implicates IL-6 not just as a primary inducer of EMT, but a link in a chain of EMT signals within the tumor microenvironment resulting in stabilization of the invasive cellular state. Possibly other EMT driving factors which act in a similar way to Twist, such as the E-cadherin repressors Snail1 and Snail2 (reviewed in [26]), may also promote IL-6 expression when overexpressed in luminal breast cancer cells. The intertwining of IL-6 and EMT, and the essential role that EMT plays in cancer invasion (reviewed in [27]) can partially explain the functional significance of increases in IL-6 within the cancer microenvironment.
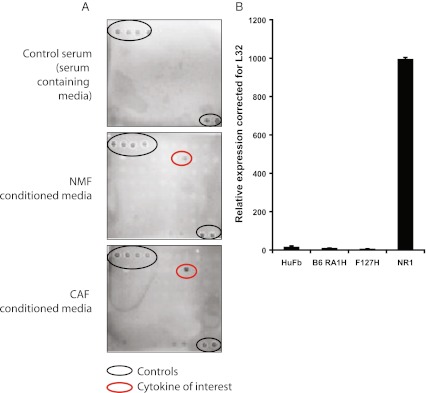
We have previously examined EMT-effects of CAF versus NMF conditioned medium on the epithelial breast cancer cell line PMC42-LA. We found that CAF-conditioned medium induced myoepithelial molecular markers and increased the migratory ability of the epithelial breast cancer cell line PMC42-LA in a 3D organoid model [15, 16]. We used the chemiarray system to examine specific soluble factor(s) in CAF-conditioned media that may be induce this EMT. A key component which we found to be present at higher amounts in the CAF-conditioned medium compared to the NMF-conditioned medium, was IL-6 (Fig. 1a). Interestingly, we observed that the CAFs expressed IL-6 mRNA to a level approximately 100 times that of the average IL-6 mRNA expression of the NMFs (Fig. 1b), whereas the protein level differences were only 2–3 fold (Fig. 1a). Additional mRNA translation controls are known for IL-6, via CPEB [28] and fungal allergens [29]. Thus the observed discrepancy between mRNA and protein levels could be explained by post-translational mechanisms being rate-limiting.
Fig. 1.
a Results of a ChemiArray Human Antibody Array (Millipore, Billerica, Massachusetts, USA) in which IL-6 was identified from the conditioned medium of NMFs or CAFs. The array was performed twice, data shown is representative of these runs. Black circles indicate controls, red circles identify IL-6 protein; b IL-6 is expressed approximately 100 fold higher in a CAF line (NR1) compared to NMFs (HuFb, B6 RA1H, F127H). QRT-PCR was performed in quadruplicate for each cell line in one experiment, error bars represent the standard deviation of the mean of quadruplicate data
Several experiments involving the application of recombinant IL-6 (in serum or serum-depleted medium) to PMC42-LA cells have so far failed to consistently reproduce the EMT effects we observed in PMC42-LA exposed to CAF conditioned medium. It is possible that IL-6 requires an additional, unidentified cofactor for its full activity, a cofactor which was present in the CAF conditioned medium, however IL-6 depletion experiments were not possible due to loss of the CAF line. Interestingly, similar observations have been made by others studying the growth of myeloma cells which grow well with IL-6 in vitro however tumor growth in vivo has been shown to require additional unknown cofactors [30].
Analysis of Species Specific IL-6 Expression in Xenografts
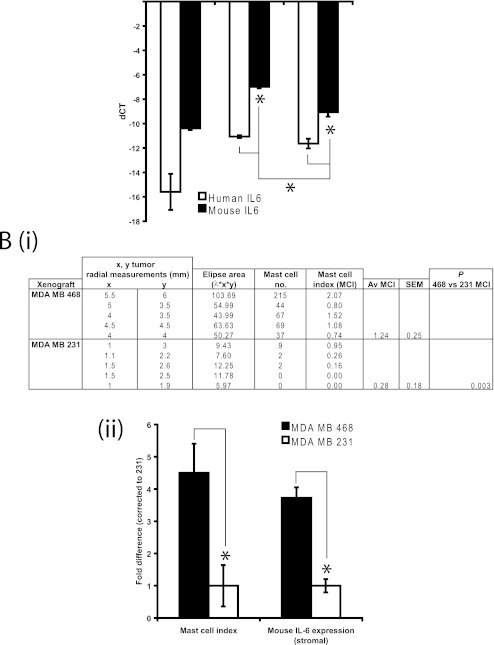
To further examine the expression of IL-6 in the stroma versus tumor in a breast cancer setting, we took advantage of the species-specificity inherent in xenografts. Species-specific qRT-PCR for human and murine IL-6 mRNA expression in MCF-7 (n = 2), MDA MB 468 (n = 6) and MDA MB 231 (n = 5) xenografts (Fig. 2a) revealed that IL-6 mRNA was more highly expressed in the stroma (mouse IL-6) compared to the tumor (human IL-6) in MDA MB 231 and MDA MB 468 xenografts (p < 0.05). MDA MB 468 xenografts contained more mouse IL-6 mRNA than MDA MB 231 determined by Tukey post tests.
Fig. 2.
a IL-6 QRT-PCR expression in stroma (mouse-specific primers) versus tumor (human-specific primers) in a human breast cancer cell (BCC) xenograft model in which MCF-7 (n = 2), MDA MB 468 (n = 5) or MDA MB 231 (n = 5) were injected into the mammary fat pad of nude Balb/C (Nu/Nu Balb/C) mice. For mouse versus human IL-6 statistical comparisons within xenograft type, the Student’s 2 tailed T test was used. ANOVA with the Tukey post test was used to determine statistical differences between xenografts. Significance [*] set at p < 0.05. Stromal IL-6 was significantly higher compared to tumor for MDA MB 468 and MDA MB 231 xenografts however stromal IL-6 of MDA MB 468 was significantly more highly expressed than stromal IL-6 of MDA MB 231; b Mast cell number was counted for each MDA MB 468 and MDA MB 231 xenograft and expressed as an index when divided by tumor size in mm; c MDA MB 468 xenograft mast cell indices and stromal IL-6 expression normalised to MDA MB 231, illustrating similar fold changes, supporting mast cell number as a distinguishing factor contributing to greater stromal IL-6 expression
Mast cells as a Potential Source of IL-6 within the Tumor Microenvironment
Mast cells are an important component of the infiltrating cell population in the inflammatory response. Their contribution to tumor growth and the prognostic significance of the presence of mast cells in the stroma adjacent to tumors is controversial [31]. On the one hand, they have been shown to correlate with low tumor grade and ER positivity in breast cancer [32] and to identify a subset of invasive cancers with a favorable prognosis [33]. Degranulating mast cell heparin has also been implicated in a fibroblast-dependent negative effect on tumor growth [34]. On the other hand, it is becoming increasingly evident that mast cells contribute to tumor growth through their positive effects on tumor blood vessel development, extracellular matrix remodelling (as a subversion to ECM remodelling in wound healing) and ability to modulate immune responses (reviewed in [35]). In addition, activated mast cells release IL-6 (reviewed in [31, 36, 37]), which could contribute to breast tumor invasiveness [25].
It has been shown that fibroblasts can mediate IL-6 dependent effects on mast cells [38]. In a model in which bone marrow derived mast cells were cultured on a NIH3T3 fibroblast monolayer, IL-6 family cytokines (Il-6, IL-11, OSM and LIF) were demonstrated to induce proliferation of mast cells, but only when the fibroblasts were present. This effect was pinpointed to the expression of stem cell factor, a well-characterized mast cell growth factor, and provides a mechanism of mast cell hyperplasia in inflammatory skin diseases. IL-6 in the bronchi can mediate inflammatory pathways by enabling class switching of B cells into IgE-producing plasma cells. Similar to the Gyotoku model [38], normal human lung fibroblasts were co-cultured with human mast cells. The fibroblasts were stimulated to produce IL-6 whereas if these cell populations were separated this production was inhibited [39]. Although these studies were not performed in the context of cancer, they indicate a mutual activation pathway between mast cells and fibroblasts involving bidirectional IL-6, which may also occur in the tumor microenvirnomental setting.
Since active, degranulating mast cells secrete IL-6 (reviewed in [31, 36, 37]) and IL-6 is important for mast cell development [40], and given the potential role of mast cells in the tumor stroma, we went back to our xenograft models to examine whether mast cells were present.
The xenograft models were based in nude mice, which are known to be partially immuno-deficient. Despite this, we were able to identify and count mast cells in sections of the tumor/stroma from each xenograft. The number of degranulating mast cells (as indicated in purple with toluidine blue) in MDA MB 468 versus MDA MB 231 xenografts was determined and this was expressed proportional to tumor size in mm (mast cell index, Fig. 2b, i). As only 2 MCF-7 xenografts were available for analysis, we excluded this line for comparison.
We found a significantly higher mast cell index (average number of mast cells per xenograft section/average tumor size) in each of the MDA MB 468 xenografts than in the MDA MB 231 xenografts (Fig. 2b, i). We observed that all of the mast cells were degranulating (active) and were located almost exclusively at the tumor-stroma interface or at the tumor periphery at the interface with healthy tissue and near blood vessels, as found in other rodent models and human cancers [32–34, 41, 42]). We also observed a large number of mast cells (as indicated by toluidine blue and c-kit staining) at the tumor-stroma interface in a small study of 11 human primary breast tumors (data not shown). In the MDA MB 231 xenografts, we observed rare occurrences of mast cells that had migrated deeper into the tumor. Taken together, the locality of the mast cells implicates them in either influencing the tumor stroma and/or the growth of the tumor itself, either in a positive or negative manner.
The tumor-associated stroma backed closely onto the epidermis of mouse underbelly (given these were palpable tumors arising from mammary fat pad injection). MDA MB 468 tumors were larger and hence contained a longer epidermal-stromal capsule. Given that mast cells are commonly found in skin especially when it is inflamed [43] this could have contributed to mast cell number.
Since IL-6 is secreted by activated mast cells, it is possible that the higher number of mast cells, almost exclusively found in the stroma, in the MDA MB 468 xenograft sections contributed to the greater stromal (mouse) expression of IL-6, given that the source of RNA for this qRT-PCR was derived from each of the xenografts that were examined for mast cells number. Indeed, when we compared the mast cell index to mouse (stromal) IL-6 expression and expressed each of these measurements as a fold change to MDA MB 231 (Fig. 2b, ii), we observed that the fold increase in mast cell number and IL-6 expression (for MDA MB 468) were similar, consistent with the idea that the mast cells contributed to stromal IL-6 expression.
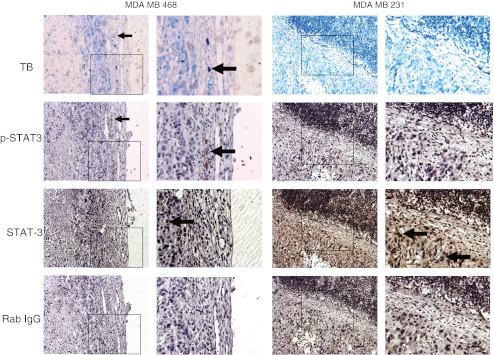
To further examine this potential association, we performed immunohistochemistry for STAT3 and p-STAT3, given that the phosphorylation of STAT3 is a direct consequence of IL-6 receptor activation by ligand. Indeed, as shown in Fig. 3, we frequently observed that the mast cells identified by toluidine blue localized with cells which stained for p-STAT3, lending further support to our hypothesis that mast cells are a source of stromal IL-6 expression. The influence of mast cells on stromal IL-6 expression is depicted schematically in Fig. 5.
Fig. 3.
Immunohistochemistry of representative xenografts stained with toluidine blue for mast cells (mast cells stain purple), p-STAT3 as a surrogate marker for IL-6 signaling, pan STAT3, and rabbit IgG as a negative control for both STAT3 antibodies. Scale bar = 200 μm; boxed area is enlarged; TB toluidine blue. Arrows indicate positively stained cells
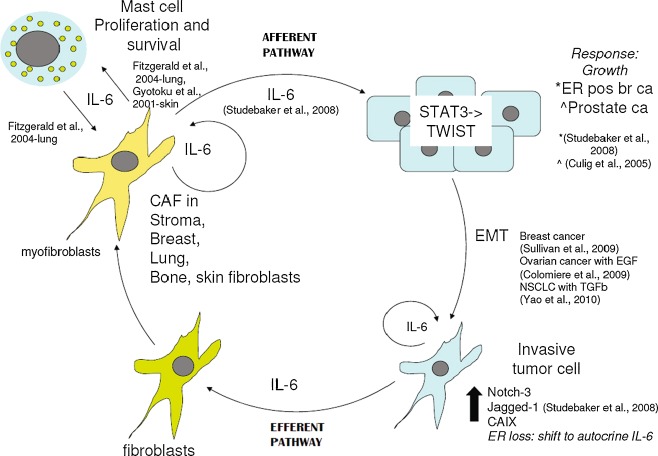
Fig. 5.
IL-6 contributes to a positive feedback cycle of growth and invasion between CAFs (myofibroblasts) and its underlying tumor (afferent pathway) and between invading tumor and the recruitment of fibroblasts to myofibroblasts (efferent pathway). Mast cells participate in this cycle through a reciprocal growth relationship with fibroblasts
Analysis of Publically Available Databases on IL-6 Expression
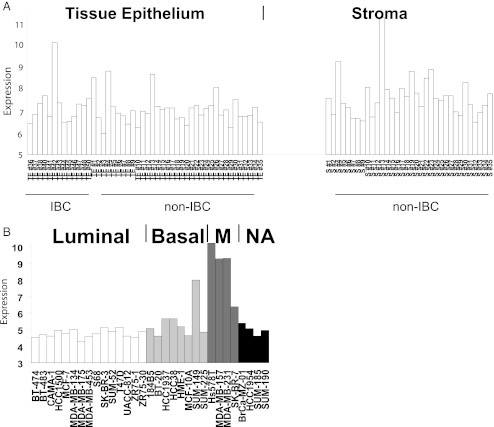
Examination of IL-6 expression from dataset GSE5847 (from GEO database) [44] revealed that IL-6 was higher in laser capture microdissected stroma compared to adjacent tissue epithelium from patients with inflammatory breast cancer (IBC) and invasive non-inflammatory breast cancer (non-IBC) (Fig. 4a).
Fig. 4.
IL-6 expression was analyzed in publicly available microarray datasets. a Laser capture microdissected stroma and adjacent tissue epithelium, dataset [44]. Original .CEL files were RMA normalized and the log2 expression calculated for the probeset ID corresponding to IL-6. IL-6 mRNA expression (log2) was higher in laser capture microdissected stroma compared to adjacent tissue epithelium from patients with IBC and invasive non-IBC combined (p = 0.0002), as determined by ANOVA statistical test using Partek Genomics Suite (v6.5). b IL-6 mRNA expression (log2) was significantly higher in Mesenchymal versus Luminal gene cluster subgroups (p < 0.0001) within the breast cancer cell line datasets as determined by ANOVA statistical test with Bonferroni post hoc test applied. Abbreviations, M Mesenchymal, NA not assigned, IBC invasive BC
Conversely, as shown in Fig. 4b, the examination of IL-6 expression across publically available datasets of breast cancer cell lines revealed that Mesenchymal (alternative name for the same subgroup which has enhanced invasive properties, [45]) versus Luminal (less invasive, with epithelial characteristics) molecular/phenotypic subgroup was significantly higher for IL-6 mRNA as determined by ANOVA statistical test with the Bonferroni post hoc test applied. Each study was normalized and analysed independently of the other datasets, thus the increased expression value of mesenchymal cell lines was not due to batch effects.
Taken together these two expression datasets support both an afferent (Fig. 4a, stroma-tumor) and efferent (Fig. 4b, tumor-stroma) pathways of IL-6 microenvironmental influence.
After a comprehensive search, we did not find any databases for tissue/protein based arrays probing for IL-6 protein that examined breast tissue/stroma/cell lines. However a forty-fold higher level of IL-6 protein in the basal B breast cancer cell line MDA MB 231 versus the luminal line MCF-7 has been demonstrated by Elisa assay [46]. These authors built upon this finding, going on to show that the IL-6 promoter is demethylated when p53 is inactivated and that IL-6 induces an epigenetic re-programming resulting in a basal-like/stem cell-like gene expression profile.
We are aware that our interpretations of the IL-6 expression database analyses, regarding afferent and efferent IL-6 microenvironmental effects (also indicated in Fig. 5), is based on the assumption that a direct, causal link exists between IL-6 mRNA and protein secretion. Despite the differences we observed between these indices in Fig. 1, we are confident that this conclusion may be drawn given the p-STAT3 immunohistochemisty presented in Fig. 3. In this figure we show that it is the mast cells which express p-STAT3, and given we have presented an association with mast cells and increased stromal IL-6 (Fig. 2), we therefore demonstrate a link between IL-6 protein and IL-6 expression. This direct link is further supported by reports in the literature [47, 48].
Conclusions
Secretion of IL-6 into the tumor microenvironment can trigger a perpetual cycle stimulating growth and invasion of tumor, mast cells and myofibroblasts (Fig. 5). Triggering of an immune response, as an initiating factor in carcinogenesis of irritation-related cancers, or as a secondary response to cancer invasion, produces a rich reservoir of pro-inflammatory cytokines including IL-6. This may be carried to the tumor microenvironment by mast cells, which are recruited along with other infiltrating inflammatory cells to halt tumor growth. Tissue remodeling occurs in the formation of fibrous tissue, where mast cells may participate through the secretion of matrix metalloproteinase 1 (MMP-1) and a disintegrin and metalloproteinases (ADAMs)-9, -10 and -17 [49]. However cancer subverts these responses and mast cell IL-6 may promote proliferation of fibroblasts, which in turn secrete IL-6 as a positive feed-forward mechanism to reinforce mast cell numbers. Stromal fibroblasts, via an afferent pathway, may secrete IL-6 that then acts on the tumor to promote growth and invasion via EMT, with the acquisition of autocrine IL-6 expression to reinforce this neoplastic change. IL-6 secretion from the tumor (efferent pathway) may then act on benign inflammatory-associated fibroblasts to transform them into myofibroblasts which then feedback to enhance tumor growth.
Actemra (tocilizumab) is an anti IL-6R antibody and has been approved by the FDA for the treatment of rheumatoid arthritis [50, 51] with potential efficacy in the rare Castleman’s disease, Crohn’s disease and bipolar disorder [52, 53]. IL-6 antagonism may potentially be successful in the treatment of cancer, and a fully humanized anti-IL-6 antibody (1339 Mab, GlaxoSmithKline, Boronia, Australia) inhibited the growth and invasion of multiple myeloma in a SCID-hu mouse model [54]. Given the central role of IL-6 in the vicious cycle of solid tumor growth, it is encouraging that phase I/II clinical trials are currently underway in the treatment of ovarian, pancreatic, colorectal, head and neck and lung cancer using a newly developed IL-6 antibody CNTO 328 (Centocor, NCT00841191) and positive reports are emerging for its use in metastatic renal cell and prostate cancer [55, 56].
Acknowledgements
The research efforts associated with this article were funded in part by the U.S. Army Medical Research and Materiel Command (BC0213201 and BC084667), the Victorian Breast Cancer Research Consortium, and the National Breast Cancer Foundation (Australia). We thank Dr. Geoff Lindeman (Walter and Eliza Hall Institute, Melbourne, Australia) for supplying us with the additional NMF populations (B6RA1H and F127H), and Geraldine Mitchell and Keren Abberton (O’Brien Institute, Melbourne, Australia) for help with mast cell identification.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Erik W. Thompson and M. Leigh Ackland contributed equally
References
- 1.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 4.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, Roy F, et al. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 5.Tse JC, Kalluri R. Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J Cell Biochem. 2007;101:816–829. doi: 10.1002/jcb.21215. [DOI] [PubMed] [Google Scholar]
- 6.Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, et al. Increased expression and secretion of interleukin-6 in patients with Barrett’s esophagus. Clin Cancer Res. 2004;10:2020–2028. doi: 10.1158/1078-0432.CCR-0437-03. [DOI] [PubMed] [Google Scholar]
- 7.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–359. doi: 10.1016/0140-6736(90)91889-I. [DOI] [PubMed] [Google Scholar]
- 8.Kai H, Kitadai Y, Kodama M, Cho S, Kuroda T, Ito M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–713. [PubMed] [Google Scholar]
- 9.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 11.Hibi M, Nakajima K, Hirano T. IL-6 cytokine family and signal transduction: a model of the cytokine system. J Mol Med. 1996;74:1–12. doi: 10.1007/BF00202068. [DOI] [PubMed] [Google Scholar]
- 12.Jazayeri JA, Carroll GJ, Vernallis AB. Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol. 2010;10:1–8. doi: 10.1016/j.intimp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebret SC, Newgreen DF, Thompson EW, Ackland ML. Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res. 2007;9:R19. doi: 10.1186/bcr1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebret SC, Newgreen DF, Waltham MC, Price JT, Thompson EW, Ackland ML. Myoepithelial molecular markers in human breast carcinoma PMC42-LA cells are induced by extracellular matrix and stromal cells. In Vitro Cell Dev Biol Anim. 2006;42:298–307. doi: 10.1290/0601004.1. [DOI] [PubMed] [Google Scholar]
- 17.Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 18.McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol. 2010;28:4022–4028. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 19.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–84. [PubMed] [Google Scholar]
- 20.Salgado R, Junius S, Benoy I, Dam P, Vermeulen P, Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- 22.Asgeirsson KS, Olafsdottir K, Jonasson JG, Ogmundsdottir HM. The effects of IL-6 on cell adhesion and e-cadherin expression in breast cancer. Cytokine. 1998;10:720–728. doi: 10.1006/cyto.1998.0349. [DOI] [PubMed] [Google Scholar]
- 23.Hussein MZ, Al Fikky A, Abdel Bar I, Attia O. Serum IL-6 and IL-12 levels in breast cancer patients. Egypt J Immunol. 2004;11:165–170. [PubMed] [Google Scholar]
- 24.Arihiro K, Oda H, Kaneko M, Inai K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer. 2000;7:221–230. doi: 10.1007/BF02967464. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugo HJ, Kokkinos MI, Blick T, Ackland ML, Thompson EW, Newgreen DF. Defining the E-cadherin repressor interactome in epithelial-mesenchymal transition: the PMC42 model as a case study. Cells Tissues Organs. 2010;193:23–40. doi: 10.1159/000320174. [DOI] [PubMed] [Google Scholar]
- 27.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 28.Groppo R, Richter JD. CPEB control of NF-kappaB nuclear localization and interleukin-6 production mediates cellular senescence. Mol Cell Biol. 2011;31:2707–2714. doi: 10.1128/MCB.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neveu WA, Bernardo E, Allard JL, Nagaleekar V, Wargo MJ, Davis RJ et al (2011) Fungal Allergen {beta}-glucans Trigger p38 MAPK-mediated IL-6 Translation in Lung Epithelial Cells. Am J Respir Cell Mol Biol. doi:10.1165/rcmb.2011-0054OC [DOI] [PMC free article] [PubMed]
- 30.Camp B, Riet I. Homing mechanisms in the biology of multiple myeloma. Verh K Acad Geneeskd Belg. 1998;60:163–194. [PubMed] [Google Scholar]
- 31.Ribatti D, Crivellato E. The controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. 2009;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- 32.Amini RM, Aaltonen K, Nevanlinna H, Carvalho R, Salonen L, Heikkila P, et al. Mast cells and eosinophils in invasive breast carcinoma. BMC Cancer. 2007;7:165. doi: 10.1186/1471-2407-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod Pathol. 2004;17:690–695. doi: 10.1038/modpathol.3800094. [DOI] [PubMed] [Google Scholar]
- 34.Samoszuk M, Kanakubo E, Chan JK. Degranulating mast cells in fibrotic regions of human tumors and evidence that mast cell heparin interferes with the growth of tumor cells through a mechanism involving fibroblasts. BMC Cancer. 2005;5:121. doi: 10.1186/1471-2407-5-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, et al. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hultner L, Szots H, Welle M, Snick J, Moeller J, Dormer P. Mouse bone marrow-derived IL-3-dependent mast cells and autonomous sublines produce IL-6. Immunology. 1989;67:408–413. [PMC free article] [PubMed] [Google Scholar]
- 38.Gyotoku E, Morita E, Kameyoshi Y, Hiragun T, Yamamoto S, Hide M. The IL-6 family cytokines, interleukin-6, interleukin-11, oncostatin M, and leukemia inhibitory factor, enhance mast cell growth through fibroblast-dependent pathway in mice. Arch Dermatol Res. 2001;293:508–514. doi: 10.1007/PL00007465. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald SM, Lee SA, Hall HK, Chi DS, Krishnaswamy G. Human lung fibroblasts express interleukin-6 in response to signaling after mast cell contact. Am J Respir Cell Mol Biol. 2004;30:585–593. doi: 10.1165/rcmb.2003-0282OC. [DOI] [PubMed] [Google Scholar]
- 40.Hu ZQ, Zhao WH, Shimamura T. Regulation of mast cell development by inflammatory factors. Curr Med Chem. 2007;14:3044–3050. doi: 10.2174/092986707782793998. [DOI] [PubMed] [Google Scholar]
- 41.Dabbous MK, North SM, Haney L, Tipton DA, Nicolson GL. Effects of mast cell-macrophage interactions on the production of collagenolytic enzymes by metastatic tumor cells and tumor-derived and stromal fibroblasts. Clin Exp Metastasis. 1995;13:33–41. doi: 10.1007/BF00144016. [DOI] [PubMed] [Google Scholar]
- 42.Ch’ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–159. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 43.Harvima IT, Nilsson G, Naukkarinen A. Role of mast cells and sensory nerves in skin inflammation. G Ital Dermatol Venereol. 2010;145:195–204. [PubMed] [Google Scholar]
- 44.Boersma BJ, Reimers M, Yi M, Ludwig JA, Luke BT, Stephens RM, et al. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer. 2008;122:1324–1332. doi: 10.1002/ijc.23237. [DOI] [PubMed] [Google Scholar]
- 45.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 46.D’Anello L, Sansone P, Storci G, Mitrugno V, D'Uva G, Chieco P, et al. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer. 2010;9:300. doi: 10.1186/1476-4598-9-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlaepfer DD, Hou S, Lim ST, Tomar A, Yu H, Lim Y, et al. Tumor necrosis factor-alpha stimulates focal adhesion kinase activity required for mitogen-activated kinase-associated interleukin 6 expression. J Biol Chem. 2007;282:17450–17459. doi: 10.1074/jbc.M610672200. [DOI] [PubMed] [Google Scholar]
- 49.Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, et al. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol. 2005;206:279–290. doi: 10.1002/path.1780. [DOI] [PubMed] [Google Scholar]
- 50.Hirabayashi Y, Ishii T, Harigae H. Clinical efficacy of tocilizumab in patients with active rheumatoid arthritis in real clinical practice. Rheumatol Int. 2010;30:1041–1048. doi: 10.1007/s00296-009-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;7:CD008331. doi: 10.1002/14651858.CD008331.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Brietzke E, Scheinberg M, Lafer B (2010) Therapeutic potential of interleukin-6 antagonism in bipolar disorder. Med Hypotheses. doi:10.1016/j.mehy.2010.08.021 [DOI] [PubMed]
- 53.Nishimoto N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010;87:483–487. doi: 10.1038/clpt.2009.313. [DOI] [PubMed] [Google Scholar]
- 54.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, et al. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S et al (2011) The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate. doi:10.1002/pros.21362 [DOI] [PubMed]
- 56.Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, et al. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2011;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]