Abstract
Background
Dysregulated cyclin-dependent kinases (CDKs) are important to the growth of some sarcomas. Flavopiridol is a pan-CDK inhibitor that has been shown to potentiate chemotherapy. As such, we explored the potentiation of doxorubicin by flavopiridol in sarcoma, in vitro and in vivo, and performed a phase I trial of flavopiridol with doxorubicin in patients with advanced sarcomas.
Design
Sarcoma cell lines and xenografts were treated with flavopiridol alone and in combination with doxorubicin. In the phase I study, doxorubicin and flavopiridol were administered on 2 flavopiridol schedules; a 1 hour bolus and split dosing as a 30 minute bolus followed by a 4 hour infusion.
Results
Pre-clinically, flavopiridol potentiated doxorubicin. In vivo, doxorubicin administered 1 hour prior to flavopiridol was more active than doxorubicin alone. Clinically, 31 patients were enrolled on protocol and flavopiridol was escalated to target dose in 2 schedules (90 mg/m2 bolus; 50 mg/m2 bolus + 40 mg/m2 infusion) both in combination with doxorubicin (60 mg/m2). Dose-limiting toxicities were neutropenia, leukopenia and febrile neutropenia but no maximum tolerated dose was defined. Flavopiridol pharmacokinetics showed increasing Cmax with increasing dose. RECIST responses included 2 partial responses however stable disease was seen in 16 patients. Of 12 evaluable patients with progressive well- and de-differentiated liposarcoma, 8 had stable disease greater than 12 weeks.
Conclusions
The sequential combination of doxorubicin followed flavopiridol is well tolerated on both schedules. Disease control was observed in well- and de-differentiated liposarcoma specifically, a disease where CDK4 is known to be amplified.
Keywords: Flavopiridol, Sarcoma, Cyclin-dependent kinase, CDK, phase I
Introduction
Cyclin-dependent kinases (CDKs) are serine/threonine kinases that are activated upon association with cyclin proteins to form cyclin-dependent kinase complexes. In response to mitogenic and stress stimuli, CDK complexes phosphorylate effector protein complexes, regulating both RNA polymerase II mediated transcription and progression through the cell cycle. CDKs are attractive targets for drug development given that certain malignancies are dependent on dysregulated cyclin activity(1) and CDK inhibition has been observed as a potent vehicle to overcome resistence to standard chemotherapy(2–4).
Flavopiridol is a pan – CDK inhibitor that specifically inhibits CDK2, CDK4, CDK6 and CDK9 at nanomolar concentrations. Inhibition of CDKs by flavopiridol blocks cell cycle progression at the G1-S or G2-M checkpoints and is associated with cell cycle arrest and subsequent apoptosis(5, 6). Flavopiridol has been tested at various dosing levels and schedules in both hematologic(7, 8) and solid tumor malignancies(3, 9, 10). To date, the most compelling data regarding clinical efficacy has been observed in hematologic malignancies, such as chronic lymphocytic leukemia(11).
The therapeutic impact of single agent flavopiridol in solid tumors has been less robust. However, preclinical models and clinical trial results suggest a utility of flavopiridol in combination with chemotherapy. For example, flavopiridol, as a pan-CDK inhibitor, blocks CDK9 leading to suppression of Rad51, an enzyme involved in homologous recombination. This defect in DNA repair then sensitizes tumor cells to p53-dependent induction of apoptosis by irinotecan(12). Flavopiridol has also been associated with the potentiation of other chemotherapies such as paclitaxel, docetaxel, gemcitabine, and doxorubicin, where flavopiridol exposure has been shown to enhance apoptosis in tumor cells(13–17).
Advanced sarcomas are a group of heterogeneous mesenchymally derived neoplasms for which clinical treatment options are limited. After progression to metastatic or unresectable disease, systemic treatment options include chemotherapies such as doxorubicin, ifosfamide, dacarbazine, gemcitabine and docetaxel. While the molecular characteristics of these diseases are increasingly being elucidated, cytotoxic chemotherapy remains the clinical standard of care. Unfortunately, response rates to these agents are low and patients rarely obtain durable clinical benefit.
Preclinically, flavopiridol has been shown to potentiate the effects of doxorubicin in a bone sarcoma (osteosarcoma), especially in an Rb null background(18). However, the ability of flavopiridol to potentiate doxorubicin in soft tissue sarcoma is unknown. Certain soft tissue sarcoma subtypes, such as well-differentiated and de-differentiated (WD and DD) liposarcoma, are especially attractive for drug targeting by flavopiridol, as CDK4 is amplified in 90% of these tumors(19). WD and DD liposarcoma has been shown to be sensitive to inhibition of CDKs in preclinical models and early phase clinical trials have hinted that targeted inhibition of CDKs may lead to impressive clinical benefit(20). Therefore, we evaluated both the in vitro and in vivo ability of flavopiridol to potentiate the effects of doxorubicin in sarcoma and performed a phase I clinical trial of the combination in patients with advanced sarcomas.
Methods
Preclinical Methods
Cell Culture
LS141 primary human cell line was derived from a patient with high-grade retroperitoneal dedifferentiated liposarcoma and the MPNST cells were derived from a patient with a high-grade peripheral nerve sheath tumor of the thigh (graciously supplied by Jonathan Fletcher, Dana-Farber Cancer Institute). These were grown in RPMI1640 supplemented with 15% heat-inactivated fetal bovine serum plus penicillin and streptomycin.
Colony Assays
MPNST cells were treated with doxorubicin, flavopiridol (graciously supplied by National Cancer Institute, Bethesda, Maryland), or the combination of the two drugs together in sequence. MPNST cells were chosen given that LS141 (and other CDK4 dependent) cells are exquisitely sensitive to CDK4 inhibition in vitro, thus making combination studies uninterruptable. MPNST cells were plated, in triplicate, at a density of 1000 cells/100 mm2 per plate. Twenty-four hours after plating, cells were treated for 24 hours with the IC50 of doxorubicin (D, 15 nM), flavopiridol (F, 150 nM), drug free media (control), or a combination of the two drugs, either concomitantly or sequentially for 24 hours each. After treatment, drug-containing medium was removed and cells were allowed to grow for 10 days to form colonies. The resulting colonies were stained with 0.01% crystal violet for 30 minutes and colonies counted using an automated colony counter (ColCount, Oxford Optronix, Oxford UK) . Results are presented as the percentage of untreated controls and the statistical significance of the experimental results was determined by the two-sided t test.
Immunoblotting
MPNST cells were lysed in RIPA buffer supplemented with protease inhibitor cocktail tablets (Complete Mini, Roche Diagnostics) and 1 mM NaVO3. Total protein concentration of the lysates was measured by Bio-Rad protein assay (Bio-Rad Laboratories), and equal amounts of protein were loaded on 4–12% PAGE gels (Invitrogen). PVDF membranes were blocked with 5% nonfat dried milk in PBS buffer containing 0.1% Tween-20 (PBST) for 1 hour and probed with antibodies for full length and cleaved PARP (Santa Cruz Biotechnology) and tubulin (Cell Signaling).
In vivo studies
LS141 xenografts were established by directly implanting into severe combined immunodeficient (SCID) mice. Once tumors reached 100 mm3 , groups of five mice were treated with the maximum tolerated dose (MTD) of flavopiridol (9 mg/kg), doxorubicin (0.9 mg/kg), or doxorubicin (0.7 mg/kg) followed by flavopiridol (7 mg/kg) at selected time points (1, 4 and 7 hours). In addition, one set of animals was treated in reverse order of flavopiridol followed by doxorubicin, administered 7 hours apart. All treatments were administered in intraperitoneal fashion, twice weekly, for a total of 5 treatments. Tumors were measured every 2 to 3 days with calipers, and tumor volumes were calculated by the formula π/ 6 × (large diameter) × (small diameter)2. Tumor volume was compared between groups of mice at various points in time based on the experiment and the statistical significance of the experimental results was determined by the two-sided t test. Given the aggressive morbidity of the tumors, animal survival data could not be estimated. Toxicity was monitored by weight loss. These studies were done in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 85-23, released 1985), under an IACUC-approved protocol.
Clinical Trial Methods
Patient Entry Criteria
All patients (age ≥18, male and female) were histologically confirmed to have metastatic or locally recurrent sarcoma. Prior treatment excluded anthracyclines but allowed up to two prior lines of therapy. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists, thalidomide or targeted therapy such as tyrosine kinase inhibitors, were not considered as prior lines of therapy. A minimum of 3 weeks from last treatment had to elapse before study entry (6 weeks for nitrosoureas and mitomycin C and 1 week for targeted agents). Patients had to have a Karnofsky performance status ≥60%, total WBC count ≥3,500/mm3, absolute neutrophil count (ANC) ≥1,500/mm3, platelets ≥100,000/mm3, and adequate hepatic, renal and cardiac function (including ejection fraction ≥50%). Patients with central nervous system metastases were not eligible. Patients with a history of significant heart disease or radiation to both the pelvis and spine were additionally excluded. The protocol was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center and all patients provided written informed consent.
Clinical Trial Design
The trial design was a non-randomized, open-label dose escalation of doxorubicin and flavopiridol. Groups of three to six patients were treated sequentially according to the dose escalation in Table 1. Treatment was every 3 weeks (1 cycle), provided the absolute neutrophil count (ANC) was ≥1,500/mm3 and platelet count ≥100,000/mm3. Doxorubicin was given intravenous (IV) push over 5–7 minutes on day 1 of each cycle at a dose of 60 or 75 mg/m2. Based on our and in vitro and in vivo preclinical model (see Results), flavopiridol was given 1 hour following doxorubicin as a 60 minute IV bolus (Cohorts 1–6), starting at a dose of 40 mg/m2 to a goal escalation dose of 70 mg/m2, the approximate MTD defined in single agent bolus schedule studies(21). This dose has also been shown to consistently achieve > 2.0 μM of flavopiridol in human plasma. In view of 90% protein binding in plasma, this achieves a therapeutically active free flavopiridol plasma level of approximately 200 nM. Given the desire to continue to increase flavopiridol exposure and the success of split dosing (bolus followed by infusion) in the treatment of chronic lymphocytic leukemia(22), further cohorts were examined using a split dosing schedule. Patients in cohorts 7–8 received flavopiridol as a 30 minute bolus followed by a 4 hour infusion on day 1 of each cycle, beginning 1 hour after the administration of doxorubicin. The target flavopiridol dose was 90 mg/m2 (Table 1); the single agent MTD with divided dose flavopiridol therapy. Because of concerns for tumor lysis syndrome with the split-dose schedule, tumor lysis blood samples were obtained, including LDH, calcium, magnesium, and phosphorous, on the day following therapy. Where indicated, dexrazoxane was given prior to each dose of doxorubicin (cumulative doxorubicin dose >300 mg/m2). Dexrazoxane was given at 10 times the dose of doxorubicin. Doxorubicin was given within 30 minutes of start of the dexrazoxane infusion. After 600 mg/m2 doxorubicin (including use of dexrazoxane), doxorubicin was discontinued and flavopiridol could be continued as a single agent until progression of disease. All treatments were administered in the outpatient setting and intra-patient dose escalation was not permitted.
Table 1.
Clinical trial dosing cohorts.
| Cohort | Doxorubicin (mg/m2) IV push over 5 minutes | Flavopiridol (mg/m2) 60 minute bolus or 30 minute bolus followed by 4 hour infusion | Number accrued to each level | Dose- Limiting Toxicity (DLT) (Cycle 1) |
|---|---|---|---|---|
| 1 | 60 | 40 | 3 | 0 |
| 2 | 60 | 50 | 3 | 0 |
| 3 | 60 | 60 | 3 | 0 |
| 4 | 60 | 70 | 3 | 0 |
| 5 | 75 | 60 | 3 | 0 |
| 6 | 60 | 70 (40/30) | 4* | 0 |
| 7 | 60 | 80 (50/30) | 6 | 1# |
| 8 | 60 | 90 (50/40) | 6 | 1+ |
1 patient withdrew prior to treatment in the fourth cohort
DLT in cohort 7 of grade 4 neutropenia
DLT in cohort 8 grade 3 febrile neutropenia, and grade 4 neutropenia and leukopenia
Toxicity was graded in accordance with the Common Toxicity Criteria Version 3.0(23). Dose-limiting toxicity (DLT) was defined as the occurrence during the first cycle of Grade 4 hematologic toxicity 21 days after treatment, Grade 4 hematologic toxicity lasting 7 days or longer, Grade 3 or 4 non-hematologic toxicity including diarrhea despite antidiarrheal prophylaxis, nausea despite maximum anti-emetic therapy, or any delay in treatment of more than two weeks. The MTD was defined as the dose one level below the dose at which two or more of the patients in the initial dose level experienced DLT during the first treatment course. Patients who experienced a DLT, or toxicity attributed to study medication, could continue to receive study treatment after recovery, with appropriate dose modifications as defined per protocol.
To be evaluable for response and to be assessable for determination of MTD, patients had to have received at least one full cycle of therapy. Otherwise, treatment responses were evaluated after every two cycles with computed tomography scans or other diagnostic tests, as appropriate. Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0, were used for response assessment and done by an independent protocol radiologist. Complete or partial responses were confirmed by repeat studies at least 4 weeks after the criteria for response were first met.
The main objective of this study was to determine the MTD, or achieve a maximal identified target dose of flavopiridol, when administered in combination with a doxorubicin. Standard 3 + 3 design was used for dose escalation. The incidence of hematologic and non-hematologic toxicities was summarized separately by flavopiridol cohort. Secondary analyses included a pharmacokinetic analysis of flavopiridol by non-compartmental methods.
Drug Supply
Flavopiridol (also known as alvocidib, HMR 1275) was supplied by Sanofi Aventis Pharmaceuticals and distributed by CTEP. Doxorubicin and dexrazoxane are commercially available.
Pharmacokinetics
Blood samples for pharmacokinetic (PK) studies were collected into heparin-coated tubes and analyzed per previously published methods(24). Flavopiridol levels were measured on the bolus schedule prior to treatment, at completion of flavopiridol (time 0) and then 0.5, 1, 2, 6 and 24 hours later. For the split dosing schedule, PK samples were collected pretreatment and at approximately 0.5, 4.5, 24 and 28 hours from initiation of flavopiridol infusion.
RESULTS
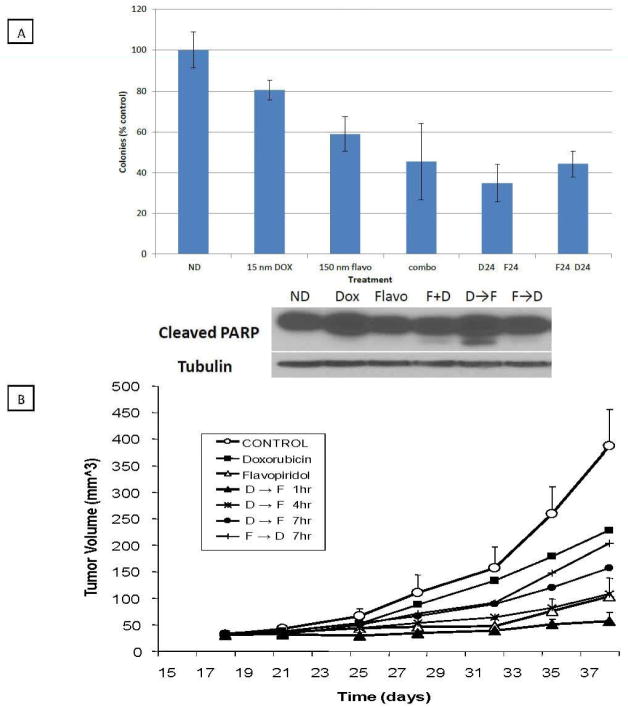
In vitro, the doxorubicin and flavopiridol combinations were statistically superior to single agent doxorubicin or flavopiridol. As shown in Figure 1A, colony formation assays revealed that in comparison to doxorubicin or flavopiridol alone, observable colonies decreased significantly with all the combinations tested. For example, colony formation decreased from 59% with flavopiridol alone to 41% with concomitant therapy (combo, p = 0.046), 35% with doxorubicin followed by flavopiridol (D24, F24, p = 4.4 × 10−5) and 44% with flavopiridol followed by doxorubicin (F24, D24, p = 3.8 × 10−5). Though there was a trend in decrease of colony formation favoring doxorubicin followed by flavopiridol, no statistically significant differences in colony formation observed for the three combinations. However, when these combinations were examined for induction of apoptosis by PARP cleavage, this was predominantly observed for the cells treated with the sequential doxorubicin followed by flavopiridol combination (Figure 1A).
FIGURE 1.
The effects of flavopiridol and doxorubicin on MPNST cell colony formation and dedifferentiated liposarcoma tumor xenograft growth. A. Colony formation after treatment of MPNST cells with doxorubicin, flavopiridol (individual, in combination or in sequence). MPNST cells were treated with doxorubicin (D) for 24 hours, flavopiridol (F) for 24 hours, concomitantly for 24 hours (combo) or sequentially such that cells were treated with D for 24 hours followed by F for 24 hours, or the reverse combination. After treatment, drug containing media was removed and colony formation was assayed 10 days later. Results are presented as percentages of untreated controls. Immunoblot analysis after treatment under these same conditions using antibody for cleaved PARP. α-tubulin is shown to confirm equal loading of protein. B. Treatment of dedifferentiated liposarcoma xenografts with doxorubicin and flavopiridol, as single agents or in sequence (separated by 1, 4 or 7 hours). LS141 xenografts (in groups of 5) were treated with doxorubicin, flavopiridol or sequentially separated by 1, 4 or 7 hours or the reverse sequence.
In vivo, human xenograft mouse models revealed that single agent flavopiridol decreased tumor growth relative to untreated controls and was significantly more efficacious than single agent doxorubicin. As we have previously reported that the timing of chemotherapy relative to flavopiridol can effect the degree of tumor regressions in vivo, we elected to treat our tumor xenografts with doxorubicin followed by flavopiridol at 1, 4, and 7 hours, as well as with the reverse combination at a 7 hour interval. As shown in Figure 1B, the only combination that was statistically superior to doxorubicin alone was doxorubicin followed 1 hour later by flavopiridol (p=0.01 at day 38). All the other combinations were either equal to (D → F4, p=0.19) or inferior to (D → F7, p=0.08 and F → D7, p=0.71) doxorubicin alone. There was no significant weight loss with the combination therapy. Interestingly, when comparing flavopiridol to the D → F1 combination, there appeared to be a trend toward lower tumor growth with the combination however this did not reach statistical significance (p=0.15). These data were consistent with prior observations that dedifferentiated liposarcoma cell lines, with amplified CDK4, are highly sensitive to flavopiridol (data not shown) as well as the clinical observation that dedifferentiated liposarcomas are generally resistant to doxorubicin.
Given these findings, we launched a phase I dose-escalation trial of flavopiridol in combination with doxorubicin. The primary objective of this trial was to determine the MTD of flavopiridol in combination with doxorubicin in patients with advanced sarcomas. Secondary objectives were to investigate the clinical pharmacokinetics of flavopiridol in combination with doxorubicin and to obtain preliminary data on the therapeutic activity of this regimen.
Clinical Trial Results
Patient Characteristics
From 10/13/2004 to 1/14/2010, 31 patients with metastatic or locally recurrent sarcoma were registered and 30 were treated. One patient was not evaluable for determining DLT as that patient did not initiate treatment after registration (withdrew consent). Three patients were not evaluable for determining response because they did not complete two cycles of treatment. The reasons were withdrawal of consent (2 patients) and clinical deterioration (1 patient).
Table 2 lists the patient characteristics of the 31 patients who were accrued to the protocol. The median age was 57 years (range, 31–72 years) and the median Karnofsky performance status was 90% (range, 70–100%). There were 20 men and 11 women. The sarcomas treated and patient numbers were liposarcoma (16: 15 of which were WD/DD, 1 pleomorphic), leiomyosarcoma (5), fibrosarcoma (3), malignant peripheral nerve sheath tumor (MPNST) (2), undifferentiated pleomorphic sarcoma (UPS) (1), osteosarcoma (extra-osseous) (1), rhabdomyosarcoma (pleomorphic) (1), gastrointestinal-stromal tumor (GIST) (1) and solitary fibrous tumor (SFT) (1). The majority of patients had not received prior chemotherapy (16% pretreated) with an overall range of 0-2 prior treatments. No patients had received prior doxorubicin and all had progressive disease at the time of enrollment with an indication to begin systemic treatment.
Table 2.
Patient characteristics.
| Characteristic | Number of patients |
|---|---|
| Total | 31 |
| Assessable for response | 28 |
| Male | 20 |
| Female | 11 |
| Age (years) | |
| Median | 57 |
| Range | 31–72 |
| KPS (%) | |
| Median | 90 |
| Range | 70–100 |
| Prior Chemotherapy | 16% |
| Number of prior lines | |
| Median | 0 |
| Range | 0–2 |
| Prior Doxorubicin | 0 |
| Sarcoma Subtype | |
| Liposarcoma (15 WD/DD, 1 pleomorphic) | 16 |
| Leiomyosarcoma | 5 |
| Fibrosarcoma | 3 |
| MPNST | 2 |
| UPS | 1 |
| Osteosarcoma | 1 |
| Rhabdomyosarcoma (pleomorphic) | 1 |
| GIST | 1 |
| Solitary Fibrous Tumor | 1 |
Toxicity
Table 3 lists the most common grade 2–4 hematologic toxicities for the first cycle of therapy (no relevant non-hematologic toxicities were observed in cycle 1). Combination treatment with 60 mg/m2 of doxorubicin and flavopiridol as a 1 hour bolus was documented to be well tolerated without DLT to the protocol-specified levels. Starting at 60 mg/m2 of flavopiridol, grade 3 lymphopenia was also observed, a common non-dose limiting hematologic toxicity of flavopiridol(21). A single cohort of patients was treated with 75 mg/m2 of doxorubicin and 60 mg/m2 of flavopiridol without DLT (cohort 5). However, there was grade 3 neutropenia in all three patients treated at this dose level, suggesting that the increased doxorubicin dose resulted in increased neutropenia.
Table 3.
Grade 2 or greater hematologic toxicity observed in cycle 1
| Cohort (evaluable pts) | Doxo | Flavo | ANC | Lymphopenia | Leukocytes | Hemoglobin | Platelets | Febrile Neutro | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |||
| 1 (3) | 60 | 40 | 1 | 1 | 1 | |||||||||||||||
| 2 (3) | 60 | 50 | 1 | 1 | ||||||||||||||||
| 3 (3) | 60 | 60 | 1 | 1 | 1 | 2 | 2 | 1 | ||||||||||||
| 4 (3) | 60 | 70 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||
| 5 (3) | 75 | 60 | 3 | 1 | 1 | |||||||||||||||
| 6 (3) | 60 | 70 (40/30) | 2 | 1 | 1 | |||||||||||||||
| 7 (5)* | 60 | 80 (50/30) | 1 | 3 | 1+ | 3 | 4 | 1+ | ||||||||||||
| 8 (5)* | 60 | 90 (50/40) | 2 | 5 | 1# | 3 | 2 | 1# | 1 | 2 | 2 | 1# | ||||||||
All dose-limiting toxicity was hematologically related
DLT including ANC and leukocytes was experienced by the same patient in cohort 7
DLT including ANC, leukocytes and febrile neutropenia was experienced by one patient in cohort 8
Starting with 70 mg/m2 of total flavopiridol in cohort 6, the combination of doxorubicin and split dosing flavopiridol was also found to be tolerable. However, DLTs were observed in the 7th and 8th cohorts. In the 7th cohort, one patient experienced DLT including both grade 4 neutropenia and leukopenia leading to expansion of the cohort to six patients. In cohort 8, one patient experienced DLT including grade 4 neutropenia and leukopenia and grade 3 febrile neutropenia. This cohort was also expanded without observance of further significant toxicity. Beyond these, no other DLTs were observed throughout the study. Dose escalation beyond cohort 8 was not pursued as the pre-specified maximal flavopiridol dose of 90 mg/m2 was achieved. As such, no MTD was formally established. However, it should be noted that there was substantial grade 3 neutropenia in cohort 7 (3 of 6 patients) and cohort 8 (5 of 6 patients) with two episodes of grade 3 thrombocytopenia. Therefore it is unlikely that further dose escalation could have been safely achieved. Notably, all DLTs were hematologic in nature and tumor lysis syndrome was not observed.
Supplementary Table 1 lists the most common grade 2–4 cumulative hematologic toxicities for all cycles of treatment. The cumulative pattern of toxicity was similar to that in cycle 1 of treatment and was principally limited to hematologic effects. For the whole study population, the most common grade 3/4 toxicities were neutropenia (45%), leukopenia (26%), thrombocytopenia (9%) and lymphopenia (6%). Grade 3/4 neutropenia occurred in 14% of patients on the bolus schedule versus 33% of those on the bolus/infusional schedule. Similarly, grade 3/4 leukopenia occurred in 4% and 23% for the bolus only and split dosing schedules, respectively. Grade 3/4 thrombocytopenia occurred in 0% and 9% of patients on the bolus only and split dosing schedules, respectively. The rates of lymphopenia and anemia were not impressively different between the two schedules.
Several significant non-hematologic toxicities occurred after cycle 1 however were generally not attributed to study treatment and were not considered dose-limiting for the protocol. The majority of these occurred with the split dosing flavopiridol schedules. The exception to this was the one death on protocol (cohort 3). This was due to a small bowel perforation and was attributed to treatment. The other toxicities (attributed as not related to treatment) included the development of grade 4 CNS ischemia and a grade 3 pulmonary embolus in 2 different patients in dose cohort 6, a grade 3 pulmonary embolus with left ventricular dysfunction (following 2 cycles of therapy) and a grade 4 small bowel perforation in two separate patients in dose cohort 7, and both a grade 3 psychosis and grade 4 confusion in a patient in dose cohort 8.
Pharmacokinetics
Blood samples from 31 patients were obtained to perform PK analyses of flavopiridol. Table 4 summarizes maximum observed plasma concentration (Cmax) across all subjects in a cohort. Flavopiridol Cmax was observed at the first time point analyzed in each schedule (0.5 hours) and ranged from 2.22 μM (SD 0.07) at a flavopiridol dose of 50 mg/m2, up to a maximum of 3.33 μM (SD 1.38) at a flavopiridol dose 90 (50/40) mg/m2. Use of the split dosing schedule did not appear to increase the Cmax of flavopiridol. There was significant inter-patient variability within some cohorts (3, 7 and 8). The dose of doxorubicin was held constant for all but one dose level thus making evaluation of any potential interaction between the two drugs difficult. Notably however, the Cmax range noted in this trial is similar to that seen in previous clinical trials combining flavopiridol with other chemotherapies(24, 25), suggesting that flavopiridol exposure is unlikely to be significantly altered when combined with doxorubicin. In cohorts 7 and 8, there was higher Cmax in patients who experienced DLT as opposed to those who did not (data not shown), but this difference did not meet statistical significance. There were insufficient PK time points available to formally evaluate the area under the curve (AUC) for either schedule.
Table 4.
Flavopiridol pharmacokinetics by dose level during cycle 1.
| Cohort | Doxo | Flavo | n | Mean Cmax (uM) | Standard Deviation (SD) |
|---|---|---|---|---|---|
| 1 | 60 | 40 | 3 | 2.27 | 0.49 |
| 2 | 60 | 50 | 3 | 2.23 | 0.52 |
| 3 | 60 | 60 | 3 | 3.29 | 1.39 |
| 4 | 60 | 70 | 3 | 2.22 | 0.07 |
| 5 | 75 | 60 | 3 | 2.46 | 0.67 |
| 6 | 60 | 70 (40/30) | 4 | 2.56 | 0.31 |
| 7 | 60 | 80 (50/30) | 6 | 3.03 | 1.29 |
| 8 | 60 | 90 (50/40) | 4 | 3.33 | 1.38 |
Clinical Activity
Twenty-eight patients were evaluable for response assessment (Table 5). There were two RECIST 1.0 partial responses and fourteen patients had RECIST stable disease as best response (median 15 weeks; range 3–99 weeks). The disease control rate (CR + PR + SD for >3 months) was 57% (16/28). There was no apparent difference by flavopiridol schedule. Of the 12 evaluable patients with well- and de-differentiated liposarcoma, five had progression of disease at first evaluation while seven had stable disease for at least three months as best response (median 20 weeks, range 12–99 weeks). One notable patient (WD and DD liposarcoma) was maintained on study for 99 weeks. This patient, treated in cohort 1, initially received the maximum allowable doxorubicin dose (including use of dexrazoxane) and was thereafter continued on single agent flavopiridol. The best response was stable disease, which was maintained through 83 weeks. At that point, the patient decided to withdrawal consent for further treatment. Thirty-three weeks later, the patient was noted to have progression of disease and under a special protocol amendment was reinitiated on single agent flavopiridol. Disease stability was recovered and the patient continued on treatment for a further 16 weeks prior to eventually progressing.
Table 5.
Time on study and response by individual patient and cohort.
| Patient # and cohort | Histology | Best Response | Reason Off-Study | Time On Study (wks) | PFS ≥12 wks | PFS ≥24 wks | Total Cycles |
|---|---|---|---|---|---|---|---|
| 1 (1) | Leiomyosarcoma | POD | POD | 7 | No | No | 2 |
| 2 (1) | Liposarcoma (WD/DD) | SD | POD | 25 | YES | YES | 9 |
| 3 (1) | Liposarcoma (WD/DD) | SD | POD | 99 | YES | YES | 31 |
| 4 (2) | Liposarcoma (Pleiomorphic) | SD | POD | 7 | No | No | 2 |
| 5 (2) | Leiomyosarcoma | SD | Clincal POD | 47 | YES | YES | 15 |
| 6 (2) | MPNST | POD | POD | 7 | No | No | 2 |
| 7 (3) | Liposarcoma (WD/DD) | POD | POD | 6 | No | No | 2 |
| 8 (3) | MPNST | SD | Clincal POD | 7 | No | No | 3 |
| 9 (3) | Leiomyosarcoma | PR | POD | 45 | YES | YES | 15 |
| 10 (4) | Fibrosarcoma | SD | Clincal POD | 6 | No | No | 2 |
| 11 (4) | Leiomyosarcoma | SD | POD | 47 | YES | YES | 2 |
| 12 (4) | Osteosarcoma | SD | POD | 12 | YES | No | 4 |
| 13 (5) | Liposarcoma (WD/DD) | SD | Withdrew Consent | 12 | YES | No | 4 |
| 14 (5) | Fibrosarcoma | SD | POD | 23 | YES | No | 8 |
| 15 (5) | Liposarcoma (WD/DD) | POD | POD | 6 | No | No | 2 |
| 16 (6) | Liposarcoma (WD/DD) | SD | Withdrew Consent | 15 | YES | No | 5 |
| 17 (6) | Leiomyosarcoma | SD | POD | 25 | YES | YES | 8 |
| 18 (6) | Liposarcoma (WD/DD) | Unevaluable | Intercurrent Illness | 3 | No | No | 1 |
| 19 (6) | Liposarcoma (WD/DD) | POD | POD | 6 | No | No | 2 |
| 20 (7) | Liposarcoma (WD/DD) | Unevaluable | Toxicity | 4 | No | No | 2 |
| 21 (7) | Fibrosarcoma | SD | POD | 73 | YES | YES | 24 |
| 22 (7) | Liposarcoma (WD/DD) | SD | Withdrew Consent | 25 | YES | YES | 7 |
| 23 (7) | Solitary Fibrous Tumor | SD | Intercurrent Illness | 12 | YES | No | 3 |
| 24 (7) | Liposarcoma (WD/DD) | SD | Withdrew Consent | 14 | YES | No | 4 |
| 25 (7) | Liposarcoma (WD/DD) | POD | POD | 6 | No | No | 2 |
| 26 (8) | Liposarcoma (WD/DD) | POD | POD | 6 | No | No | 2 |
| 27 (8) | Rhabdomyosarcoma | PR | POD | 38 | YES | YES | 12 |
| 28 (8) | Liposarcoma (WD/DD) | Unevaluable | Not Treated | 0 | No | No | 0 |
| 29 (8) | GIST | SD | Toxicity | 3 | No | No | 1 |
| 30 (8) | Liposarcoma (WD/DD) | SD | POD | 15 | YES | No | 4 |
| 31 (8) | UPS | POD | POD | 6 | No | No | 2 |
Patients are listed in order of accrual to the protocol and treatment cohorts are designated by shading (i.e. – cohort 1 includes patients 1–3 while cohort 8 includes patients 26–31).
Abbreviation: Progression of disease (POD), weeks (wks)
Discussion
We examined the utility of flavopiridol in potentiating the effects of doxorubicin on the growth of malignant sarcoma both in vitro and in vivo. Preclinically, we documented in MPNST cells that flavopiridol potentiates doxorubicin, when compared to single agents alone. Further, we demonstrated that flavopiridol is active in vivo both as a single agent and in combination with doxorubicin in liposarcoma xenograft with amplified CDK4.
Given these findings, we conducted a phase I dose-escalation clinical trial of flavopiridol plus doxorubicin in patients with advanced sarcomas. Biologically active and therapeutic doses of flavopiridol (90 mg/m2; 50 mg/m2 bolus followed by 40 mg/m2 infusion) and doxorubicin (60 mg/m2) were combined without reaching a MTD. The achieved dose of flavopiridol was similar to that shown to be tolerable in combination with other chemotherapies, and the PK at most of the dose levels tested were in the active range based on pre-clinical data(13, 26). Hematologic DLTs, constituted by neutropenia, leukopenia, lymphopenia and thrombocytopenia, were observed by the combination of flavopiridol and anthracycline chemotherapy. Adverse events were generally tolerable, with the appearance of febrile neutropenia in only one instance. We conclude that flavopiridol can be combined with doxorubicin safely at biologically active doses.
Based on the results of the clinical study, it is not possible to make a definite determination whether the bolus schedule or the split dosing schedule is preferred for future clinical development of flavopiridol in combination with doxorubicin or more generally in the treatment of sarcoma. Regarding safety, no MTD was reached. Dose-limiting hematologic toxicity was increased with the split dosing regimen and this became more evident with cumulative dosing. Non-hematologic toxicity also became more apparent with cumulative dosing on the divided dose flavopiridol schedule. Unlike studies utilizing a split-dose schedule for the treatment of hematologic malignancies, no evidence of tumor lysis syndrome was observed in this study.
In regards to efficacy, there were two partial responses, as well as stable disease as long as 99 weeks. Disease control (PR+SD > 3 months) was documented at various dose levels and was independent of dosing schedules of flavopiridol. Inter-patient variability, especially in dose levels 3, 7 and 8, somewhat confounds the use of PK to determine the most efficacious dose and schedule. The flavopiridol Cmax generally increased with increasing total flavopiridol dose with all dose cohorts having Cmax greater than 2 μM. Given this, it also does not appear that continuous flavopiridol exposure via infusion added significant clinical benefit. In view of the overall increased toxicity with the divided dose schedule, the bolus schedule would seem to be preferred for future development of flavopiridol in combination with doxorubicin for the ambulatory treatment of sarcoma.
The combination of flavopiridol and doxorubicin provided a substantial disease control in this study, with 68% (19/28) achieving PR or SD as best result. This is especially interesting given that 12 of the 28 evaluable patients had a diagnosis of WD and DD liposarcoma, a disease that is generally non-chemotherapy responsive but where CDK4 is frequently amplified(27). Within this sub-population, the disease control rate was 67%. For the entire study, the progression- free survival at 12 weeks (PFS12weeks) was 57% (16/28) and progression-free survival at 24 weeks (PFS24weeks) was 32% (9/28). Given the heterogeneity of soft tissue sarcomas, the European Organization for Research and Treatment of Cancer (EORTC) has developed standards for evaluating new treatments for soft tissue sarcoma in the phase II setting(28). These standards incorporate progression-free survival and clinical benefit in evaluation of new agents. Notably, in this study, the PFS12weeks and PFS24weeks compare favorably to these reference standards and suggest that this regimen may be worth further exploration in this patient population.
While the benefit of flavopiridol based therapy in the treatment of WD and DD liposarcoma could be hypothesized to be a function of its CDK4 amplification, other sarcoma types are not as clearly linked to dysregulated apoptosis. In this study we note prolonged SD in various tumor types such as leiomyosarcoma, fibrosarcoma and pleomorphic rhabdomyosarcoma. While these tumors are also associated with chemotherapy responsiveness to anthracyclines, it is possible that doxorubicin was potentiated by flavopiridol. Recent literature has suggested that the predominant mechanism of flavopiridol efficacy is through inhibition of CDK9(29). This results in suppression of critical anti-apoptotic molecules and may lead to the potentiation of chemotherapy. Considering this, a rational mechanism for chemotherapy potentiation by flavopiridol would appear to be promotion of a death response in tumor cells after insult by chemotherapy. Given these observations, further study of flavopiridol in the treatment of WD and DD liposarcomas, and soft tissue sarcomas more generally, is warranted.
Supplementary Material
Statement of Translational Relevance.
Doxorubicin is part of standard therapy for the treatment of soft tissue sarcomas. Flavopiridol, the pancyclin dependent kinase (CDK) inhibitor, has been shown to enhance the effects of chemotherapy. We report that flavopiridol potentiates the effects of doxorubicin in soft tissue sarcoma cell lines both in vitro and in vivo. Based on these results, we designed and conducted a phase I clinical trial of fixed dose doxorubicin followed by escalating doses of flavopiridol on two different flavopiridol schedules. Our results indicate that the combination is well tolerated with clinical benefit at biologically active doses. These studies provide a foundation upon which to further examine the role of CDK inhibitors in augmenting doxorubicin. This is particularly applicable in diseases where CDKs are known to be oncogenic drivers and in which doxorubicin is active, including certain types of liposarcomas, lymphomas and leukemias.
Acknowledgments
Research Grant Support: Soft Tissue Sarcoma Program Project grant P01 CA 047179
References
- 1.Goy A, Kahl B. Mantle cell lymphoma: The promise of new treatment options. Critical reviews in oncology/hematology. 2010 doi: 10.1016/j.critrevonc.2010.09.003. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 2.Komina O, Nosske E, Maurer M, Wesierska-Gadek J. Roscovitine, a small molecule CDK inhibitor induces apoptosis in multidrug-resistant human multiple myeloma cells. Journal of experimental therapeutics & oncology. 2011;9(1):27–35. Epub 2011/02/01. [PubMed] [Google Scholar]
- 3.Yenugonda VM, Deb TB, Grindrod SC, Dakshanamurthy S, Yang Y, Paige M, et al. Fluorescent cyclin-dependent kinase inhibitors block the proliferation of human breast cancer cells. Bioorganic & medicinal chemistry. 2011;19(8):2714–25. doi: 10.1016/j.bmc.2011.02.052. Epub 2011/03/29. [DOI] [PubMed] [Google Scholar]
- 4.Richard C, Matthews D, Duivenvoorden W, Yau J, Wright PS, Th'ng JP. Flavopiridol sensitivity of cancer cells isolated from ascites and pleural fluids. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(9):3523–9. doi: 10.1158/1078-0432.CCR-04-2507. Epub 2005/05/04. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266(5192):1821–8. doi: 10.1126/science.7997877. Epub 1994/12/16. [DOI] [PubMed] [Google Scholar]
- 6.Zhai S, Senderowicz AM, Sausville EA, Figg WD. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in clinical development. The Annals of pharmacotherapy. 2002;36(5):905–11. doi: 10.1345/aph.1A162. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 7.Blum W, Phelps MA, Klisovic RB, Rozewski DM, Ni W, Albanese KA, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010;95(7):1098–105. doi: 10.3324/haematol.2009.017103. Epub 2010/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin TS, Blum KA, Fischer DB, Mitchell SM, Ruppert AS, Porcu P, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(3):418–23. doi: 10.1200/JCO.2009.24.1570. Epub 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramaswamy B, Phelps MA, Baiocchi R, Bekaii-Saab T, Ni W, Lai JP, et al. A dose-finding, pharmacokinetic and pharmacodynamic study of a novel schedule of flavopiridol in patients with advanced solid tumors. Investigational new drugs. 2010 doi: 10.1007/s10637-010-9563-7. Epub 2010/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Italiano A, Bianchini L, Gjernes E, Keslair F, Ranchere-Vince D, Dumollard JM, et al. Clinical and biological significance of CDK4 amplification in well-differentiated and dedifferentiated liposarcomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(18):5696–703. doi: 10.1158/1078-0432.CCR-08-3185. Epub 2009/09/10. [DOI] [PubMed] [Google Scholar]
- 11.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(35):6012–8. doi: 10.1200/JCO.2009.22.6944. Epub 2009/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosini G, Seelman SL, Qin LX, Schwartz GK. The cyclin-dependent kinase inhibitor flavopiridol potentiates the effects of topoisomerase I poisons by suppressing Rad51 expression in a p53-dependent manner. Cancer research. 2008;68(7):2312–20. doi: 10.1158/0008-5472.CAN-07-2395. Epub 2008/04/03. [DOI] [PubMed] [Google Scholar]
- 13.Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, et al. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(12):4209–19. Epub 2001/12/26. [PubMed] [Google Scholar]
- 14.Jung CP, Motwani MV, Schwartz GK. Flavopiridol increases sensitization to gemcitabine in human gastrointestinal cancer cell lines and correlates with down-regulation of ribonucleotide reductase M2 subunit. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7(8):2527–36. Epub 2001/08/08. [PubMed] [Google Scholar]
- 15.Wall NR, O'Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer research. 2003;63(1):230–5. Epub 2003/01/09. [PubMed] [Google Scholar]
- 16.Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(7):1876–83. Epub 1999/08/03. [PubMed] [Google Scholar]
- 17.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer research. 1997;57(16):3375–80. Epub 1997/08/15. [PubMed] [Google Scholar]
- 18.Li W, Fan J, Bertino JR. Selective sensitization of retinoblastoma protein-deficient sarcoma cells to doxorubicin by flavopiridol-mediated inhibition of cyclin-dependent kinase 2 kinase activity. Cancer research. 2001;61(6):2579–82. Epub 2001/04/06. [PubMed] [Google Scholar]
- 19.Dei Tos AP, Doglioni C, Piccinin S, Sciot R, Furlanetto A, Boiocchi M, et al. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. The Journal of pathology. 2000;190(5):531–6. doi: 10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. Epub 2000/03/23. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) British journal of cancer. 2011;104(12):1862–8. doi: 10.1038/bjc.2011.177. Epub 2011/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senderowicz AM, Headlee D, Stinson SF, Lush RM, Kalil N, Villalba L, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(9):2986–99. doi: 10.1200/JCO.1998.16.9.2986. Epub 1998/09/17. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Lin TS, Dalton JT, Wu D, Phelps MA, Fischer B, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109(2):399–404. doi: 10.1182/blood-2006-05-020735. Epub 2006/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in radiation oncology. 2003;13(3):176–81. doi: 10.1016/S1053-4296(03)00031-6. Epub 2003/08/07. [DOI] [PubMed] [Google Scholar]
- 24.Dickson MA, Shah MA, Rathkopf D, Tse A, Carvajal RD, Wu N, et al. A phase I clinical trial of FOLFIRI in combination with the pan-cyclin-dependent kinase (CDK) inhibitor flavopiridol. Cancer chemotherapy and pharmacology. 2010;66(6):1113–21. doi: 10.1007/s00280-010-1269-1. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathkopf D, Dickson MA, Feldman DR, Carvajal RD, Shah MA, Wu N, et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(23):7405–11. doi: 10.1158/1078-0432.CCR-09-1502. Epub 2009/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah MA, Kortmansky J, Motwani M, Drobnjak M, Gonen M, Yi S, et al. A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(10):3836–45. doi: 10.1158/1078-0432.CCR-04-2651. Epub 2005/05/18. [DOI] [PubMed] [Google Scholar]
- 27.Lorigan P, Verweij J, Papai Z, Rodenhuis S, Le Cesne A, Leahy MG, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(21):3144–50. doi: 10.1200/JCO.2006.09.7717. Epub 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 28.Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38(4):543–9. doi: 10.1016/s0959-8049(01)00398-7. Epub 2002/03/02. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Fischer PM. Cyclin-dependent kinase 9: a key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends in pharmacological sciences. 2008;29(6):302–13. doi: 10.1016/j.tips.2008.03.003. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.