Abstract
Background
Superficial thrombophlebitis can produce pain and result in deep-vein thrombosis if not treated. Conservative therapies including prescription of non-steroidal anti-inflammatory medications and heat have been standard care. Recently, studies have been published reporting efficacy and safety of low-molecular weight heparin for treatment of superficial thrombophlebitis. However, there are few comparative trials to conservative therapy. We studied the effectiveness and safety of treatment with dalteparin compared to ibuprofen in patients with confirmed superficial thrombophlebitis.
Methods
Consecutive patients were randomized to receive daily dalteparin versus ibuprofen three times daily for up to 14 days. The primary outcome measure was the incidence of extension of thrombus or new symptomatic venous thromboembolism during the 14 day and 3 month follow-up period. The secondary outcome was reduction in pain. The outcome measure of safety was the incidence of major and minor bleeding.
Results
Of 302 consecutive patients screened, 72 were enrolled. Four patients receiving ibuprofen compared to no patients receiving dalteparin had thrombus extension at 14 days (p=0.05), however, there was no difference in thrombus extension at 3 months. Both treatments significantly reduced pain. There were no episodes of major or minor bleeding during the treatment period.
Conclusions
Dalteparin is superior to the NSAID ibuprofen in preventing extension of superficial thrombophlebitis during the 14 day treatment period with similar relief of pain and no increase in bleeding. However, questions concerning the optimal treatment duration should be explored in future trials.
Keywords: superficial vein thrombosis, thrombophlebitis, extremity, therapy
Introduction
Superficial thrombophlebitis is a common clinical problem affecting up to 50% of patients with varicose veins (1). In the absence of treatment, superficial thrombophlebitis may cause its greatest morbidity by extension of thrombus into the deep venous system with resultant risk of pulmonary embolism (2, 3). In addition, patients may suffer severe pain and erythema over the affected venous segment and commonly seek relief of these symptoms (4). Current standard therapy for superficial thrombophlebitis consists of local heat, elevation of the extremity, and non-steroidal anti-inflammatory medication (NSAID) (5). However, few studies to date have adequately evaluated the effectiveness of this therapy despite persistence and recurrence of symptoms of superficial thrombophlebitis in many patients. Surgical ligation and extraction has been used in the past for symptomatic relief and prevention of recurrence, but is expensive, invasive and inconvenient (6, 7). Without treatment, the extension rate of superficial thrombophlebitis is estimated at 40 to 50% with a risk of subsequent deep-vein thrombosis of 3.5 to 11% (2,8) and pulmonary embolism of 0.5% (4,9). There is growing interest in the use of low-molecular weight heparin (LMWH) with its anti-inflammatory and antithrombotic effects for the treatment of superficial thrombophlebitis, and recent studies indicate that it may be effective (10). However, it remains unclear if LMWH is more efficacious than NSAIDS (11).
This study evaluated the outcome of patients with objectively confirmed superficial thrombophlebitis of the upper or lower extremity who were treated with NSAID therapy versus those treated with low-molecular weight heparin (dalteparin sodium) according to a pre-defined treatment regimen.
Our hypothesis was that the LMWH dalteparin given in the dose of 200 units/kg sc at presentation followed by fixed dose dalteparin 10,000 units sc daily for an additional 6-13 days was superior to NSAID Ibuprofen 800mg given orally three times daily in halting thrombus progression and preventing VTE. In addition, we hypothesized that the dalteparin treatment regimen was superior to ibuprofen in alleviating pain associated with superficial thrombophlebitis without an increase in clinically significant bleeding.
Methods
The study was performed at the two hospitals and ambulatory clinic on the University of Oklahoma Health Sciences Center campus: OU Medical center and, Veterans Administration Medical Center, and the Vascular Center in the OU Physicians Medical Building. The study was approved by the institutional review board of the University of Oklahoma Health Sciences Center. The study was approved and annually reviewed by the FDA (IND No. 66032, NCT 00264381).
Consecutive patients, both inpatient and outpatient, who had been objectively tested by non-invasive vascular laboratory diagnostic ultrasound imaging and found to have superficial thrombophlebitis of the lower or upper extremities in the absence of a current intravenous catheter were eligible for the study. Patients were screened, consented and randomized in most cases at time of diagnosis, however, patients who received imaging for suspected superficial thrombophlebitis after hours were identified by vascular laboratory log review the next business day and consented if eligible.
Patients were ineligible if they had one or more of the following: receiving anticoagulant therapy including warfarin, heparin, or low-molecular weight heparin for more than 24 hours duration, concurrent deep-vein thrombosis, active, clinically significant bleeding, known hypersensitivity to NSAIDS, heparin or derivatives, currently pregnant or < 1 week post-partum, history of bleeding gastric or duodenal ulcer in past year, history of hemorrhagic cerebrovascular event in past year, platelet count less than 100,000, known inherited or acquired bleeding disorder, serum creatinine >2mg/dl, blood pressure > 180/110 at time of enrollment, weight < 40 kg or > 135 kg, or unable to return for repeat diagnostic testing or follow-up visits.
Each consented patient underwent a complete baseline assessment including demographic characteristics, history of present illness, past medical history, physical examination findings, and medication profile recorded on a standard data form. A complete blood count was obtained at baseline and at 5 to 7 day follow-up. Evaluation of pain severity at presentation and during the 14 day treatment period was performed using the 11-point Box Pain Scale as described below.
The study was a randomized, controlled, double-blind, double-dummy trial. Figure 1 summarizes the study design. Randomization was performed using balanced randomization blocks of four each consisting of equal numbers of dalteparin treatments and ibuprofen treatments, for example, IDID, IIDD, DIDI, and so on. Randomization was stratified by hospital site. The patient, research assistant and principal investigator were blinded to treatment group. Randomization of patient to treatment group was performed by the investigational medication pharmacist within 24 hours of presenting with a confirmed diagnosis of superficial thrombophlebitis. Patients were randomized to one of the following treatment groups: Experimental Group (dalteparin 200 units/kg sc first dose then 10,000 units sc daily for 6 additional doses + placebo given orally three times daily for 7 days) or Control Group (ibuprofen 800mg given orally three times daily for 7 days + one placebo injection given daily for 7 days). The anticoagulant dose of dalteparin chosen was based on providing maximum anti-thrombotic efficacy in the first 24 hours after presentation, followed by a regimen of daily injections intended to minimize the risk of bleeding. In addition, the treatment regimen chosen was simple, convenient and practical to maximize patient compliance. Dalteparin was dosed based on actual body weight. There was a ceiling dose of 18,000 units for obese patients with weights to 135 kg. Patients with weights between 40 kg and 50 kg were given a minimum dose of 10,000 units. Patients with weights greater than 135 kg or less than 40 kg were excluded. If symptoms of superficial thrombophlebitis were not resolved at day 7 to 9, in the absence of thrombus extension, the patient received an additional 7 days of the blinded therapy. Compression stockings were not routinely prescribed.
Figure 1.
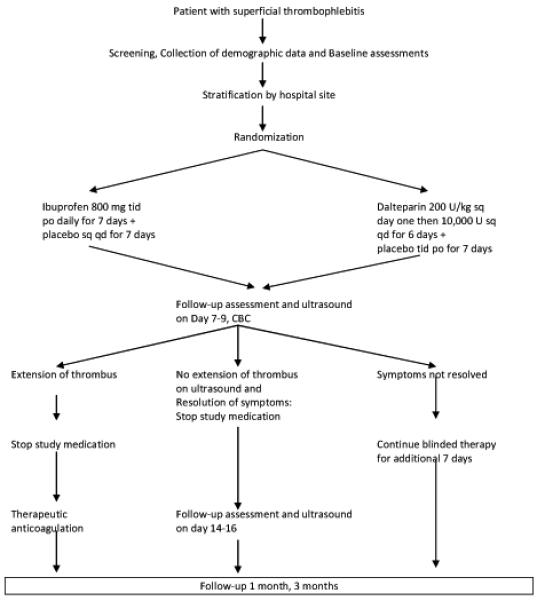
Treatment algorithm
If at any time thrombus was found to extend either superficially or into the deep venous system, experimental therapy was discontinued and the patient received full therapeutic anticoagulation with intravenous heparin or low-molecular weight heparin using standard dosing guidelines, unless contraindicated.
The outcome in each of the two treatment groups was assessed by follow-up at day 7-9, 14-16, 1 month and 3 months. On day 7-9 and 14-16, the patient returned to the non-invasive vascular laboratory to have reassessment of the area of superficial thrombophlebitis by ultrasound (technique described under ultrasound testing). In addition, a complete blood count was obtained. Patients were instructed to return immediately to the clinic or emergency room if they developed symptoms or signs suggesting deep-vein thrombosis (DVT) or pulmonary embolism (PE). Those with suspected DVT underwent testing with compression ultrasound. Those with suspected PE underwent nuclear lung scanning or helical CT scan for evidence of new perfusion defects.
At routine follow-up visits, an interim history was taken, addressing general health and specific symptoms, hospital admission, use of anticoagulants, and performance of the 11-point Box Pain Scale. Compliance was assured by pill counts of dispensed ibuprofen and placebo, and having patients return empty vials of dalteparin injection and placebo at their day 7-9 and day 14-16 follow-up visits.
Compression ultrasound testing was performed at time of diagnosis by certified vascular technicians using real-time duplex ultrasonography. Ultrasound testing at presentation of the lower extremity included documentation of the distance of the thrombus from the saphenofemoral junction or lesser saphenous-popliteal junction as well as measurement of length of thrombus. Testing for deep-vein thrombosis was performed using the simplified approach of Birdwell et al. (12). Evaluation of the upper-extremity was performed using the technique of Prandoni et al. (13). Distance of the thrombus from the cephalic-axillary vein junction for cephalic vein thrombosis and distance to the antecubital fossa for basilic, ulnar, and radial vein superficial thrombophlebitis in the forearm was documented as well as length of thrombus. Follow-up ultrasound testing was performed on day 7 to 9 and 14 to 16 using the Sonosite or Terason portable ultrasound by the principal investigator who has registered vascular technologist certification.
The 11-point Box Pain Scale consisted of 11 numbers (0 through 10) surrounded by boxes (14). The patient was told that the 0 represented no pain and the 10 represented extreme pain, and was asked to place an “X” through the number representing his or her pain level. The pain scale was recorded daily during the first 14 days.
The primary outcome measure of efficacy was the cumulative proportion of patients with thrombus extension or symptomatic objectively confirmed deep-vein thrombosis as assessed by objective ultrasound imaging including deep veins on day 14-16 and at 3 month follow-up. The secondary outcome measure was the improvement in pain as assessed as the change in the daily 11-point Box Pain Scale from baseline to day 7 and to 14-16 day follow-up. The outcome measures of safety were major and minor bleeding. Bleeding was defined as major if overt and associated either with a decrease in the hemoglobin concentration by at least 2.0 g per deciliter or with the need for the transfusion of 2 or more units of blood, or the bleeding was intracranial or retroperitoneal. Bleeding was defined as minor if it is overt but did not meeting criteria for major bleeding. Bleeding was defined as trivial (clinically insignificant) if it resolved spontaneously, and no medical or additional intervention was required. Thrombocytopenia was defined as present if the platelet count dropped from the normal range (150,000-450,000) to less than 100,000 or dropped by > 50% of the baseline level at presentation.
Results
Demographics
Of 302 consecutive patients screened, 72 patients were consented, 206 were ineligible and 22 refused consent. The reasons for ineligibility were: 52 currently receiving anticoagulant therapy for more than 24 hours, 19 with concurrent DVT, 14 with active, clinically significant bleeding, 8 with known hypersensitivity to NSAIDS, 12 currently pregnant or < 1 week postpartum, 2 with history of bleeding gastric or duodenal ulcer in past year, 4 with history of hemorrhagic cerebrovascular event in past year, 7 with platelet count < 100,00, 30 with serum creatinine > 2mg/dl, 5 with weight < 40kg or > 135kg, 14 unable to return for repeat diagnostic testing or follow up visits, 3 non-English speaking, 2 transplant patients, 11 at increased risk for bleeding, 2 on NSAIDS, 15 with altered mental status, 3 minors, 1 inmate, and 2 dialysis patients. Patient demographics are given in Table 1. There were no significant differences between treatment groups. Table 2 presents the reported symptoms and documented signs of superficial thrombophlebitis. The systolic and diastolic blood pressure in the dalteparin group was significantly lower compared to the ibuprofen group. No other physical examination findings were significantly different. Since only 5 patients were enrolled at the VA site, patients from both sites were combined for the purpose of analysis.
Table 1. Demographic characteristics.
| Dalteparin n=37 | Ibuprofen n=35 | |
|---|---|---|
| Male/Female | 13/24 | 8/27 |
| Mean age (Age range) | 51 (28-88) | 52 (19-85) |
| Outpatient/Inpatient | 36/1 | 32/3 |
| White/Black/Hispanic/Nat Amer | 29/4/1/3 | 30/4/0/1 |
| Upper/Lower extremity | 10/27 | 5/30 |
| Recent IV | 15 | 12 |
| Varicose veins | 15 | 17 |
| History of Superficial Thromb | 2 | 5 |
| History of DVT | 3 | 1 |
| History of PE | 1 | 0 |
| Recent hospitalization | 11 | 10 |
| Recent surgery | 9 | 9 |
| Recent travel | 13 | 15 |
| Malignancy | 3 | 4 |
Table 2. Signs and Symptoms of Superficial Thrombophlebitis at Enrollment.
| Limb arm/leg | Dalteparin | Ibuprofen |
|---|---|---|
| Redness | 2/21 | 5/22 |
| Warmth | 2/14 | 1/16 |
| Swelling | 8/39 | 6/39 |
| Tenderness | 10/43 | 6/44 |
Primary and secondary outcomes
There were 11 patients with suspected venous thrombotic events given in Table 3. For the initial primary outcome of thrombosis progression at 14 days, dalteparin was statistically superior (p=0.05) compared to ibuprofen. Four patients receiving ibuprofen had extension of the superficial thrombus during the two week treatment period. No patients receiving dalteparin experienced superficial thrombus progression or deep-vein thrombosis while receiving study medication. Two additional patients for a total of six patients (17%, 95% CI 6.5-33.6%) randomized to ibuprofen had thrombus progression during the 3 month follow up period after study medication had been discontinued. Four patients randomized to dalteparin had thrombus progression including one patient with pulmonary embolism within the 3 month follow up period after study medication had been discontinued. There was no statistical difference in thrombus progression between ibuprofen and dalteparin during the 3 month follow-up period. (p=0.51)
Table 3. Suspected Venous thromboembolic events at 3 month follow up.
| Study ID |
Treatment | Baseline | Thrombus progression while receiving study med Y/N |
5-7 days | 1 month | Comment |
|---|---|---|---|---|---|---|
| S010 | Ibuprofen | L GSV | Y | Progression | Thrombus from ankle to distal thigh extended to SFJ. Started on full dose dalteparin |
|
| S013 | Ibuprofen | R GSV trib | Y | Progression | Thrombus from GSV trib below knee extended distally. Started on therapeutic anticoagulation |
|
| S020 | Ibuprofen | R SSV | Y | Progression | Thrombus from ankle to 3 cm from confluence extended to 0.5cm from confluence. Started on therapeutic enoxaparin. Metastatic ovarian ca |
|
| S028 | Ibuprofen | L below knee tributary |
Y | Progression | No further extension compared to 7 day f/u |
Thrombus in tributary (not named) below knee extended proximally above knee at 1 day post enrollment. Started on therapeutic enoxaparin at 7days. Developed black stools. Enoxaparin discontinued with no further thrombus progression at day 14. |
| S049 | Ibuprofen | L GSV | N | Progression | 25 days post enrollment complained of increased swelling. Thrombus from 4cm from SFJ extended distally from ankle into foot |
|
| S058 | Ibuprofen | R GSV and tributary |
N | 2 mo post enrollment developed right thigh pain and tenderness (taking ibuprofen 400bid) Found to have recurrent superficial thrombosis symptoms, although US findings unchanged from enrollment. Patient started on enoxaparin 1mg/kg q12h and therapeutic warfarin. |
||
| S009 | Dalteparin | L GSV | N | No change | Left cephalic vein thrombosis from antecubital fossa to hand at 2 mo follow up |
|
| S038 | Dalteparin | R SSV | Y | Progression off study med |
3 weeks post entry, developed LLE pain (off study med). Pt with R SSV thrombus. Given enoxaparin and warfarin which she continued. Heterozygous FVLeiden |
|
| S040 | Dalteparin | R GSV trib below knee |
N | 6 weeks post enrollment, pt had pain (off study drug) and found to have thrombus extending from GSV tributary into posterior tibial vein |
||
| S045 | Dalteparin | L GSV | N | 2 mo post enrollment, developed new pain left thigh. Found to have extension of thrombus in superficial vein. Started on therapeutic enoxaparin and warfarin. Varicose veins present. Found to be FVLeiden heterozygous. |
||
| S056 | Dalteparin | L GSV and tributary |
N | 6 weeks post enrollment developed SOB and tachy. US neg for DVT. CTA showed RML subsegmental PE + consolidation c/w pneumonia in RML. Pt found to have uterine adenoca after heavy vaginal bleeding |
L=left, R=right, GSV=great saphenous vein, SSV=small saphenous vein
Both treatment groups show a statistically significant reduction in pain from baseline (day 1) to day 7, and from baseline to day 14 (Figure 2). However, there were no differences between the treatment groups in pain at baseline, or change in pain at day 7 or 14.
Figure 2.
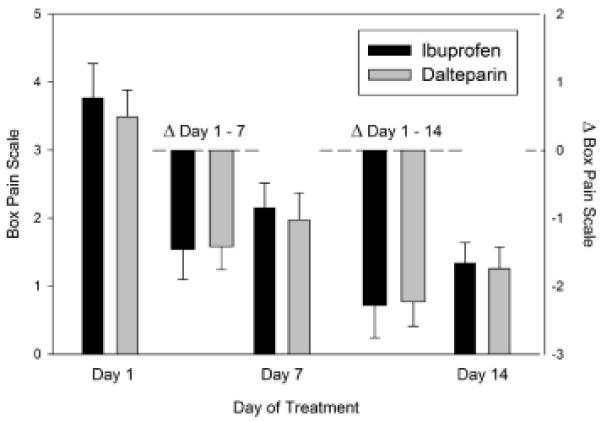
Change in pain scores during initial 14 day treatment
Adverse events
One patient (S028), originally randomized to ibuprofen, was found to have thrombus progression at the 7 day follow-up and was prescribed enoxaparin 1.5mg/kg/day and developed black stools seven days post initiation of enoxaparin. Enoxaparin was subsequently discontinued and follow up ultrasound seven days later revealed no further superficial thrombus progression and no evidence of DVT.
One patient receiving ibuprofen had a decrease in platelet count of > 50% of baseline at day 7 with a nadir platelet count of 92,000 that recovered spontaneously. No patients receiving dalteparin had a greater than 50% decrease in the platelet count. Overall, no patients receiving either treatment developed clinically overt bleeding.
One patient randomized to ibuprofen developed tinnitus on day 3 of therapy. The tinnitus worsened and ibuprofen was discontinued at 1 week follow up. Upon further questioning, the patient reported a history of tinnitus with caffeine consumption. At 3 month follow up visit, patient reported that she had lessened caffeine consumption and tinnitus was resolved.
Two patients, both randomized to dalteparin, developed rash. The first patient developed a rash on the back and neck one day post enrollment. Dalteparin was discontinued and the rash resolved by day 3 post enrollment. The second patient developed a diffuse maculopapular pruritic rash 6 days post enrollment. The patient was also receiving vancomycin which was discontinued. The rash persisted and dalteparin was discontinued at day 9 post enrollment. The patient was started on enoxaparin 80mg daily and then reduced to 40mg daily. The rash resolved.
One patient died at two month post enrollment from ovarian adenocarcinoma. This patient had been placed under hospice care one month post enrollment.
Discussion
The results of this study reveal four key findings. First, dalteparin appeared superior to ibuprofen in preventing extension of thrombus in patients with superficial thrombophlebitis of the upper or lower extremity during the two week study medication administration period. However, at 3 months, there was no significant difference in thrombus progression including development of VTE. Second, extension of thrombus including clinically apparent VTE occurring in the time period after ibuprofen and dalteparin were discontinued suggests that the treatment duration may have been too brief, or there is a rebound phenomenon that occurred after medication was halted.
Third, both treatment regimens of ibuprofen and dalteparin significantly reduced symptoms of pain during the treatment period, however, there was no statistical difference between them in pain alleviation. Fourth, both regimens appear safe as there were no episodes of major or minor bleeding during the period of study medication administration. It is important to note that major bleeding is rare even among those receiving therapeutic anticoagulation, and the study was not powered to detect a difference in bleeding complications. Dalteparin was associated with rash in two patients. The causative effect between ibuprofen and tinnitus could not be confirmed.
Since 2003, three randomized trials have been published evaluating medical treatment of superficial thrombophlebitis with only one including an NSAID treated group. The first was the double-blind STENOX trial where 427 patients were randomized to receive enoxaparin 40mg, enoxaparin 1.5mg/kg, tenoxicam 20mg or placebo for treatment of superficial thrombophlebitis of the lower extremities given for 8 to 12 days (10). The primary outcome was the development of deep-vein thrombosis by ultrasound imaging at the end of treatment and the secondary outcome was extension of the superficial thrombosis. All treatment groups significantly reduced the incidence of DVT and superficial thrombus extension compared to placebo, and there was a trend towards enoxaparin in either dose being superior to tenoxicam. They also found that most venous thromboembolic events occurred during the medication administration period for placebo and tenoxicam, but mainly after study medication discontinuation for the two enoxaparin groups. There were no episodes of death or major hemorrhage in any treatment group.
The VESALIO investigators randomized 164 patients with documented superficial thrombophlebitis of the great saphenous vein to nadroparin at a fixed dose (2850 units) or therapeutic body-weight adjusted dose once daily for one month (14). The study outcome was the rate of asymptomatic and symptomatic extension of superficial thrombosis and development of VTE during a 3 month follow-up period. The study found no difference in superficial thrombosis extension or VTE between the two treatment groups and there were no episodes of major bleeding. Importantly, most of the events that occurred in the therapeutic dose nadroparin group occurred after the medication was discontinued suggesting that the 1 month treatment duration may provide more effective protection against superficial thrombophlebitis progression, but the effect may be lost after medication discontinuation.
Most recently, a longer duration of therapy has been explored by the CALISTO study group (15). In a double-blind, double-dummy trial, 3002 patients with acute, symptomatic superficial thrombophlebitis of the legs were randomized to receive fondaparinux 2.5mg daily given subcutaneously versus placebo for 45 days. The primary efficacy outcome was the composite of death from any cause or pulmonary embolism, or symptomatic DVT, extension of thrombus to the saphenofemoral junction or symptomatic recurrence of superficial thrombosis at day 47, although patients were followed to day 77. Fondaparinux treatment was associated with an 85% risk reduction in the primary efficacy outcome at day 47 and 77. There was one episode of major bleeding in each group. Of note, there were relatively few thrombotic events between day 47 and 77 suggesting that a longer duration of therapy may be more efficacious. However, the trial excluded high risk patients including those with cancer, those with a recent history of VTE in the past 6 months, those with superficial thrombophlebitis associated with an intravenous catheter or those with thrombosis within 3 cm of the saphenofemoral junction. In addition, the relative cost of this lengthened therapeutic regimen was not evaluated. A 2007 Cochrane systematic review found that low molecular weight heparin and NSAIDS were more effective than placebo in reducing superficial thrombophlebitis extension or recurrence.
Our study had several strengths including minimization of bias. Selection bias was avoided by screening consecutive patients and adhering to predefined eligibility criteria of the two treatment cohorts. Bias in treatment allocation was avoided by block randomization. Bias in the assessment of outcomes was avoided by choosing the “hard” outcome of objectively documented extension of superficial thrombophlebitis and deep-vein thrombosis as the primary outcome measure, and by blinding the investigator, nurse and patient to treatment allocation. In addition, patients with superficial thrombophlebitis related to a recent intravenous catheter, patients with malignancy and those with either upper or lower extremity superficial thrombophlebitis were included making the results highly generalizable.
The study’s findings are limited by the small sample size, highlighting the challenge in enrolling eligible patients despite over 300 patients identified with superficial vein thrombosis, and the results should be considered hypothesis generating. The small sample size prohibits any subgroup analysis especially for those at higher risk for thrombosis extension or recurrence including cancer patients and those with proximal great saphenous vein thrombosis. Since patients with body weight <40kg or >135kg were excluded, the results may not be generalizable to this population. In addition, repeated ultrasounds were not evaluated by blinded adjudication, however, ultrasound imaging was performed by an experienced operator who was blinded to treatment allocation. The results of this study also do not provide definitive evidence for length of treatment, but similar to the STENOX trial, suggest that treatment longer than 14 days may be warranted.
Conclusions
In conclusion, this study provides evidence that fixed dose intermediate dose low molecular weight heparin dalteparin is superior to the NSAID ibuprofen in preventing extension of superficial thrombophlebitis during the treatment period with similar relief of pain and no increase in bleeding. However, questions remain concerning the optimal treatment duration. Additional clinical trials are needed to confirm our findings and should include high risk subgroups of those with cancer or with superficial thrombophlebitis near the saphenofemoral junction.
Acknowledgement
We acknowledge the support of the University of Oklahoma General Clinical Research Center grant M01 RR14467 from the National Center for Research Resources, National Institutes of Health, and Pfizer Inc. for provision of dalteparin sodium, ibuprofen, and nurse personnel salary support.
Footnotes
Financial disclosure: SR (none), CA (none), TW (none)
Contributor Information
Christopher E. Aston, Research Associate Professor, Department of Pediatrics, University of Oklahoma Health Sciences Center
Thomas L. Whitsett, Professor of Medicine, Cardiovascular section, University of Oklahoma Health Sciences Center.
References
- 1.Sullivan V, Denk P, Sonnad S, Eagleton M, Wakefield T. Ligation versus Anticoagulation: Treatment of Above-Knee Superficial Thrombophlebitis not Involving the Deep Venous System. J Am Coll Surg. 2001;193:556–62. doi: 10.1016/s1072-7515(01)01043-2. [DOI] [PubMed] [Google Scholar]
- 2.Bounameaux H, Reber-Wasem M. Superficial Thrombophlebitis and Deep Venous Thrombosis. Arch Intern Med. 1997;157:1822–4. [PubMed] [Google Scholar]
- 3.Blumenberg R, Barton E, Gelfand M, Skudder P, Brennan J. Occult deep venous thrombosis complicating superficial thrombophlebitis. J Vasc Surg. 1998;27:338–43. doi: 10.1016/s0741-5214(98)70364-7. [DOI] [PubMed] [Google Scholar]
- 4.Belcaro G, Nicolaides A, Errichi B, et al. Superficial Thrombophlebitis of the Legs: A Randomized, Controlled, Follow-up Study. Angiology. 1999;50:523–9. doi: 10.1177/000331979905000701. [DOI] [PubMed] [Google Scholar]
- 5.Messmore H, Bishop M, Wehrmacher W. Acute venous thrombosis: Therapeutic choices for superficial and deep veins. Postgraduate Medicine. 1991;89:73–7. doi: 10.1080/00325481.1991.11700941. [DOI] [PubMed] [Google Scholar]
- 6.Husni E, Williams W. Superficial thrombophlebitis of the lower limbs. Surger. 1982;91:70–4. [PubMed] [Google Scholar]
- 7.Lohr J, McDevitt D, Lutter K, Roedersheimer L, Sampson M. Operative Management of Greater Saphenous Thrombophlebitis Involving the Saphenofemoral Junction. Am J Surg. 1992;164:269–75. doi: 10.1016/s0002-9610(05)81084-0. [DOI] [PubMed] [Google Scholar]
- 8.Chengelis D, Bendick P, Glover J, Brown O, Ranval T. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg. 1996;24:745–9. doi: 10.1016/s0741-5214(96)70007-1. [DOI] [PubMed] [Google Scholar]
- 9.Krause U, Kock J, Kroger K, Albracht K, Rudofsky G. Prevention of deep venous thrombosis associated with superficial thrombophlebitis of the leg by early saphenous vein ligation. Vasa. 1998;27:34–8. [PubMed] [Google Scholar]
- 10.Decousas H. A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med. 2003;163:1657–63. doi: 10.1001/archinte.163.14.1657. [DOI] [PubMed] [Google Scholar]
- 11.Downing LJ, Strieter RM, Kadell AM, Wilke CA, Greenfield L, Wakefield T. Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg. 1998;28:848–54. doi: 10.1016/s0741-5214(98)70060-6. [DOI] [PubMed] [Google Scholar]
- 12.Birdwell B, Raskob G, Whitsett T, et al. The Clinical Validity of Normal Compression Ultrasonography in Outpatients Suspected of Having Deep Venous Thrombosis. Ann Intern Med. 1998;128:1–7. doi: 10.7326/0003-4819-128-1-199801010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Arch Intern Med. 1997;157:57–62. [PubMed] [Google Scholar]
- 14.Prandoni P, High vs. low doses of low-molecular weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind, randomized trial. J Thromb Haemost. 2005;3:1152–7. doi: 10.1111/j.1538-7836.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- 15.Decousas H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–32. doi: 10.1056/NEJMoa0912072. [DOI] [PubMed] [Google Scholar]
- 16.Di Nisio M, Wichers I, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database of Systematic Review. 2007;(2) doi: 10.1002/14651858.CD004982.pub6. Art. No: CD004982. [DOI] [PMC free article] [PubMed] [Google Scholar]


