Abstract
The Streptococcus mutans ComX-regulon encompasses >200 mostly uncharacterized genes, including cinA. Here we report that cinA is regulated by ComX in the presence of the competence stimulating peptide (CSP), wherein loss of CinA (strain SmuCinA) results in reduced transformability with or without added CSP by 74- and 15-fold, respectively (p<0.003). In CSP-supplemented cultures, a 2-fold increase in cell viability was noted for SmuCinA relative to UA159 (p<0.002), suggesting CinA’s involvement in the CSP-modulated cell killing response. Relative to UA159, loss of CinA also rendered the mutant hypersensitive to killing by methyl methanesulfonate (MMS), which impairs homologous recombination. Despite our use of a non-polar mutagenesis strategy to knockout cinA, which is the first gene of the multicistronic operon harboring cinA, we noted a drastic reduction in recA expression. By using a CinA-complemented mutant, we were able to partially, but not completely restore all phenotypes to UA159 levels. Complementation results suggested that although cinA participates in modulating competence, viability and MMS tolerance, genes downstream of the cinA transcript may also regulate these phenotypes, a finding that warrants further examination. This is the first report that describes a role for S. mutans’ CinA in contending with DNA damage, genetic transformation and cell survival.
Keywords: Streptococcus mutans, cinA, comX, CSP, genetic competence, cell death
Introduction
Genetic competence is a transient physiological state that facilitates horizontal gene transfer that enables recipient bacteria to acquire novel genes by the uptake of exogenous DNA from the environment (Claverys & Martin, 1998). The dental biofilm harbors copious amounts of DNA that is released via cell lysis, which acts as a major component of the biofilm matrix and contributes both to its structural integrity and provides a source of genetic material for naturally transformable plaque bacteria such as Streptococcus mutans (Perry, et al., 2009).
S. mutans is an opportunistic pathogen considered as one of the principle etiological agents of dental caries. Natural genetic transformation of this bacterium was shown to be modulated by a quorum sensing (QS) signaling system comprised of a ComDE two component signaling system, which responds to a peptide signaling molecule designated the competence stimulating peptide (CSP) (Li, et al., 2001). In addition to eliciting the competence phenotype, the CSP signaling pathway also contributes to proper biofilm formation, bacteriocin production and stress tolerance in S. mutans (Senadheera & Cvitkovitch, 2008). Intriguingly, the CSP-induced genetic transformation pathway also modulates cellular lysis in a fraction of the population in S. mutans cultures (Qi, et al., 2005, Perry, et al., 2009). Development of genetic competence is directly correlated with activation of an alternate sigma factor, ComX, which depends on ComE activity and that of another regulatory protein, ComR that responds to an internalized signaling peptide called XIP (Mashburn-Warren, et al., 2010). Recently, it was demonstrated that ComX was expressed only in a fraction of the CSP-induced population, which resulted in the bifurcation of the population into fractions undergoing competence or cell death (Mashburn-Warren, et al., 2010, Lemme, et al., 2011). Although transcriptome analysis has shown the regulation of nearly 240 genes by ComX (Perry, et al., 2009), most of these putative “late competence genes” modulating competence and cell lysis remain uncharacterized to date. Here, we studied a ComX-regulated gene designated the competence induced protein A (cinA) in S. mutans. Recently, Okinanga et al. showed that the HdrRM system regulated expression of cinA via ComX in S. mutans (Okinaga, et al., 2010). While cinA’s putative functions have not been closely examined in S. mutants, in S. pneumoniae, its ortholog belongs to the ComX-activated “late competence” regulon (Masure, et al., 1998, Mortier-Barriere, et al., 1998). In pneumococci, cinA is part of the rec locus, which includes recA that facilitates homologous recombination between single- and double-stranded DNA during genetic transformation (Kowalczykowski, 1994, Camerini-Otero & Hsieh, 1995). While CinA in S. pneumoniae was shown to facilitate transport of RecA to the membrane during genetic transformation (Masure, et al., 1998), studies in Bacillus subtilis suggested that CinA is not specific to competence, but instead is a nucleoid-associated protein that serves a general role in cells entering stationary phase (Kaimer & Graumann, 2010).
Here we report that cinA transcription is modulated by ComX in response to CSP, and that cinA is required for optimal genetic transformation in S. mutans. Deficiency of cinA drastically reduces the ability of synthetic CSP (sCSP) to induce cell lysis within the population, and to survive DNA damaging conditions induced by methyl methanesulfonate. This is the first report that describes functional roles for cinA in S. mutans.
Materials and Methods
Bacterial Strains and culture conditions
S. mutans wild type UA159 strain (J. Ferretti, University of Oklahoma), its isogenic CinA deficient mutant (SmuCinA, this study) and a CinA complimented mutant (strain SmuCinA+pCinAHis, this study) were utilized (Table 1). All strains were grown overnight at 37°C in a 5% (vol/vol) CO2 atmosphere as standing cultures in Todd-Hewitt-yeast extract (THYE) broth (Becton Dickinson, Sparks, MD). Strains were propagated on THYE plates supplemented with agar 1.5% (wt/vol) agar (Bioshop, Burlington) in the presence or absence of 10 μg/ml erythromycin.
Table 1.
Strains and primers used in this study
| Primer | Sequence (5′ - 3′) | Description |
|---|---|---|
| cinA-P1 | GAAGGAACGACTGATACG | cinAmutagenesis |
| cinA-P2 | ggcgcgccCAGATTTCATTCTAACCTCC | cinAmutagenesis |
| cinA-P3 | ggccggccGAACAATGGCAATCAAAGTG | cinAmutagenesis |
| cinA-P4 | ACGGACATCAAGACGCACAG | cinAmutagenesis |
| erm-F | ggcgcgccCCGGGCCCAAAATTTGTTTGAT | Erythromycin cassette |
| erm-R | ggccggccAGTCGGCAGCGACTCATAGAAT | Erythromycin cassette |
| ProHisCinA-F | CAgagctcCTATGTGTGATGGTGATGATGAATTTTTATGAGATAATAAAGT | pCinAHisA complimentation |
| ProCinA-R | AGgaattcAAGAGGCTTAACTAGCTCAA | pCinAHisA complimentation |
| cinAprobe-F | CCTATGGGCTTTTGTTGATAAACG | cinA probe, Northern blot |
| cinAprobe-R | GCAGTTGGTGATAATGAAGAACGC | cinA probe, Northern blot |
| recAprobe-F | TTGCCACGGACATCAAGACG | recA probe, Northern blot |
| recAprobe-R | ACCAGATTCAGGAGAACAGGGTC | recA probe, Northern blot |
| cinArt-F | GCGTAACACACGAGAATAAAGC | cinA qRT-PCR |
| cinArt-R | TTGGAGGCAGATGGAGTGAC | cinA qRT-PCR |
| recArt-F | TATCTCCGTCAATCTCCGCACG | recA qRT-PCR |
| recArt-R | CTATGCTGCTGCTCTTGGTG | recA qRT-PCR |
| 16sRNArt-F | CTTACCAGGTCTTGACATCCCG | 16S qRT-PCR |
| 16sRNArt-R | ACCCAACATCTCACGACACGAG | 16S qRT-PCR |
| Strain | Relevant Charateristics | Reference/source |
| UA159 | S. mutans wild-type strain | J. Ferretti Univ. of Oklahoma |
| SmuCinA | Δsmu.2086; Emr | This study |
| SmuCinA+pCinAHIs | Δsmu.2086; Emr + pCinAHis; Specr | This study |
| Plasmids | Relevant Charateristics | Reference/source |
| pDL277 | Specr | (LeBlanc, et al., 1992) |
| pDL289 | Kanr | (Buckley, et al., 1995) |
Note: Restriction Enzyme sites are in lower case AscI - ggcgcgcc; FseI – ggccggcc; EcoRI – gaattc; SacI – gagctc. Penta-His Tag is underlined.
Construction of mutant strains
S. mutans wild type UA159 was used to construct a cinA knockout mutant (strain SmuCinA) using PCR-ligation mutagenesis with primers in Table 1, as described previously (Lau, et al., 2002). Briefly, 5′ and 3′ flanking regions of cinA (NCBI gene ID: SMU.2086) were ligated to an ermr cassette, which were then amplified and transformed into UA159. From these, an Ermr transformant was selected and successful deletion of cinA was validated using PCR and nucleotide sequence analysis. The SmuCinA complimented strain (SmuCinA+pCinAHis) was constructed by amplifying cinA from the UA159 genome with its corresponding 129 bp promoter sequence upstream of the ATG start site. A penta His-tag sequence was also added to the 3′ end of the reverse primer (Table 1). PCR amplicons were then cloned into pDL277Spec (LeBlanc, et al., 1992) and the plasmid construct (pCinAHis) was transformed into DH5α Escherichia coli cells (Invitrogen). Following plasmid extraction, successful cloning was confirmed using DNA sequencing and SmuCinA was transformed with pCinAHis using standard in-house transformation protocols.
Northern Blot hybridization
Total RNAs were isolated from UA159 and SmuCinA using the Trizol method as described previously (Senadheera, et al., 2007) and used for Northern hybridization according to the protocol outlined in the DIG High Prime DNA labeling and Detection Starter Kit II (Roche) with the following modifications. To prepare RNA probes, 330bp and 558bp fragments of the cinA and recA genes were PCR amplified, respectively, using primers listed in Table 1 and labeled according to the DIG High Prime DNA Labeling Starter Kit (Roche Applied Science). Total RNA was separated using a 3.5% polyacrylamide gel, which was electro-transferred to a Sensiblot Plus Nylon membrane (Fermentas). Hybridization, washing and detection were all performed using appropriate protocols and solutions in the Detection Starter Kit II (Roche Applied Science). Images were captured every 5 min using BioRad ChemiDoc Gel Docking System and Quantity One software (BioRad, Hercules, CA, USA). A second hybridization was performed by stripping the same blot with NaOH and reprobing with a recA RNA probe (Table 1).
Transcriptional Analysis
Quantitative real-time PCR (qRTPCR) was performed using cells grown to mid-exponential phase (OD600 ~ 0.4) in THYE medium, in the presence or absence of 1μg/ml of CSP. Cells were harvested by centrifugation and snap frozen in liquid nitrogen, and used for cDNA synthesis as described previously (Senadheera, et al., 2007). Fold expression of a target gene was calculated relative to the no-CSP control or wild-type levels set at a user-defined value of 1.0. Expression was calculated using three to five biological replicates, each subjected to triplicate amplifications.
Genetic Transformation
Cultures treated without CSP (natural transformation) or supplemented with 1μg/ml of CSP were grown to early exponential phase (OD600 0.1) and transformation frequency (TF) assays were conducted using streptococcal vector pDL289 as described previously (Senadheera, et al., 2007).
Growth kinetics
Overnight cells were diluted 1:30 in pre-warmed sterile THYE with or without 1μg/ml of synthetic CSP. Growth was monitored as described previously (Senadheera, et al., 2007) using a Bioscreen microbiology workstation (Bioscreen C Labsystems, Finland).
Cell viability
For cell viability assays, S. mutans strains were grown to stationary phase (OD600 0.8 to 1.0) in the presence or absence of 1μg/ml CSP. Following incubation, cells were sonicated, serially diluted, and grown on THYE plates at 37°C in 5% CO2 for 48 h. Percentage survival was calculated as CFU of cells treated with CSP divided by cells not treated with CSP, times 100. Statistical significance was calculated using the Student’s t-test using results from 3 independent experiments.
Determination of sensitivity to DNA damaging agents
To assess sensitivity to DNA damaging agents, mitomycin C (MMC, 0.05 μg/ml ) and methyl methanesulfonate (MMS, 0.1%) were added to cells in mid-log phase (OD600 0.4). MMC-treated cells were incubated for 20 and 60 min while MMS-treated cells were incubated for 90 min. Untreated cells were used as controls. Following incubation, cells were sonicated, serially diluted, spotted on THYE agar plates in triplicate and incubated at 37°C in 5% CO2 for 48 h. Percentage survival was calculated by counting CFUs of treated cells divided by untreated cells, times 100.
Results and Discussion
CinA locus
The cinA locus (NCBI ID SMU.2086) is framed by several genes primarily involved in DNA recombination and repair, processes important for genetic competence (Figure 1a). In the vicinity of cinA, two terminator sequences were identified downstream of SMU.2083c and SMU.2090c (WebGesTer DB: http://pallab.serc.iisc.ernet.in/gester/dbsearch.php), suggesting that cinA may be a component of a 7-gene operon as indicated in Figure 1A. It was previously shown that in S. pneumoniae, the cinA transcript was only present during genetic competence induced by CSP and was co-transcribed with recA (Martin, et al., 1995). Since repeated attempts at determining the nature of transcripts originating from the putative cinA promoter using a series of reverse-transcription PCR provided inconclusive results, we employed northern blot analysis to determine whether cinA and recA were co-transcribed during CSP-induced competence development. Using a cinA probe, we identified 2 transcripts in CSP-supplemented UA159 cells, wherein one corresponded to ~ 1.2 kb cinA transcript alone and the other corresponded to ~ 2.4 kb cinA-recA transcript (Figure 1B). No transcripts were identified in SmuCinA mutant cells grown in the presence of CSP (negative control) and also when UA159 was grown in the absence of CSP. In UA159 cells without CSP supplementation, it was likely that we could not detect bands due to the low abundance of the transcripts without added CSP. To validate that cinA and recA were indeed co-transcribed, we further probed CSP-supplemented RNAs with a recA probe, which resulted in a single transcript corresponding to a size representing the cinA-recA transcript (Figure 1B). Hence, these results suggested that cinA and recA were co-transcribed under conditions favoring DNA uptake, and that cinA was likely to produce transcripts in excess of recA when CSP was added.
Figure 1.

(A) The genetic locus of cinA (SMU.2086c) and its neighboring genes are highlighted by the dark arrows and include genes that are primarily involved in DNA recombination and repair. Two predicted termination sequences represented by hairpin loop structures were found in the vicinity of SMU.2080 and SMU.2090 (grey arrows). Classical promoter elements including a ribosomal binding site (RBS), transcriptional start site (+1), −10 TATAAT box and −35 TTGACA box, were detected in the complementary strand harboring the putative cinA promoter (bold and underlined). A putative Cin-Box or Com-Box (Rathsam, et al., 2005) was identified 27 bp upstream of the ATG start site. (B) Northern analysis of the CinA null mutant and UA159 strains probed with cinA or recA. All strains were grown to OD600 0.4 in the presence or absence of 1ug/ml of synthetic CSP. The initial blot was probed for cinA, chemically stripped, and then re-probed for the recA transcript. The arrows correspond to a predicted transcripts representing cinA (1,256 bp) and cinA-recA (2,407 bp).
cinA and recA are upregulated by CSP and are transcriptionally regulated by ComX
In S. pneumoniae, the cinA and recA orthologs belong to the ComX-activated “late competence” regulon (Masure, et al., 1998, Mortier-Barriere, et al., 1998). Our search of the cinA promoter in S. mutans revealed a putative ComX binding site (Figure 1), suggesting that cinA and recA were perhaps part of the CSP-inducible ComX regulon (Peterson, et al., 2004, Rathsam, et al., 2005). To test this, we examined cinA and recA expression using cDNAs derived from S. mutans UA159 grown in the absence or presence of CSP. In CSP-supplemented UA159 cells, the expression of cinA and recA were increased by 5.5- and 2.4- fold, respectively, relative to the no-CSP control (Figure 2). Without added CSP, fold-expression of recA was reduced by 63% (i.e. ~ 0.37) relative to that in UA159, suggesting a polar effect on recA transcription by cinA mutagenesis (Figure 2). Supplementing the SmuCinA strain with CSP increased recA expression to 0.64, which still reduced recA transcription by 36% compared with wild type levels. Taken together, these results can be used to summarize that CinA is independently and highly driven by its own promoter, likely in the presence of CSP, and that the recA is co-transcribed with cinA, but not transcribed independently.
Figure 2.

Real-time gene expression of cinA and recA in S. mutans strains UA159 wild-type, SmuCinA and SmuComX. Each strain was grown in the presence or absence of 1μg/ml of sCSP Fold-expression was calculated using three biological replicates and normalized to the house keeping gene 16sRNA. Graphs represent mean ± SE.
To understand the regulatory role of ComX on cinA and recA expression, we also performed qRTPCR using cDNAs isolated from a comX-deficient mutant (SmuComX) and its wild type parent grown in the presence of CSP. Compared with UA159 supplemented with CSP, we could not detect cinA and recA transcripts in the comX mutant (Figure 2). These results are in accordance with previous finding by Okinaga et al. (2010), which suggested that the alternate sigma factor ComX was necessary for transcription of cinA and recA in the presence of CSP. As shown in Figure 1A, a conserved com-box sequence was identified in the cinA promoter, suggesting that ComX directly binds to the cinA promoter for transcriptional regulation, although more research is warranted to validate this finding.
CinA modulates genetic transformation
In pneumococci, ComX occupies a central role in the latter stages of the transformation process, by facilitating uptake of extracellular DNA and modulating genetic recombination (Luo & Morrison, 2003, Peterson, et al., 2004). Since we showed that ComX regulated cinA expression increases dramatically in response to CSP, we investigated the role of CinA in genetic transformation by assessing the transformation frequency (TF) of S. mutans UA159 wild type and SmuCinA in the presence and absence of synthetic CSP. Relative to wild type, the natural transformability and TF with added CSP of SmuCinA was significantly decreased by 15-fold and 74-fold, respectively (p<0.001) (Figure 3).
Figure 3.
Transformation frequencies (TF) of S. mutans strains UA159, SmuCinA and SmuCinA+pCinAHis cells. Experiments were performed using cells in early-log phase (OD600 0.1) supplemented with 1μg/ml of CSP or without CSP (natural TF). All experiments were performed with a minimum of three biological replicates and Student’s t-test was used to calculate statistical significance (* p<0.001; ** p<0.003). Graphs represent mean ± SE.
Since we showed that cinA was co-transcribed with recA under competence-inducing conditions, and because deletion of cinA caused polar effects on recA expression, we constructed a CinA complemented strain SmuCinA+pCinAHis that was used in TF assays to validate CinA’s role in genetic transformation. In the CinA complemented strain, although transformability was drastically increased relative to the CinA-deficient mutant, TF was not restored to wild type levels as observed under no-CSP and plus-CSP conditions (Figure 3). More specifically, an approximate 5-fold decrease in TF was observed in the SmuCinA+pCinAHis strain relative to UA159 in the presence or absence of CSP (p<0.001) (Figure 3).
Despite polar effects on recA as judged by expression analysis using SmuCinA and UA159 strains, partial restoration of the competence phenotype by the CinA complemented strain demonstrates a clear role for cinA in genetic transformation in S. mutans. However, we cannot ignore the possible contribution of recA to the transformation results we observed. RecA is a major component of the bacterial homologous recombination apparatus and is essential for the transformation of both plasmid and chromosomal DNA in S. pneumoniae (Mortier-Barriere, et al., 1998). Our inability to fully complement the CinA deficiency was likely caused by diminished recA expression in the SmuCinA mutant.
Recently Mashburn-Warren, et al. showed that S. mutans ComR serves as the proximal regulator of ComX, that ComR is activated by exogenous XIP (Mashburn-Warren, et al., 2011). Hence, it is likely that the ComRS system also regulates cinA transcription by activating ComX. Examination of other two component signaling systems in S. mutans suggests that in addition to the CSP-activated ComDE, other systems including RelRS, CiaRH, and VicRK also modulate ComX activity [(Ahn, et al., 2006), unpublished data], thus affecting expression of cinA. While here we focused on understanding ComX-mediated effects on cinA transcription and function, the regulatory roles of these other systems on ComX and CinA also warrant additional experimentation.
CinA modulates CSP-mediated cell death
Since cinA was up-regulated in the presence of CSP, and CSP was shown to modulate cell death via activity of ComX (Perry, et al., 2009, Lemme, et al., 2011), we hypothesized that CinA participated in CSP-induced cell lysis. To test this, we first monitored the growth of S. mutans UA159, SmuCinA and SmuCinA+pCinAHis in the absence and presence of 1μg/ml of sCSP. While growth in the absence of CSP was not drastically affected by the loss of cinA (Figure 4A), supplementing CSP resulted in an increased growth yield of SmuCinA relative to UA159 (Figure 4B). In fact, the negative effect of CSP on growth was partially abolished when CinA was complemented (Figure 4B), suggesting that killing effects of CSP was modulated by comX via the cinA. To validate cinA’s role in cell lysis, we performed cell viability assays in the presence of synthetic CSP. As expected, a significant increase in CFUs was observed in SmuCinA (54%) relative to UA159 (24%) (p<0.002, Figure 4C). Complementation of cinA did not bring the percentage survivors to wild type levels, although percentage viability of the SmuCinA+pCinAHis strain was substantially reduced to 35% relative to wild type (p<0.01). These results clearly demonstrate a role for CinA in CSP-induced cell lysis in S. mutans.
Figure 4.
Growth kinetics of S. mutans strains UA159, SmuCinA and SmuCinA+pCinAHis in THYE (A) and THYE supplemented with 1 μg/ml of CSP (B). Graphs represent mean ± SE (n=5). (C) Cell viability assays were performed by growing each strain to post-exponential phase in the presence and absence of 1μg/ml CSP. Statistical significance was calculated using Student’s T-test wherein * p<0.002 and **p<0.01. Graphs represent mean ± SE (n=4).
A role for CinA in cell lysis of pneumococci was previously suggested by Novak et al who showed that a zinc metalloprotease (ZmpB) mutant had a lysis defect when treated with penicillin (Novak, et al., 2000). It was suggested that this defect was caused by co-localization of the autolysin LytA with CinA within the cytoplasm, wherein LytA was normally located in the cell membrane (Novak, et al., 2000), a finding that could not be confirmed by a different group (Berge, et al., 2001). Despite these conflicting results in S. pneumoniae, the possibility of CinA interacting with a putative autolysin protein in S. mutans to initiate cell lysis should be considered.
Loss of CinA affects sensitivity to DNA damaging agents
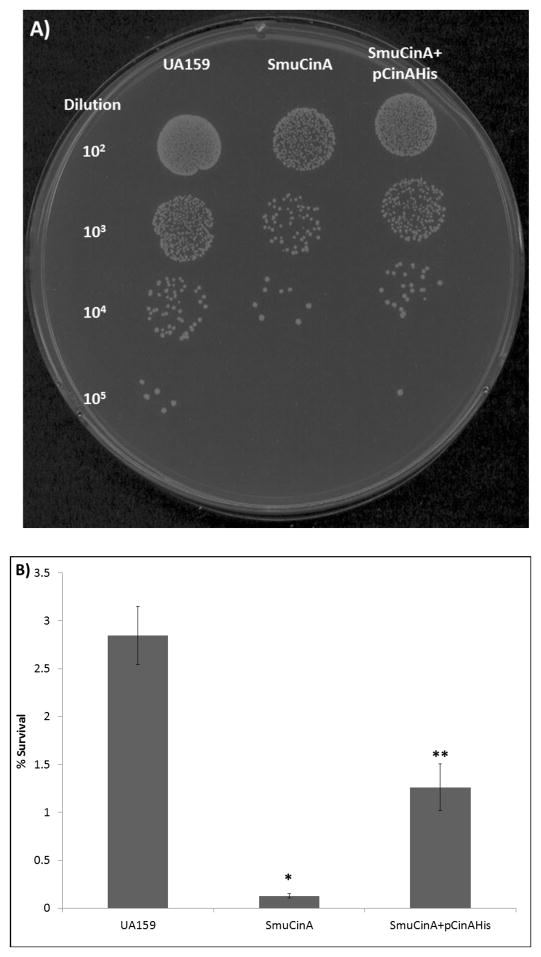
In S. pneumoniae, competent cells or those exposed to DNA damaging agents produced a 5.7 kb polycistronic transcript that included cinA and recA (Martin, et al., 1995). From this transcript, the product encoded by recA serves a critical step during transformation and DNA repair where it identifies homologous regions of incoming DNA and incorporates them into the host chromosome (Kowalczykowski, 1994). Martin et al also demonstrated that CinA and RecA interacted to modulate genetic competence and facilitate survival under DNA damaging conditions (Martin, et al., 1995). Hence, we next studied CinA’s role in contending with DNA damage by assessing cell survival under chemical agents that either damaged DNA directly or disrupted the replication process. We used mitomycin C (MMC) which inhibits growth by causing DNA cross-linkage (Tomasz, 1995) and methyl methanesulfonate (MMS) that stalls the replication fork in areas where homologous recombination occurs (Lundin, et al., 2005). Following MMC treatment, survival of SmuCinA was not significantly altered relative to wild type (data not shown), which was similar to the results obtained for the CinA mutant in B. subtilis (Kaimer & Graumann, 2010). In contrast, a 22-fold reduction in survival was observed in SmuCinA, when exposed to 0.1% MMS for 90 minutes as compared to UA159 (p<0.0002, Figure 5). The growth was partially restored by complementation with cinA resulting in percentage survival of a 2.3-fold reduction as compared with the wild-type strain (p < 0.007, Figure 5). The loss of CinA, therefore, enhances the mutant’s sensitivity to killing by MMS, which is likely caused by diminished expression of recA in our SmuCinA mutant or due to a possible interaction with RecA at the DNA replication fork. However, our ability to partially restore viable CFUs by using the CinA complemented strain clearly suggests an important role for CinA in contending with MMS-induced stress in S. mutans.
Figure 5.
A) Effects of 0.1% methyl methanesulfonate (MMS) on viability of S. mutans strains UA159, SmuCinA and SmuCinA+pCinAHis.B) Graphical representation of results from (A) subjected to statistical analysis using Student’s T-test; * p<0.0002 and ** p<0.007. Graphs represent mean ± SE (n=4).
Conclusions
Here we have demonstrated that cinA is transcriptionally regulated by ComX, which in turn, modulates genetic competence and cell death in S. mutans. Although, we only investigated CSP’s effects on cinA upregulation, it is likely that cinA also transcriptionally responds to XIP, which was shown to activate ComX (Mashburn-Warren, et al., 2010, Lemme, et al., 2011). In addition to ComDE, we know that other signaling systems also modulate ComX activity (e.g. ComRS, LiaRS, VicRK) (Mashburn-Warren, et al., 2010; unpublished data). Hence, it stands to reason that ComX-dependent transcription of cinA relies on multiple signaling inputs for optimal activity. Further, our results support the findings of Lemme et al, who showed that ComX can modulate cell death versus competence depending on its activity (Mashburn-Warren, et al., 2010, Lemme, et al., 2011). Here, we have further shown that these ComX-regulated phenotypes are, at least in part, regulated via CinA. In this report, we also showed that S. mutans’ ability to withstand DNA damage induced by MMS was also dependent on CinA. Taken together, we have demonstrated novel roles for the CinA in S. mutans in modulating genetic transformation, cell viability and tolerance to MMS.
Acknowledgments
We would like to thank Martha Cordova for assistance with Northern blots. DGC is a recipient of NIH grant R01DE013230-03 and CIHR-MT15431.
References
- 1.Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berge M, Garcia P, Iannelli F, Prere MF, Granadel C, Polissi A, Claverys JP. The puzzle of zmpB and extensive chain formation, autolysis defect and non-translocation of choline-binding proteins in Streptococcus pneumoniae. Mol Microbiol. 2001;39:1651–1660. doi: 10.1046/j.1365-2958.2001.02359.x. [DOI] [PubMed] [Google Scholar]
- 3.Buckley ND, Lee LN, LeBlanc DJ. Use of a novel mobilizable vector to inactivate the scrA gene of Streptococcus sobrinus by allelic replacement. J Bacteriol. 1995;177:5028–5034. doi: 10.1128/jb.177.17.5028-5034.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camerini-Otero RD, Hsieh P. Homologous recombination proteins in prokaryotes and eukaryotes. Annu Rev Genet. 1995;29:509–552. doi: 10.1146/annurev.ge.29.120195.002453. [DOI] [PubMed] [Google Scholar]
- 5.Claverys JP, Martin B. Competence regulons, genomics and streptococci. Mol Microbiol. 1998;29:1126–1127. doi: 10.1046/j.1365-2958.1998.01005.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaimer C, Graumann PL. Bacillus subtilis CinA is a stationary phase-induced protein that localizes to the nucleoid and plays a minor role in competent cells. Arch Microbiol. 2010;192:549–557. doi: 10.1007/s00203-010-0583-7. [DOI] [PubMed] [Google Scholar]
- 7.Kowalczykowski SC. In vitro reconstitution of homologous recombination reactions. Experientia. 1994;50:204–215. doi: 10.1007/BF01924003. [DOI] [PubMed] [Google Scholar]
- 8.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods. 2002;49:193–205. doi: 10.1016/s0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc DJ, Lee LN, Abu-Al-Jaibat A. Molecular, genetic, and functional analysis of the basic replicon of pVA380–1, a plasmid of oral streptococcal origin. Plasmid. 1992;28:130–145. doi: 10.1016/0147-619x(92)90044-b. [DOI] [PubMed] [Google Scholar]
- 10.Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. Subpopulation-Specific Transcriptome Analysis of Competence-Stimulating-Peptide-Induced Streptococcus mutans. J Bacteriol. 2011;193:1863–1877. doi: 10.1128/JB.01363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo P, Morrison DA. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J Bacteriol. 2003;185:349–358. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B, Garcia P, Castanie MP, Claverys JP. The recA gene of Streptococcus pneumoniae is part of a competence-induced operon and controls lysogenic induction. Mol Microbiol. 1995;15:367–379. doi: 10.1111/j.1365-2958.1995.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 15.Martin B, Garcia P, Castanie MP, Glise B, Claverys JP. The recA gene of Streptococcus pneumoniae is part of a competence-induced operon and controls an SOS regulon. Dev Biol Stand. 1995;85:293–300. [PubMed] [Google Scholar]
- 16.Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2011;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masure HR, Pearce BJ, Shio H, Spellerberg B. Membrane targeting of RecA during genetic transformation. Mol Microbiol. 1998;27:845–852. doi: 10.1046/j.1365-2958.1998.00732.x. [DOI] [PubMed] [Google Scholar]
- 19.Mortier-Barriere I, de Saizieu A, Claverys JP, Martin B. Competence-specific induction of recA is required for full recombination proficiency during transformation in Streptococcus pneumoniae. Mol Microbiol. 1998;27:159–170. doi: 10.1046/j.1365-2958.1998.00668.x. [DOI] [PubMed] [Google Scholar]
- 20.Novak R, Charpentier E, Braun JS, Park E, Murti S, Tuomanen E, Masure R. Extracellular targeting of choline-binding proteins in Streptococcus pneumoniae by a zinc metalloprotease. Mol Microbiol. 2000;36:366–376. doi: 10.1046/j.1365-2958.2000.01854.x. [DOI] [PubMed] [Google Scholar]
- 21.Okinaga T, Xie Z, Niu G, Qi F, Merritt J. Examination of the hdrRM regulon yields insight into the competence system of Streptococcus mutans. Mol Oral Microbiol. 2010;25:165–177. doi: 10.1111/j.2041-1014.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 22.Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol Microbiol. 2009;72:905–917. doi: 10.1111/j.1365-2958.2009.06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson SN, Sung CK, Cline R, et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 24.Qi F, Kreth J, Levesque CM, et al. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol Lett. 2005;251:321–326. doi: 10.1016/j.femsle.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Rathsam C, Eaton RE, Simpson CL, Browne GV, Valova VA, Harty DW, Jacques NA. Two-dimensional fluorescence difference gel electrophoretic analysis of Streptococcus mutans biofilms. J Proteome Res. 2005;4:2161–2173. doi: 10.1021/pr0502471. [DOI] [PubMed] [Google Scholar]
- 26.Senadheera D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. Adv Exp Med Biol. 2008;631:178–188. doi: 10.1007/978-0-387-78885-2_12. [DOI] [PubMed] [Google Scholar]
- 27.Senadheera MD, Lee AWC, Hung DCI, Spatafora GA, Goodman SD, Cvitkovitch DG. The Streptococcus mutansvicX gene product modulates gtfB/C expression, biofilm formation, genetic competence, and oxidative stress tolerance. J Bacteriol. 2007;189:1451–1458. doi: 10.1128/JB.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz M. Mitomycin C: small, fast and deadly (but very selective) Chem Biol. 1995;2:575–579. doi: 10.1016/1074-5521(95)90120-5. [DOI] [PubMed] [Google Scholar]