Abstract
Lipids are often considered membrane components whose function is to embed proteins into cell membranes. In the last two decades, studies on brain lipids have unequivocally demonstrated that many lipids have critical cell signaling functions; they are called “bioactive lipids”. Pioneering work in Dr. Robert Ledeen’s laboratory has shown that two bioactive brain sphingolipids, sphingomyelin and the ganglioside GM1 are major signaling lipids in the nuclear envelope. In addition to derivatives of the sphingolipid ceramide, the bioactive lipids discussed here belong to the classes of terpenoids and steroids, eicosanoids, and lysophospholipids. These lipids act mainly through two mechanisms: 1) direct interaction between the bioactive lipid and a specific protein binding partner such as a lipid receptor, protein kinase or phosphatase, ion exchanger, or other cell signaling protein; and 2) formation of lipid microdomains or rafts that regulate the activity of a group of raft-associated cell signaling proteins. In recent years, a third mechanism has emerged, which invokes lipid second messengers as a regulator for the energy and redox balance of differentiating neural stem cells (NSCs). Interestingly, developmental niches such as the stem cell niche for adult NSC differentiation may also be metabolic compartments that respond to a distinct combination of bioactive lipids. The biological function of these lipids as regulators of NSC differentiation will be reviewed and their application in stem cell therapy discussed.
Keywords: sphingolipids, eicosanoids, lysophospholipids, steroids, embryonic stem cells, neural progenitor cells, differentiation
Overview
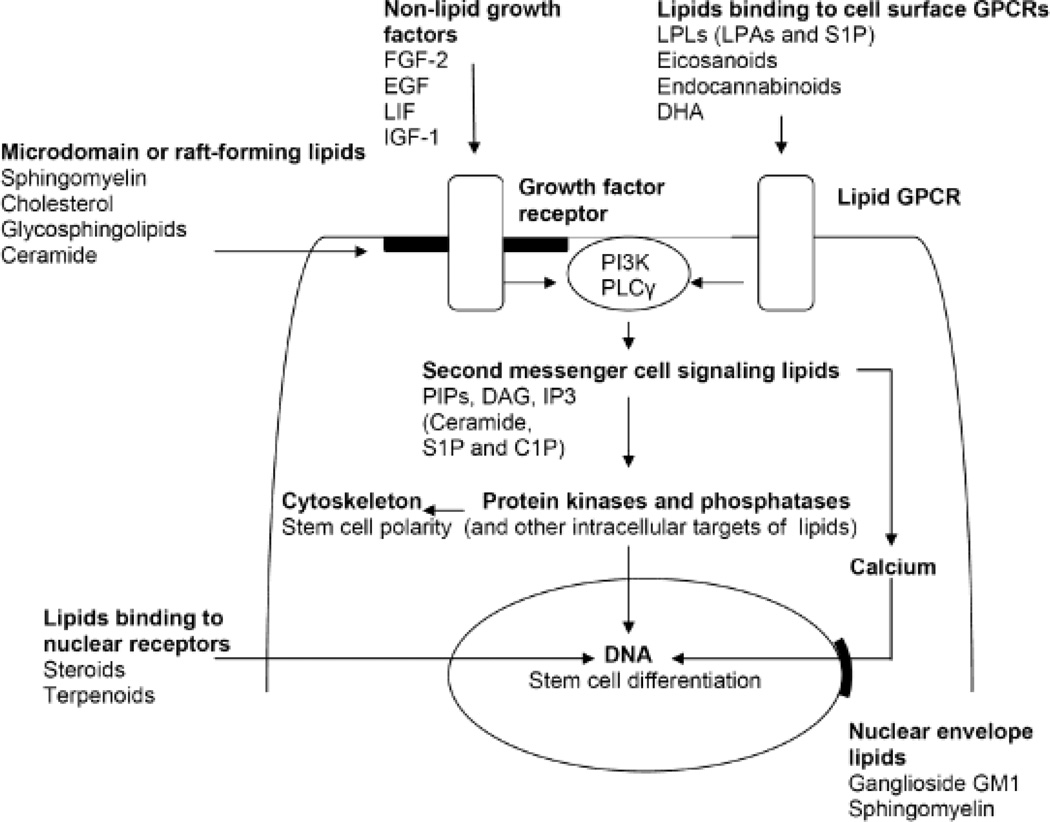
The signal transduction mechanism of bioactive lipids is not always exclusively defined by one pathway. There are lipids that specifically act through receptors such as endocannabinoids, but then there are others that can bind to receptors and intracellular protein targets such as S1P. Some lipids can affect the same receptor or signaling protein even twice, by direct binding and affecting the composition of lipid rafts such as ceramide (Fig. 1). Therefore, the classification of signaling lipids into three categories (bioactive lipids signaling through receptors; bioactive lipids binding to protein kinases, phosphatases, and other signaling proteins; and bioactive lipids regulating lipid rafts and raft-associated proteins) and is not always based on a clear-cut distinction. Likewise, individual lipids do not have exclusive functions in NSC differentiation. For example, LPA and S1P can promote proliferation and self-renewal in one population of stem cells, while their main function is induction of differentiation in another one. These differences, which may very well be species-dependent (mice vs. men) will also be discussed in greater detail. Finally, bioactive lipids may not be sufficiently effective by themselves, but they significantly enhance the function of other growth factors and differentiation inducers, e.g., in the case of raft-associated receptors. Therefore, it is rather a specific “cocktail” of bioactive lipids and growth factors that ultimately determines the fate of differentiating stem cells. It is the goal of the review to discuss the underlying mechanisms for this lipid-dependent NSC differentiation and how lipids can then be used to steer stem cell differentiation toward one lineage.
Figure 1.
Cell signaling pathways regulated by bioactive lipids
1. Bioactive lipids signaling through receptors
A variety of bioactive lipids is known to specifically interact with receptors in the cell membrane, the endoplasmic reticulum (ER), and the nucleus (Fig. 1). The lipid classes acting through receptors in the cell membrane are lysophospholipids (e.g., LPA and S1P), eicosanoids (e.g. prostaglandins), and endocannaboids (e.g., anandamide). These lipids activate G-protein coupled receptors (GPCRs) of the Gi, Gq, and G12/13 type. Downstream of these lipid-activated GPCRS are the PI3K-to-Akt (Gi), Ras-to-ERK (Gi, Gq), and PLC-to-PKC (G12/13) cell signaling pathways for neural stem cell proliferation and differentiation. In most cases, the specific cellular response (e.g., proliferation vs. differentiation) is orchestrated by a particular combination of bioactive lipids. Likewise, several lipids bind to nuclear receptors such as the terpenoid retinoic acid, and steroid hormones and glucocorticoids. Recently, lipid receptors in the ER have gained attention because they can affect ER stress and redox balance, two metabolic regulators of neural stem cell differentiation. In particular, sigma receptors are interesting in that they can bind to sphingolipids and neurosteroids as specific ligands, but are also lipid raft-associated. Therefore, many bioactive lipids can regulate stem cell differentiation in two different ways, by binding to specific receptors and modulating the membrane environment of receptors associated with lipid rafts. In this chapter, we will first discuss the receptor specific binding of these lipids and elaborate on the metabolic effect (ER stress and redox potential) and lipid raft-dependent stem cell differentiation in the subsequent chapters.
1.1. Nuclear receptors: Terpenoids and steroids
Terpenoids or isoprenoids are a structurally rich family of lipids based on the ene-addition of isoprene units, which are known to give rise to linear, branched, and cyclic compounds including cholesterol and other steroids. Retinoic acid (RA) is one of the terpenoids with a long tradition of usage in neural stem cell differentiation. In 1982, it was for first time described that RA induces neuronal and glial differentiation of P19 embryonic carcinoma cells [1]. Five years later, it was shown that RA induces motoneuron differentiation of mouse embryonic stem (ES) cells and it was speculated that it is a physiological inducer of the Hox2.3 gene [2]. It is now well established that RA is critical for the spatially regulated expression of Hox genes during embryonic brain development. This is achieved by the tissue-specific expression of retinal aldehyde dehydrogenase 2 (Raldh2), an enzyme converting retinaldehyde (retinal) into RA. In contrast to Aldh1, a commonly used marker for undifferentiated stem cells, RAldh2 is missing in undifferentiated ES cells preventing the undue expression of RA as differentiation inducer when stem cells are in contact with retinal or retinol (vitamin A) [3]. Interestingly, incubation of mouse ES cells with retinol/vitamin A resulted in the promotion of self-renewal, suggesting a novel cell signaling pathway for maintenance of pluripotency by vitamin A. While it has been found that this signaling pathway involves the insulin growth factor 1 receptor (IGF-1R)-to-PI3K-to-Akt-to-mTORC1 signal transduction pathway, it is not clear how vitamin A activates this pathway [3, 4].
In contrast to vitamin A, its derivative RA triggers the expression of Hox genes by activation of nuclear RA receptors or RARs. RA binds to RAR in heterodimer with RXR, the retinoid X receptor, and releases repression of a histone demethylase, which in turn activates the expression of genes for neural differentiation such as the homeobox transcription factor gene dlx5 [5]. Another heterodimer binding partner of RXR, the oxysterol receptor LXR, has also been shown to promote neural differentiation, in particular of dopaminergic hindbrain neurons, while it prevents glial differentiation [5].
In addition to terpenoids, steroids bind to nuclear receptors that promote neural differentiation, for example the estrogen receptor (ER). Estrogen is a female sex steroid hormone, which originates in the same biosynthetic pathway as terpenoids, the mevalonate or HMG-CoA reductase pathway. It is not surprising that certain statins, inhibitors of HMG-CoA reductase and cholesterol-lowering drugs, inhibit self-renewal of ES cells, although the mechanism is not via nuclear steroid receptors but by preventing isoprenylation (geranylgeranylation) of RhoA [6]. Interestingly, simvastatin not only prevents ES cell self-renewal, but also induces osteogenic differentiation [7]. Estrogen, on the other hand, promotes differentiation and survival of dopaminergic neurons and therefore, it belongs to a group of neurogenic and neuroprotective steroids (neurosteroids) with great potential to be used in the clinics [8]. Another steroid of this group is the pregnancy hormone progesterone, which has been shown to alleviate neuronal damage in traumatic brain and spinal cord injury, to attenuate astrogliosis, and to enhance oligoendrocyte differentiation and myelination [9, 10]. While it is not clear whether these neurosteroid effects are solely induced by nuclear receptors or other non-nuclear receptors are involved, it is known that the majority of lipids in stem cell differentiation act via receptors in other subcellular compartments, in particular the cell membrane. This holds also true for cholesterol, the common precursor for the biosynthesis of steroid hormones.
Cholesterol is not only interesting as a metabolic precursor, but directly affects stem cell differentiation via two distinct mechanisms: it constitutes a post-translational modification covalently linked to one of the key morphogens in embryogenesis, sonic hedgehog (Shh), and it is a critical component of lipid rafts [11–14]. Sonic hedgehog has many important functions during embryonic and adult neurogenesis. In the embryo, it acts as a morphogen regulating the ventral patterning of the brain, while in the adult brain; it specifies the ventral position of neural stem cells. It has been shown that during embryogenesis, cholesterylation of sonic hedgehog is critical for establishing a morphogenetic gradient [15]. When administered to ES cells, Shh can stimulate the differentiation of two distinct progenitor populations: when combined with RA it promotes differentiation of ES cells to motor neurons, while subsequent incubation of RA+Shh-treated ES cells with fibroblast growth factor-2 (FGF-2) induces the generation of oligodendrocyte precursors (OPCs) [16, 17]. It is not clear whether this remarkably precise editing of in vitro ES cell differentiation recapitulates a physiologically significant process and which role cholesterylation of Shh may play. However, it becomes clear from this example that bioactive lipids not only act on distinct receptors, but can also modulate the activity of receptors for growth factors that are not specific for lipids. In particular, cholesterol is an important modulator of raft-associated growth factor receptors, which will be discussed in chapter 3.
1.2. Cell surface receptors: Lysophospholipids, eicosanoids, and endocannabinoids
Receptors for bioactive lipids can reside within all membranes or subcellular organelles of neural stem cells. So far, nuclear receptors for terpenoids and steroids have been discussed as inducers or suppressors of gene expression in neural differentiation. However, nuclear receptors are “blind” toward spatial cues. In contrast, receptors at the cell membrane can be activated by gradients of bioactive lipids (Fig. 1). This is important for the synchronization of neural differentiation with cell polarity, e.g., during neural migration or process formation. The most prominent examples for lipids associated with cell polarity are lysophospholipids (LPLs) and phosphatidylinositolphosphates (PIPs). This section focuses on LPLs, while PIPs will be discussed in chapter 2.
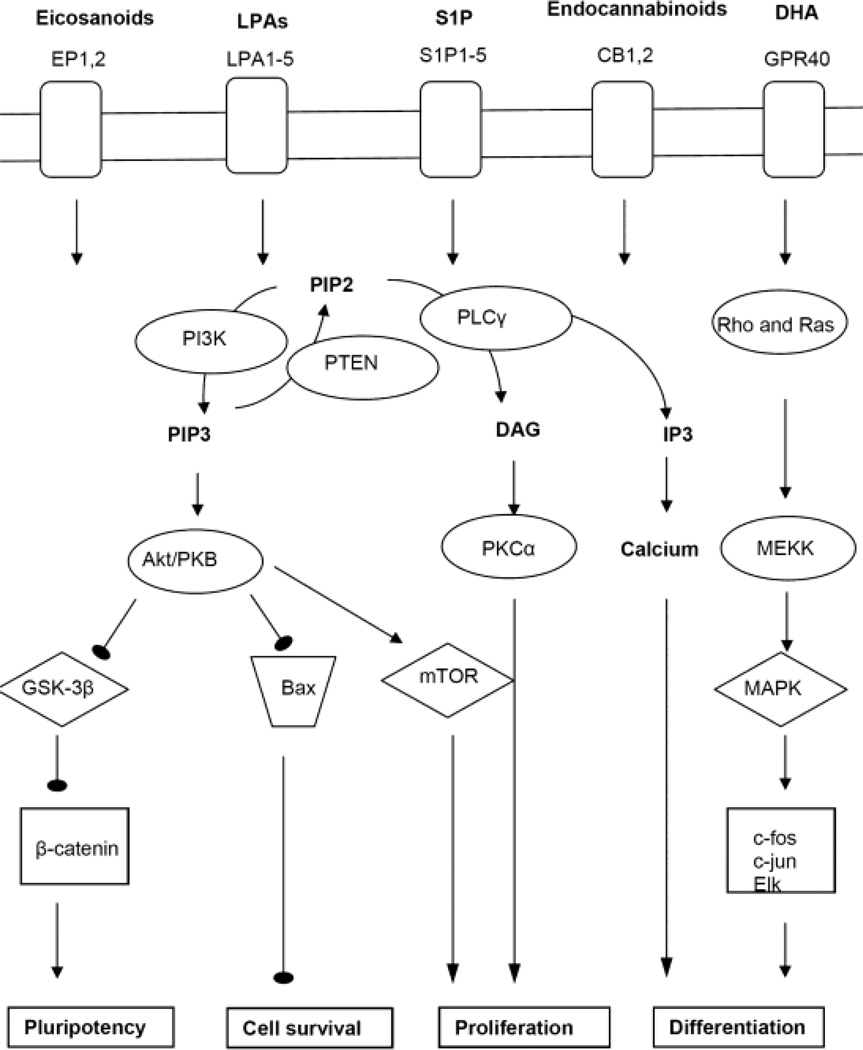
LPLs are bioactive lipids that can be generated by hydrolytic cleavage of fatty acid from glycerophospholipids, a reaction catalyzed by phospholipases. Distinct phospholipases cleave off either one of the two (PLA1 and PLA2) or both (PLB) fatty acid residues, or they cleave off the phosphate-containing head group (PLC) or the alcohol (PLD) [18–26]. For example, PLD cleaves off the choline residue from phophatidylcholine (PC) giving rise to phosphatidic acid (PA). PLA1 and PLA2 then generate lysophosphatidic acid (LPA) by cleaving off one of the two fatty acids from PA. Another enzyme, lysophospholipase D or autotaxin catalyzes a reaction similar to PLD; it generates LPA from lyso-PC, which is derived from PC by cleaving off one fatty acid residue, a reaction catalyzed by PLA2 [23, 27, 28]. While the enzymatic reactions generating LPA are well known, it is less clear which cells release LPA that can affect NSCs. In blood, activated platelets are a rich source for LPA and lesions or injuries, including those of the brain, may acutely release LPA to stimulate cell growth or migration. However, a systemic supply with LPA through the bloodstream as in the developing embryo would only affect NSC differentiation independent of spatial cues. In contrast, LPLs including LPA and S1P have been shown to act as chemotaxins, which is clearly demanding for defined albeit unidentified cellular source for neural development. LPA receptors are critical in cell proliferation and tumorigenesis and have recently been shown to promote proliferation of human neural precursor cells [22, 29–33]. In fact, LPA seems to inhibit neuronal lineage specification in favor of olidendroglial differentiation. LPA receptors are GPCRs, which is a receptor family that also encompasses receptors for other bioactive lipids such as S1P, eicosanoids, and endocannabinoids (Fig. 2).
Figure 2.
Cell signaling pathways activated by lipid-specific GPCRs in NSCs
The biosynthesis of eicosanoids is initiated by the activation of PLA2, an enzyme that has a dual function: the generation of lyso-PC from PC and LPA from PA, but also the release of arachidonic acid, a fatty acid that is the precursor for the generation of eicosanoids [34–39]. Arachidonic acid is converted to a variety of pro-inflammotory eicosanoids among which prostaglandins, thromboxanes, and leukotrienes are the most important signaling lipids. The effect of eicosanoids on ES cells is not well understood and research is mostly limited to results with mouse ES cells. Due to their activity on GPCRs, LPA and eicosanoids such as prostaglandin E2 (PGE2) appear to activate similar downstream cell signaling pathways, mainly the PI3K-to-Akt/PKB, MAPK, and Wnt/GSK-3β pathways [30, 32, 33, 40–43] (Fig. 2). The eicosanoid and LPL (LPA and S1P) receptors belong to the family of Class A Rhodopsin-like GPCRs [20, 22, 24, 32, 33, 44]. They mediate the activation of downstream cell signaling pathways through different types of GTPases, mainly Gi, Gq, and G12/13, acting upon PI3K-to-Akt/PKB (Gi), Ras-to-ERK (Gi, Gq) Rho (G12/13), and PLC-to-PKC (Gq) cell signaling pathways for pluripotency and cell survival (Akt/PKB), proliferation (Rho and PKC), and differentiation/specification (MAPK) pathways (Fig. 2).
The combination of cell signaling lipids with cytokines or growth factors such as LIF or FGF-2 activating similar effectors has been found to be useful in directing stem cell fate toward pluripotency, proliferation, or differentiation, respectively [24, 29, 30, 45, 46]. In contrast to activation of the receptors for LIF and FGF-2, LIFRα or FGFR2, however, stimulation of Akt/PKB by PGE2 has not been reported to sustain pluripotency, but is rather anti-apoptotic/cell protective and promotes stem cell proliferation. This may not be surprising since generation and conversion of arachidonic acid is often a response to hypoxic insults, which can damage mitochondria and induce apoptosis. Notably, inhibition of eicosanoid biosynthesis reduces the potential of mouse and human ES cells to self-renew, indicating a role of eicosanoids in stem cell maintenance or pluripotency [47]. Thromboxane has not been described to play a role in stem cell differentiation, maybe because its main function is rather confined to platelet aggregation. In contrast, prostacyclin, a similar eicosanoid in platelet aggregation has been shown to promote cardiogenic differentiation from human ES cells [48, 49]. In addition to prostacyclin, leukotriene of the LTD4 type has been used in several studies to promote proliferation and cardiovascular differentiation of mouse ES cells [39, 50, 51].
Most recently, two additional classes of bioactive lipids, endocannabinoids and particular poly-unsaturated fatty acids have been shown to stimulate gliogenesis and neuroblast migration (endocannabinoids) or promote neuronal differentiation from NSCs (docosahexanenoic acid (DHA)), respectively [52–55]. Endocannabinoids such as anandamide (N-arachidonylethanolamine) and 2-arachidonoylglycerol bind to the CB1 receptor and activate the Gαi subunit upstream of PI3K-to-Akt, while DHA binds to GPR40, a Gαq subunit upstream of PLCγ (Fig. 2). Its activation generates DAG and IP3, a signaling lipid mediating Ca2+ release from the ER. At this point, it becomes clear that many GPCRs, although binding specifically distinct bioactive lipids will activate very similar downstream cell signaling pathways, which may not be completely surprising considering that LPA, eicosanoids, endocannabinoids, and PUFAs are generated by interconnected metabolic pathways initiated by phospholipases. In this view, metabolically interconnected pathways constitute cell signaling niches since conversion of metabolic intermediates generates bioactive lipids with similar function for NSC differentiation. This view cannot be generalized when applied to S1P, another LPL triggering GPCRs. S1P is not generated in the phospholipase pathway, but it is derived from ceramide or sphingosine in sphingolipid metbolism. S1P and other bioactive sphingolipids will be discussed in chapter 2.
1.3. Receptors in the ER and other subcellular organelles
In addition to nuclear and cell membrane-resident receptors, bioactive lipids may also bind to receptors in other subcellular organelles. One of these receptors discussed in the context of NSC differentiation is the sigma receptor in the ER. The sigma receptor is highly enriched in mitochondria-associated membranes (MAM) of the ER and associates tightly with cholesterol, a lipid usually found at rather low concentration in the ER [56, 57]. It has been shown that the interaction with cholesterol is amino acid specific. However, since cholesterol is embedded in lipid microdomains or ER rafts this interaction is considered a lipid raft association. In addition, the sigma receptor can also bind to other steroids and sphingosine, but not to S1P [58]. Recently, the sigma receptor has gained attention because it binds to a multitude of psychopharmacological agents including opiates, antipsychotics, anti-depressants, and neurosteroids. This is consistent with the high abundance of this receptor in neurons and oligodendrocytes. Most surprisingly, however, is the ability of the sigma receptor to elevate and redistribute cholesterol and gangliosides, thereby reconstituting lipid rafts [59]. It is currently being investigated how this receptor-dependent remodeling of lipid rafts can regulate neuronal or glial function. One of these functions has been shown to directly involve oligodendrocyte differentiation and myelination [60]. The observation that cholesterol- and galactosylceramide-associated sigma 1 receptor is critical for the differentiation of oligodendrocyte precursors may lead to a novel drug target for treatment of dys- or demyelination diseases. The role of sigma receptors in the developing brain is not known.
2. Lipid mediators that affect protein kinase cascades and energy metabolism
The bioactive lipids discussed so far bind to specific lipid receptors and therefore, are mostly distinct from those binding to intracellular cell signaling proteins. The two lipids discussed here are phosphatidylinositols (PIPs) and ceramide. PIPs, in particular PI(3,4)P2 and PI(3,4,5)P3 bind to specific protein domains such as the pleckstrin homology (PH) domain. These domains are present on a variety of protein kinases and other proteins that are regulated by PIPs. There also binding domains for ceramide, however, these have not been described as consensus sequences in protein kinases or phosphatases yet. Therefore, the specific effect of ceramide on these proteins, although well documented in many studies, is still lacking a clear molecular mechanism. Ceramide is distinct from PIPs in that it is a metabolic precursor for many other bioactive lipids that act via different mechanisms such as receptor binding (S1P) or lipid raft formation (glycosphingolipids or GSLs). This metabolic conversion makes it difficult to distinguish the biological effect of ceramide from that of its derivatives. One of these derivatives, S1P is unique in that it activates cell membrane receptors (S1P-specific GPCRs) as discussed in the previous chapter, but it can also bind to a variety of intracellular targets that will be discussed in this chapter.
PIPs and ceramide are signaling intermediates that are generated in response to intrinsic or extrinsic cues (Fig. 1). Extrinsic cues elevating these two bioactive lipids are often related to the regulation of the energy metabolism of an organism, organ, or cells within a tissue. For example, PIP3 is generated by PI3K in response to insulin or IGF-1, and therefore, a lipid signaling saturation and availability of energy and reduction equivalents. In contrast, ceramide generated by activation of sphingomyelinase is rather a signal for starvation and oxidative stress. With this thought in mind, the interdependence of lipid mediators for NSC proliferation vs. differentiation and energy metabolism will be discussed in a new way that is often paid only little attention.
2.1. Phosphatidylinositols (PIPs)
The phosphatidylinositols PI(3,4)P2 and PI(3,4,5)P3 generated by class I phosphatidyinositol-3-kinase (PI3K) upon induction of tyrosine receptor kinases or G-protein coupled receptors (GPCRs) are known to be the major activators of the Akt/PKB cell signaling pathway for cell survival and differentiation [33, 45, 61–64] (Fig. 2). There are three functionally distinct groups of receptors activating PI3K in neural differentiation. The first group encompasses the GPCRs binding to other bioactive lipids, namely LPL (LPA and S1P), eicosanoid, endocannabinoid, and DHA receptors. Then there are receptors for growth factors such as FGF-2 and LIF, which are well known effectors of stem cell self-renewal and differentiation. Finally, there are receptors for growth factors such as insulin and IGF-1, which are not confined to stem cell differentiation, but interconnect NSC survival and differentiation with functions in general metabolism. Since the first group has already been discussed in the previous chapter, this section will focus on the second and third group of receptors activating PI3K.
In the cultivation process of ES cells, LIF and FGF-2 are used to maintain pluripotency of mouse or human ES cells respectively. These two growth factors elevate the expression of the transcription factors Oct-4 and Nanog, which is essential for maintenance of pluripotency [65, 66]. It has been shown that two cell signaling pathways are critical for this regulation: the janus kinase/signal transducer and activator of transcription 3 (Jak/Stat3) and the Akt/PKB signaling pathways [62, 67]. In the cultivation of mouse ES cells, the most important growth factor activating Stat3 and Akt/PKB is LIF (leukemia inhibitory factor), an interleukin 6 class cytokine binding to LIF receptor α (LIFRα) [68–72]. In vitro, LIF is added to the medium when cultivating undifferentiated mouse ES cells on feeder fibroblasts and in feeder-free culture. In vivo, LIF is generated by the trophoectoderm from where it penetrates the inner cell mass, the source of pluripotent ES cells in the pre-implantation embryo. In human ES cells, the role of LIF as “guardian” of pluripotency is taken over by FGF-2 [73, 74]. Binding of FGF-2 to the FGF receptor 2 (FGFR2) activates similar cell signaling pathways in human ES cells as stimulated by LIF in mouse ES cells: in particular MAPK and Akt/PKB [73, 74]. However, FGFR-dependent signaling is very diverse and it depends on individual receptor protein complexes which specific response is elicited by FGF. For example, in mouse ES cells, FGF-2 is used to maintain the multipotent neuroprogenitor stage and to prevent further neuronal differentiation. In human ES cells, supplementation of the serum-free cell culture medium with FGF-2 is critical to prevent apoptosis and to maintain pluripotency.
The balance between PIP3 and PIP2 is regulated by the enzyme “phosphatase and tensin homolog deleted on chromosome 10” (PTEN). PTEN catalyzes the hydrolysis of PIP3 to PIP2, which leads to inactivation of the Akt/PKB cell signaling pathway [75–77]. It is a tumor suppressor mutated in many types of cancer and it is critical for the controlled growth of embryonic tissue and ES cells. Consistent with this function, deletion of PTEN activates Akt/PKB-dependent cell signaling pathways [75]. PTEN mutations are often found in human cancers such as glioblastoma, prostate cancer, and breast cancer. Loss of function of this tumor suppressor gene results in the up-regulation of the Akt/PKB-to-β-catenin pathway (Fig. 2A) [76]. Akt/PKB phosphorylates and inactivates glycogen synthase-3β (GSK-3β), a protein kinase in the Wnt signaling pathway that phosphorylates β-catenin [78–80]. The oncogene β-catenin is an important adhesion protein and transcription factor for genes involved in proliferation. When phosphorylated by GSK-3β, β-catenin (in a protein complex with adenomatous polyposis coli or APC) is proteolytically degraded and thus, adhesion lost and proliferation reduced. Consistent with this function, deletion of β-catenin results in loss of pluripotency and early embryonic death of the respective knockout mouse [81]. Likewise, deletion of PTEN results in increased β-catenin levels and increased pluripotency or malignancy [75]. Therefore, the PTEN vs. PI3K-to-Akt/PKB antagonism is interesting in two biological contexts with respect to stem cell differentiation: maintenance of pluripotent stem cells and tumorigenesis of cancer stem cells. In the first context, inhibition of PTEN, activation of PI3K and Akt/PKB, or inhibition of GSK-3β will be useful to maintain pluripotent ES cells. In the second context, activation of PTEN, inhibition of PI3K and Akt/PKB, or activation of GSK-3β may be a useful strategy to eliminate cancer stem cells.
In addition to using natural lipids as ligands, ES cell and NSC differentiation can also be modulated by pharmacologic reagents that are either lipid analogs, inhibitors of enzymes in lipid metabolism, or drugs targeting downstream effectors of lipid-regulated cell signaling pathways. Two drugs that are inhibitors of protein kinases in the LIFRα and FGFR2 pathways have been tested on their effect on pluripotency: LY294002 and indirubin-3-monoxime, two inhibitors specific for PI3K and GSK-3β, respectively [62, 65, 77, 82–86]. The PI3K inhibitor LY294002 has been shown to reduce the capacity of mouse and human ES cells to self-renew and to undergo subsequent steps of lineage specification and differentiation [62]. These effects are likely to involve differentiation stage-specific (contextual) other cell signaling pathways downstream (or parallel) to the PI3K-to-Akt/PKB signaling axis. While it may not be desired to interfere with ES cell pluripotency, LY294002 and other PI3K and Akt/PKB inhibitors are currently tested for cancer treatment, in particular for targeting cancer stem cells [87, 88]. If one desires to sustain self-renewal of ES cells, GSK-3β inhibitors such as indirubin-3-monoxime or BIO are attractive candidates. BIO has been successfully used to maintain pluripotency in human ES cells [65]. Additional effectors targeting GSK-3β are synthetic agonists of the Wnt receptor frizzled, however, their use in stem cell differentiation is not yet sufficiently investigated [86].
Interestingly, inhibitors of the MAPK pathway such as the MAPK kinase (MEK) inhibitor PD98059 have been used with mouse ES cells to promote self-renewal or pluripotency [74, 89]. This appears paradoxical since LIFRα as well as FGFR2 are known to activate MAPK, which suggests that activation of MAPK is involved in pluripotency. However, only transient MAPK activation to promote G1 re-entry is useful for self-renewal while prolonged activation will promote differentiation. Therefore, a combination of LIF with the MEK inhibitor PD98059 activating PI3K-to-Akt/PKB while inhibiting MAPK signaling has been successfully used to promote pluripotency in mouse ES cells, but also to enhance the generation of induced pluripotent stem (iPS) cells [74, 86]. The situation in human ES cells, however, is different. In contrast to mouse ES cells, inhibition of the MAPK cell signaling pathway reduces the potential of undifferentiated human ES cells to self-renew, indicating that FGFR2-mediated activation of Ras/Raf-to-MEK-to MAPK is critical for human ES cell pluripotency [90]. A similar role has been found for Bmp4, which promotes pluripotency in mouse and differentiation in human ES cells [91–93]. It is quite possible that this difference depends on which other pathways for pluripotency are co-activated such as Jak/Stat3 in mouse or activin in human ES cells. Bioactive lipids are important in that they co-regulate several cell signaling pathways critical for pluripotency and differentiation of ES cells and NSCs (Fig. 2), in particular MAPK and PI3K downstream of ClassA/Rhodopsin-like GPCRs, as discussed in the previous chapter.
2.3. Ceramide and S1P
Ceramide is a fascinating lipid. Its level is highly regulated by three interconnected metabolic pathways, de novo biosynthesis from serine and palmitoyl-CoA, the sphingomyelin cycle in the cell membrane releasing ceramide from sphingomyelin, and the salvage pathway regenerating ceramide from sphingosine that was released by endo-lysosomal hydrolysis of sphingomyelin [94–96]. Moreover, this intensive metabolic turnover allows for fast remodeling of the fatty acid species in ceramide by six (dihydro)ceramide synthases in the ER (CerS1/lass1, C18 (dihydro)ceramide; CerS2/lass2, C20, C22, C24, and C26 (dihydro)ceramide; CerS3/lass3, C24 and C26 (dihydro)ceramide; CerS4/lass4, C18, C20, and C22 (dihydro)ceramide; CerS5/lass5, C16 (dihydro)ceramide; and CerS6/lass6, C14 and C16 (dihydro)ceramide) [97]. Since C18 ceramide is a major ceramide species in the brain it is not surprising that the mRNA expression level of CerS1 is about 50% of the total CerS mRNAs. Consistently, the CerS1 knockout mouse shows severe neurodevelopmental effects, particular in the cerebellum [98]. It has been reported that the portion of C18 ceramide increases in differentiating ES cells [99].
However, the attempt to correlate specific ceramide species such as C18 ceramide with a specific function in development is difficult since the CerS1 knockout mouse shows the up-regulation of another ceramide species (C16 ceramide), most likely as a compensatory effect. Therefore, the developmental defects may result from the loss of C18 ceramide or from the elevation of the other ceramide species. A similar effect was observed for the CerS2 knockout mouse that also shows neurodevelopmental defects and up-regulation of C16 as well as C18 ceramide [100–102]. It may be possible to get a better idea of the function of ceramide in development once double-knockouts for CerS1 and CerS2 (or other CerSs) have been generated.
In our laboratory, we have examined the function of ceramide in neural differentiation of ES cells with respect to the downstream targets of ceramide, in particular cell signaling pathways important for NSC differentiation. Three major regulators of differentiation signaling pathways have been shown to be activated by ceramide: protein phosphatase 1 (PP1) and PP2a (de-phosphorylates a variety of signaling protein kinases or other proteins), kinase suppressor of Ras (upstream of Erk1/2), and atypical protein kinase C (aPKC, upstream of Akt and GSK-3β) [103–116]. Based on our data obtained with in vitro differentiating ES cells showing the critical function of the ceramide/aPKC interaction for NSC differentiation, we have focused on aPKC.
Atypical PKCs comprise a family of three protein kinase C isoforms, PKCδ (zeta), λ (lambda), and ι (iota). Knockout mice for each of these enzymes have shown that these isoforms have overlapping as well as highly specific functions in development. For example, the conventional knockout for aPKCλ/ι is embryonic lethal, while aPKCδ-deficient mice survive, but show impaired insulin, NF-κB, and IL-4 signaling [117, 118]. Conditional knockout of aPKCλ/ι has tissue-specific effects: in the retina this aPKC isoform is indispensible for retinal development and photoreceptor cell polarity, although another study has found that aPKCδ can functionally substitute for the λ/ι isoform [119, 120]. Interestingly, the specific function of aPKC for stem cell differentiation and embryo development seems to be cell- and tissue-specific: while a double-knockout of the δ and λ isoform does not affect hematopeoitic stem cell polarity and differentiation to blood cells, inhibiting all isoforms of aPKC prevents lineage commitment of ES cells and sustains self-renewal [121, 122]. This observation is important with respect to our research since ceramide appears to bind and activate all of the aPKC isoforms (unpublished).
Prior to our ceramide-aPKC binding studies, it was known from in vitro enzyme assays that ceramide can activate (at low dose) or inactivate (at high dose) aPKC [112, 113]. It was not known whether this activation/inactivation reaction is mediated via a ceramide binding site and occurs in cells. It was also known that aPKC interaction with an endogenous inhibitor protein, prostate apoptosis response-4 (PAR-4) previously discovered in prostate cancer cells, induces apoptosis [123–125]. We demonstrated for the first time that ceramide activates and enhances autophosphorylation of aPKC in cells, but also induces inactivation of aPKC when PAR-4 expression is elevated [108, 111, 126–129]. Moreover, we showed that this mechanism (sensitizing cells to ceramide by expressing PAR-4) eliminates teratoma (stem cell tumor)-forming stem cells from differentiating ES cells and may also control the number of neural progenitors during brain development [126, 128]. Later on, we found that there is a ceramide-binding site in the C-terminus of aPKC, which is distinct from the modified C1 domain in the regulatory moiety of the enzyme [110, 130].
The C1b domain in aPKC is similar to the C1 domain in classical PKCα, which mediates binding to DAG and activation of PKCα. Prior to our study; it was hypothesized that the C1b domain may bind ceramide because of its structural similarity to DAG [131]. However, until recently, it has never been shown that ceramide can actually bind to the C1b domain. First evidence that this binding occurs comes from a study with KSR, which contains a C1b domain mediating activation of the enzyme by ceramide [107]. Therefore, it is likely that aPKC has two binding domains for ceramide, the C1b domain and the C-terminal domain identified in our studies. Although this idea is speculative, but two binding sites may tremendously enhance ceramide binding of aPKC since the free enthalpy for binding will be the sum of that for each binding site (or the combined dissociation constant the product of the individual constants). Binding of ceramide to aPKC may proceed in a similar way as binding occurs between DAG and PKCα or PKD: the C2 domain (in PKCα) binds to Ca2+/PIP2 or phosphatidylserine (PS) and promotes binding of the C1a domain to DAG [132–134]. The pseudosubstrate domain unmasks the catalytic site and client proteins can be phosphorylated. The DAG binding part of the C1a domain consists of prong-like β-sheets that form a binding pocket partially penetrating the inner leaflet of the cell membrane. While this pocket almost completely engulfs one DAG residue, it forms a tight interaction with hydrophobic and anionic lipid residues at its outside. One may envision a similar “hollow rivet”-like mechanism for binding of ceramide to the C1b domain.
We have hypothesized that that ceramide binding of aPKC is associated with PIP2 or PIP3 binding of Cdc42, a small GTPase interacting with the N-terminus of aPKC via the polarity protein Par6 [135–137]. Moreover, this mechanism could initiate a larger sphingolipid-protein scaffold (SLIPS) regulating cell polarity, which is known to critically depend on the aPKC-Par6-Cdc42 interaction [130, 135–142]. Finally, mutually exclusive binding of ceramide-aPKC to either PAR-4 or Par6-Cdc42 may constitute a “cell fate switch” we have proposed to control the balance between apoptosis (PAR-4) and cell polarity (Par6-Cdc42) in NSCs [126, 135–137, 143, 144]. Therefore, it is not surprising that ceramide elevation is concurrent with apoptosis in “unwanted” or surplus stem cells and cell polarity of neural progenitor cells at the same differentiation stage [126, 128, 135–137, 144]. We have found that one mechanism distinguishing these two opposite cell fates is the unequal distribution of PAR-4 to the two daughter cells of a dividing stem cell: the one daughter cell that inherits PAR-4 is going to die, while the other one differentiates to a neural progenitor cell with ceramide sustaining cell polarity instead of inducing apoptosis [126].
How can ceramide affect cell signaling pathways in NSC differentiation apart from regulating cell polarity and apoptosis? We have proposed that two rheostats between ceramide and its derivatives are involved in this regulation: the balance between ceramide and S1P and that between ceramide and GSLs, the latter ones being discussed in chapter 3 of this review [135, 143–145]. Since elevating ceramide in polarized cells puts these cells at risk to undergo apoptosis, it is important to counteract the pro-apoptotic ceramide signal downstream of its client proteins. We have shown that ceramide activation of aPKC enhances phosphorylation and thereby inactivation of GSK-3β, a mechanism critical for cell polarity [136]. However, ceramide is also known to inactivate Akt, which compromises GSK-3β phosphorylation and reduces cell survival [106, 146, 147]. These two opposite effects of ceramide on GSK-3β can be regulated by the ceramide derivative S1P. We have discussed in the previous chapter that S1P binding to GPCRs activates Akt, which then in turn phosphorylates and inactivates GSK-3β (Fig. 2). Inactivation of GSK-3β entails hypohosphorylation and therefore, stabilization of β-catenin, a protein with dual function. As transcription factor it is critical for neural differentiation of stem cells, while as cell polarity-associated protein it sustains adhesion of neural progenitors to the basal lamina in the subventricular zone (SVZ) of the embryonic brain.
It appears as if fine-tuning of the ceramide/S1P rheostat may actually imply that these two lipids with opposite function cooperate in favor of NSC differentiation. While ceramide sustains cell polarity and promotes differentiation, S1P increases cell survival. The critical role of ceramide for neural progenitor cell polarity has been reported in our recent studies showing that chemically induced ceramide deficiency completely abrogates the distribution of β-catenin to the basal lamina of the SVZ [136]. The β-catenin dislocation leads to aberrant neural progenitor migration and defective cortical lamination. Consistently, a water-soluble ceramide analog for the first time synthesized in our laboratory (N-oleoyl serinol or S18) rescues the β-catenin distribution, neural progenitor migration, and cortical lamination in ceramide-deficient embryos [108, 136, 148]. We have tested the combinatorial cell signaling effects of ceramide and S1P in neural progenitor cells. When differentiating ES cells are incubated with S18 and S1P or its prodrug analog FTY720, residual pluripotent, teratoma-forming stem cells are eliminated while the survival and differentiation of neural progenitor cells is enhanced [126]. Elimination of teratoma-forming stem cells is due to their high expression level of PAR-4, which is only weakly expressed in neural progenitor cells. The enhanced survival of progenitor cells is due to their cell surface expression of the S1P receptor S1P1, which is missing in the tumor stem cells [144].
Interestingly, further incubation with S1P or FTY720 promotes differentiation of neural progenitor cells toward oligodendrocyte precursor cells (OPCs), while prolonged incubation with S18 promotes neuronal differentiation [128, 143, 144]. This ceramide and S1P-regulated switch between neuronal and glial differentiation of neural progenitor cells is consistent with other studies showing that RA-induced neuronal differentiation of ES cells is concurrent with elevation of ceramide and that S1P and S1P1 is critical for survival and oligodendroglial differentiation of OPCs in primary cell culture [127, 149–152]. While our results and studies from other groups suggest that the effect of S1P (and also LPA) on ES cells and NSCs is mediated by GPCRs [29, 30, 143, 144, 153], it is quite likely that intracellular targets of S1P are also involved in stem cell differentiation. For example, the inhibition of histone deacetylase 1 (HDAC1) by S1P synthesized by shingosine kinase 2 in the nucleus is certainly an important regulator for cancer cell proliferation, but may also promote differentiation of NSCs [154]. Therefore, it is expectable that many bioactive lipids such as ceramide and S1P regulate NSC differentiation by targeting several distinct mechanisms with similar outcome. This involves activation of lipid-specific receptors, binding of various intracellular cell signaling proteins, or forming lipid microdomains modulating the activation of growth factor receptors. Plus, lipids can be metabolically interconverted, turning them into highly dynamic and versatile regulators of various cell signaling pathways in NSC differentiation. The next section will elaborate further on this specific property of bioactive lipids.
2.4. Metabolic regulation of NSC differentiation by PIPs and ceramide
In recent years, it has become evident that the energy and redox state of stem cells, including NSCs is a critical factor controlling stem cell differentiation. As a rule of thumb: the more oxidized a stem cell the more likely it will undergo differentiation. Since the early days of ES cell culture, this was evident when pluripotent ES cells were maintained in the presence of β- mercaptoethanol thereby promoting the reduced redox state of the undifferentiated stem cell. In light of the previous sections, this curious observation can be embedded into a more scientifically grounded framework of connecting cell signaling pathways regulating stem cell differentiation with lipid metabolism. One of these pathways is the PI3K-to-Akt and Akt-to-GSK-3β and mTOR cell signaling axis (Fig. 2). This has also been evident from the early days of ES cell culture since insulin is a critical component in N2, ITS, and B27 supplements added to serum-free media for the cultivation of ES cells and NSCs. Insulin triggers PI3K activation via the insulin receptor, which signals a saturated state: glucose is transported into the cell and converted into energy (ATP) and reduction equivalents (NADPH2) promoting anabolic metabolism in NSCs [49, 77, 155]. In turn, cell survival (via Akt) and protein translation (via mTOR) is up-regulated, a prerequisite for the propagation of neural progenitors. IGF-1 can substitute for insulin with respect to PI3K activation and induction of neural differentiation [156, 157].
However, aerobic production of energy puts cells at risk to generate oxygen radicals or so-called reactive oxygen species (ROS). The most efficient way to eliminate ROS is the cellular production of reduced glutathione (GSH), which needs high levels of NADPH2 for its regeneration. Therefore, low GSH levels due to high ROS or metabolic malfunction reducing NADPH2 levels will promote aberrant differentiation of stem cells. This has been impressively demonstrated in studies showing that GSH sustains glial progenitor propagation while oxidation will promote the differentiation to reactive astrocytes, an inflammatory process commonly found in brain tissue lesion and neurodegenerative diseases such as Alzheimer’s disease [158–162]. This mechanism also explains why maintenance of pluripotent stem cells or multipotent progenitor cells is often performed by cultivation of cells at low oxygen levels: while glucose supply is plenty for the production of reduction equivalents, generation of ROS is reduced. Further, astrocytes maintain a natural barrier between NSCs and neurons toward the oxygen-rich blood stream, while at the same time, providing these cells with GSH [163]. This separation is compromised in the case of brain tissue lesion, stroke, and neurodegenerative diseases leading to a sudden increase of ROS and the generation of reactive astrocytes that form a “glial scar” (gliosis).
How can lipids regulate this process? The regulation of metabolically induced proliferation vs. differentiation of stem and progenitor cells is related to two aspects of bioactive lipids: lipid metabolism and cell signaling. In particular, PIPs and other glycerophospholipids, and sphingolipids are important key players in the metabolic interconversion of bioactive cell signaling lipids. Diacylglycerides are synthesized from DAG, either by activating DAG itself (CDP-DAG) or the alcohol/aminoalcohol such as choline (CDP-choline) or ethanolamine. For example, PIPs can be made from CDP-DAG and inositol, while phosphatidylcholine can be generated from DAG and CDP-choline. Phosphatidylcholine is then a precursor for the generation of sphingomyelin from ceramide, a reaction that is known to reduce inflammatory and pro-apoptotic cell signaling pathways [164]. Most recently, we have found that CDP-choline-driven detoxification of ceramide by its conversion to sphingomyelin can also protect neural crest stem cells and may be useful for treatment or prevention of fetal alcohol syndrome [165]. These studies and their implications for the metabolic compartmentalization of stem cell niches will be discussed in Conclusions.
At this point, one will consider these metabolic intermediates as bioactive cell signaling lipids that distinguish a saturated metabolism from a starving one. DAG as a sensor for ample supply with nutrients will directly activate PKCα and stimulate cell proliferation, while at the same time, enable the elevation of PIP3 and reduction of ceramide, thereby increasing stem cell survival via activation of Akt. On the other hand, loss of nutrients and reduced activation of metabolic intermediates such as DAG or choline will deprive cells of precursors for phospholipid (i.e., phosphatidylcholine and sphingomyelin) biosynthesis, eventually leading to elevation of ceramide. As the consequence, stem cells will stop dividing and undergo differentiation or induce apoptosis. This assumption is in full agreement with our observations made with differentiating ES cells and embryonic mouse brain. At the transition from pluripotent EB-derived cells to multipotent neural progenitor cells or in embryonic mouse brain at gestation day E14.5, neural progenitors up-regulate ceramide, which is then converted to GSLs, in particular gangliosides (see chapter 3) [126, 127]. As discussed in the preceding section, we found that the peak of ceramide elevation does not induce apoptosis because neural progenitors show only low expression of PAR-4, the protein sensitizing cells to ceramide-induced apoptosis. Instead, ceramide is likely to function as a regulator of cell polarity in neural progenitors, thereby synchronizing NSC differentiation with the establishment of cell polarity.
The case of PIP2 is a little bit more complicated since it is cleaved by PLCγ to DAG and IP3, theoretically replenishing DAG in glucose-starved cells potentially activating mitogenic PKCα. However, IP3 will bind to the IP3 receptor (IP3R) and induce the release of Ca2+ from the ER, which is a strong differentiation inducer as already discussed as one of the effect of GPCR-dependent activation of PLCγ. From this chain of events, it becomes evident that the cooperation of specific lipids with Ca2+ may be an important signal combination for NSC differentiation. There are at least three Ca2+ receptors or channels that can be regulated by bioactive lipids: IP3R (IP3), the ryanodine receptor (RyR, sphingosine and ceramide), the Na+/Ca2+ exchanger(s) (sphingosine, ceramide, and GM1), and the transient receptor potential channel 5 (TRPC5, LPC, S1P, and GM1) [166–169]. Most recently, ceramide has been found to regulate Ca2+ homeostasis in mitochondria by blocking the permeability transition pore, however, the exact mechanism is not clear yet [170]. Studies in Bob Ledeen’s group have focused on TRPC5 and the Na+/Ca2+ exchanger in the nuclear membrane, which he has shown to be controlled by a specific ganglioside, GM1 [171–178]. This group of lipids and their function in neurogenesis and stem cell differentiation will be discussed in chapter 3.
3. Raft-forming lipids and raft-associated receptors in neural stem cells
The previous two chapters discussed bioactive lipids that are known to act through lipid receptors or binding proteins. Many lipids regulate cell signaling pathways through a mechanism known as “lipid rafts” or “lipid microdomains”. Lipid rafts are areas in the cell membrane (or intracellular membranes) that emerge from the self-assembly of lipids in an ordered (Lo) structure in the liquid phase of the membrane [14, 135, 179–184]. The concept of lipid rafts goes back to studies by Gerrit van Meer and Kai Simons showing that GSLs are asymmetrically transported to the apical membrane of MDCK cells [185–187]. Simons then formulated the functional raft hypothesis in 1997 [14, 188]. This concept was preceded by that of lipid microdomains, which dates back twenty years earlier and was based on studies that are of importance for today’s lipid raft research and understanding. In the mid 1970s, detergent-insoluble membranes enriched in cholesterol, sphingomyelin, and GSLs were isolated for the first time, a technique still used to prepare lipid rafts for the analysis of raft-associated lipids and proteins [189, 190]. About ten years later, fluorescence microscopy studies using filipin, a cholesterol cluster-binding reagent showed that the lipid distribution in the cell membrane is inhomogeneous with lipid microdomains of less and those of higher ordered structure [191–193].
The term “lipid microdomains” is now used interchangeably with that of lipid rafts, although rafts are commonly regarded as lipid microdomains of higher ordered structure. The minimum lipid composition of a raft is cholesterol and sphingomyelin, which is associated with GSLs or a glycerophospholipid such as phosphatidylcholine. In addition to lipid rafts, there are other highly ordered GSL-enriched microdomains (GEMs), so-called glycosynapses Glycosynapses mediate cell adhesion and immunological responses and have been first described by Hakomori about 25 years ago [194–196]. Finally, caveolae, lipid rafts enriched with caveolin and important for endocytosis have also been found to contain GSLs. However, the functional significance of their presence is unclear since blocking GSL biosynthesis appears not to obliterate caveolae formation or function [197–199]. Although caveolae have not been found in neurons, genetic ablation of caveolin affects neural progenitor cell proliferation, indicating a role of this protein independent of caveolae formation or alternatively, a role of this type of rafts in NSCs prior to terminal neuronal differentiation [181, 200].
Lipid rafts are believed to show high affinity to specific cell signaling proteins such as growth factor or cytokine receptors, which leads to clustering and activation of these receptors (Fig. 1). Therefore, bioactive lipids can affect stem cell differentiation in two different ways: direct interaction with lipid receptors such as GPCRs and lipid raft-dependent activation of growth factor or cytokine receptors such as LIFRα, epidermal growth factor receptor (EGFR), IGFR, or FGFR2 [13, 135, 201–206]. In addition, receptor-associated signal transduction proteins such as Ras can be modified with fatty acids (palmitoylation) or terpenoids (farnesylation, geranylation) and glycophosphatidylinositol (GPI anchor), which tremendously increases membrane binding and raft association [14, 207–209]. It has been shown that particular GSLs termed gangliosides can regulate ES cell differentiation by the activation of FGFR2 and other receptors in lipid rafts [145, 205]. An example for this mechanism is the corrective activity of the ganglioside GM1 on the effect of the fungus toxin fumonisin B1, which causes neural tube defects by inhibiting sphingolipid biosynthesis [210, 211]. Gangliosides such as GM1 are commonly found in lipid rafts, although there is heterogeneity in their lipid composition potentially giving rise to distinct types of lipid rafts with specific function in neural differentiation. This review will focus on lipid rafts composed of sphingomyelin, cholesterol, and GSLs.
3.1. Glucosyl- and galactosylceramide
Phospholipids such as sphingomyelin as well as cholesterol are commonly found in highly ordered lipid rafts. Sphingomyelin can be hydrolyzed to ceramide, which disturbs this order and displaces cholesterol [212]. The resulting ceramide-containing microdomains or rafts have been proposed to have specific functions in cancer and neural differentiation of stem cells [135, 137, 213, 214]. Studies from our laboratory have shown that ceramide microdomains bind to cell polarity-associated aPKC, which has been discussed in chapter 2. The structural and functional variety of different rafts often stems from the composition of specific GSLs. GSLs are synthesized by a multitude of ER- or Golgi-resident enzymes, which sequentially assemble a glycan structure to the polar head group of ceramide [145, 215–221]. The simplest GSLs are glucosyl- and galactosylceramide, more complex GSLs contain sialic acid residues and are called gangliosides. These will be discussed in the following section.
Galactosylceramide is the principal GSL in the brain. It comprises 23% of the total mass of myelin lipids [222]. However, in addition to its function in myelin, it has also been shown to be critical prior to myelin formation. In OPCs, galactosylceramide forms lipid rafts with cholesterol and sphingomyelin in the ER, which is instrumental for the activity of the sigma receptor [60] and OPC differentiation. Galactosylceramide is also the precursor for the biosynthesis of sulfatides, which comprise another 4% of the total lipids in myelin. Sulfatide and galactosylceramide are specific markers for OPC differentiation stages, which are recognized by the antibodies O1 (anti-galactosylceramide) and O4 (anti-galactosulfatide) widely used to enrich for pre- (O4(+)/O1(−)) or immature (O4(+)/O1(+)) oligodendrocytes, respectively. Interestingly, the O4 (but not O1) antibody is able to block terminal differentiation of OPCs, which indicates a functional role of cell surface sulfatide in oligodendrocyte differentiation [223]. It should be noted, however, that the function of the differentiation-stage specific expression of most GSLs and whether they act primarily as adhesion molecules or lipid raft modifiers in cell signaling pathways is still not known.
In contrast to galactosylceramide, which is synthesized in the ER, glucosylceramide is the precursor of a multitude of GSLs originating in the Golgi, including globosides and gangliosides (see next section). Cell surface glucosylceramide has been implicated in a variety of functions with respect to lipid raft and receptor regulation [181, 205, 224–226]. For example, rafts consisting of cholesterol, sphingomyelin, and glucosylceramide (CSG rafts) have been found to be associated with the activation of multidrug resistance (MDR) proteins in cancer [225]. We have suggested a novel concept of cancer treatment by breaking this CSG raft-dependent resistance in cancer stem cells [227]. Whether CSG rafts are also important in NSC differentiation is not known. However, it is known that stem cells persist throughout the whole life, which demands for a specific protection against environmental toxins. Therefore, it is not surprising that many stem cells express high levels of MDR proteins that may then be activated by CSG rafts.
Further insight into the biological function of glucosylceramide came from the use of specific pharmacologic inhibitors of glucosyltransferase, the enzyme synthesizing glucosylceramide from ceramide, and the generation of glucosyltransferase knockout mice. These two approaches clearly indicate the critical role of glucosylceramide transferase in insulin signaling and neural development, although this is likely to depend on the reduced biosynthesis of the ganglioside GM3 [228, 229]. Glucosylceramide is converted to lactosylceramide and then further sialylated to GM3, the precursor of a multitude of simple and complex gangliosides discussed in the next section.
3.2. Globo- and ganglio-series GSLs
One of the most frequently used surface markers for undifferentiated human ES cells is the globoside Gb5, also known as stage-specific embryonic antigen 3 (SSEA-3) [230–236]. It consists of a linear pentasaccharide structure (Glc-Gal-Gal-GalNAc-Gal) linked to ceramide. It can be converted to SSEA-4 by attaching a terminal sialic acid residue. Interestingly, in mouse, SSEA-4 is a marker for more differentiated (ectodermal) stem cells, while it is characteristic for the undifferentiated state in mouse ES cells [232, 237, 238]. A more thorough analysis of differentiation-stage specific GSLs showed that during differentiation of human ES cells, surface GSLs undergo a change from lacto- or globo- to ganglio-series, indicating an extension of the oligosaccharide to more complex GSLs in more differentiated cells [239]. It has been reported that Gb5 is associated with lipid rafts, although it has not been shown to regulate their formation [240]. For globosides as well as gangliosides, it is still unclear how they affect NSC differentiation. One potential function could be that they serve as direct attachment sites for cell-to-cell contacts, a mechanism already discussed for glycosynapses. However, more likely is the GSL-dependent modulation of growth factor receptors, potentially within lipid rafts enriched with particular GSLs.
The simplest ganglioside, GM3 is synthesized by the sialylation of lactosylceramide, which comprises a ceramide residue linked to the disaccharide lactose. GM3 and its derivative GM1 are GSLs that are characteristic for GSL-containing lipid rafts. Knocking out GM3 biosynthesis leads to enhanced insulin sensitivity, which has been suggested to indicate a direct inhibition of the insulin receptor by lipid raft-associated GM3 [228]. Double knockouts deficient in the two glycosyltransferaseses that convert GM3 to GM2 (GM2/GD2 synthase) and GD3 (GD3 synthase) have not been reported to show enhanced insulin resistance, which may be due to a lack of GM3 accumulation in these mice [229]. However, these mice show neurodegeneration in peripheral motor and sensory nerves progressing with age, and reduced brain weight and cortical thickness, indicating a critical role of GM3-derived gangliosides in neural tissue development, maintenance or repair.
Gangliosides show amazingly profound and rapid changes during early embryonic brain development. Between gestational day E12 and E14, a large portion of GM3 is converted to GD3 and complex b-series gangliosides, which are further sialylated derivatives of GD3 [127, 215, 218, 220, 221, 241]. Later in embryonic and early post-natal brain development, GM3 is converted to its sialylated derivatives in the a-series complex gangliosides, in particular GM1. These observations suggest that increasingly complex sialylation of gangliosides is critical for NSC differentiation and brain development. Surprisingly, knockout mice deficient in sialyltransferases that synthesize these complex gangliosides did not show the severe phenotypes expected from them, although clear symptoms of aberrant neural function and neurodegeneration have been observed. For example, Bob Ledeen’s group has shown that GM2/GD2-deficient mice that lack all complex gangliosides are far more susceptible to kainite-induced seizures and neuronal apoptosis than their wildtype counterparts [242]. Interestingly, the phenotype is rescued by the administration of LIGA 20, a synthetic and cell membrane-permeant analog of GM1. In the last 20 years, Ledeen’s group has focused on the role of GM1 in neuronal function and found exciting results indicating a critical role of GM1 in the regulation of Ca2+ influx to the nucleus [169, 171, 172, 175, 176, 178, 243].
GM1 is the classical ganglioside used for staining of GSL-containing lipid rafts on the cell surface with cholera toxin subunit B. GM1 in rafts of the nuclear envelope was a novel concept brought forward and investigated by Bob Ledeen’s group. He found that GM1 tightly associates with the Na+/Ca2+ exchanger in the nuclear envelope and potentiates its ability to increase Ca2+ influx into the nucleus. LIGA 20 can restore this function in GM1-deficient knockout mice and protects neurons against kainite-induced seizures [242]. This example clearly demonstrates that gangliosides are abundant in many subcellular compartments and may regulate the activation of membrane-resident receptors, ion exchanges, and other proteins in lipid rafts distinct from those found in the cell membrane.
The generation of double knockouts for various pathways in ganglioside biosynthesis has now reached a stage that the naturally occurring redundancy in the function of sialylated GSLs preventing overt phenotypes can be avoided. A double knockout of GM3 synthase and GM2/GD2 synthase does not allow for compensation of a- or b-series complex gangliosides by the elevated biosynthesis of o-series gangliosides as observed with the GM3 single knockout mouse [244]. These mice are devoid of any ganglio-series GSLs and develop a catastrophic neurodegenerative disease that results in death shortly after weaning. The phenotype observed, shrinkage of the brain, astrogliosis, and neuronal apoptosis in the cortex clearly indicates the essential role of gangliosides for maintenance of axon-glial interactions and other neural functions. This phenotype suggests a function of gangliosides similar to that proposed for sulfatides, as axon-glial attachment sites in the node of Ranvier, most likely by the interaction of GSLs with myelin-associated glycoprotein (MAG), which is essential for myelin stability [245, 246].
There is still much to discover for gangliosides, in particular for the function of GD3, GT3, and b-series complex gangliosides in NSC differentiation and brain development. The comparison of single with double-knockouts demonstrates that there is a very efficient compensation mechanism within the family of ganglio-series GSLs. Since during ES cell differentiation, the complexity of GSLs increases from lacto- and globo- to ganglio series GSLs, it is likely that more primitive states of NSC differentiation rely on the compensation within the families of simpler GSLs, e.g., globosides or sulfatides that could compensate for simpler gangliosides such as GD3. It is remarkable that some simpler gangliosides such as GD3 are highly specific for distinct NSC differentiation stages. Rober Yu’s group discovered that GD3 is specifically expressed on the cell membrane of neural progenitor cells derived from the SVZ of embryonic mouse brain [215, 247, 248]. This group also showed that complex b-series gangliosides, in particular GD1b can protect neural cells against ceramide-induced apoptosis by regulating IGF-1 receptor-dependent Ca2+ influx [127]. Therefore, it is very likely that other functions of gangliosides will be found in NSC differentiation and brain development.
4. Conclusions and hypothesis: Developmental niches may be metabolic compartments for the generation of specific cell signaling lipids in stem cell differentiation
NSC differentiation can be regulated by a multitude of bioactive lipids that act on receptors, protein kinases/phosphatases and other cell signaling proteins, or lipid rafts in the cell membrane or other subcellular sites. At any given stage during differentiation, it is a cocktail of lipids that is simultaneously acting on stem cells. However, most of the research performed on NSCs neglects or overlooks the effects of lipids because of the artificial environment in which cells are cultivated in vitro. With the exemption of linoleic and linolenic acid, lipids are not considered essential in many media used for cell culture, although most companies specialized on stem and neural cell culture provide a lipid supplement of non-disclosed composition.
Lipids can become conditionally essential in vitro as well as in vivo depending on the metabolic capabilities of the cells. Like neurons, NSCs are living in a tissue niche, a compartment separating cells from unrestricted supply with blood-born nutrients and providing a defined environment with growth factors. For example, long-term cultivation of neurons in vitro requires serum-free Neurobasal medium with B27 supplement, a mixture of ingredients including lipids such as linoleic and linolenic acid, corticosterone and progesterone, and vitamin A and tocopherol. In vivo, however, neurons are far more needy and rely on supply with a multitude of intermediates such as citrate, lactate, serine, and glutathione provided by astrocytes [249]. A recent study found that neurons also receive their cholesterol from astrocytes, which secrete apoE type lipoproteins that are endocytosed by neurons [250]. While the metabolic compartmentalization between astrocytes and neurons has been intensely studied, only little is known about are potentially similar compartmentalization between NSCs and surrounding tissue in their developmental niche. The stem cell niches in the subventricular and subgranular zone (SVZ and SGZ) may very well be metabolically compartmentalized: developmental niches may also be metabolic niches or compartments.
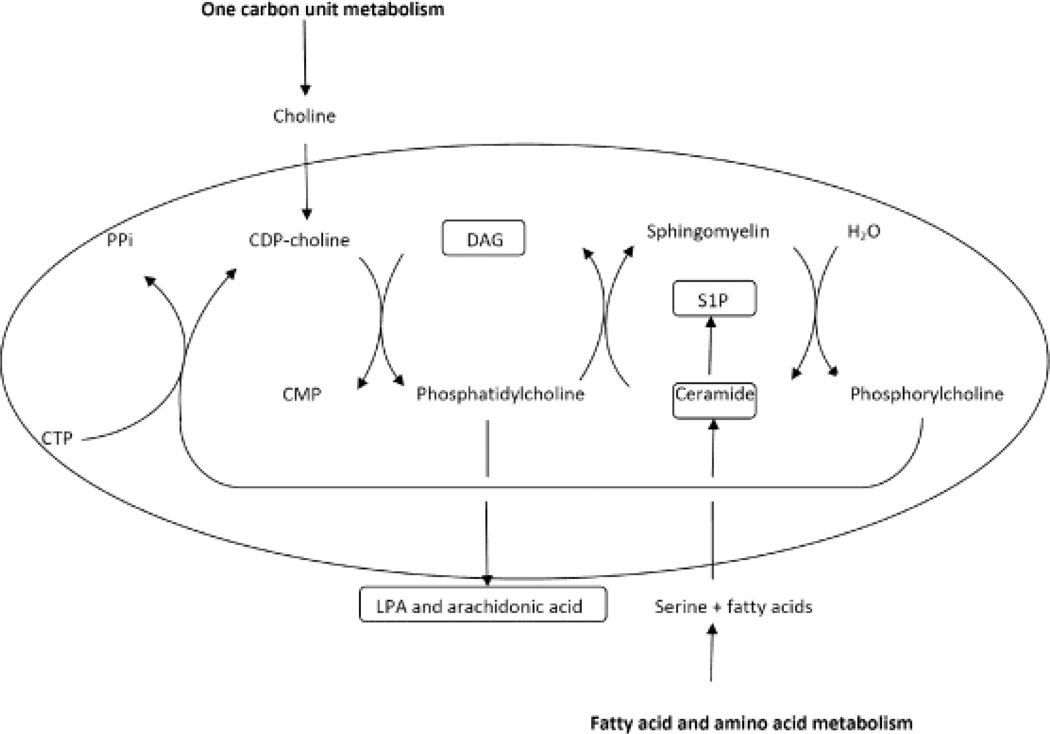
Sphingolipid metabolism is likely to be subject to compartmentalization in developmental niches such as the SVZ or SGZ niches for NSCs (Fig. 3). This hypothesis stems from our observation that neural crest-derived stem cells show ceramide elevation and apoptosis when exposed to ethanol, a potential cause for fetal alcohol syndrome and concurrent abnormalities of neural crest-derived tissues such as facial bone malformation [165]. Interestingly, in vitro, CDP-choline or citicoline, a precursor for phosphatidylcholine and subsequently, sphingomyelin biosynthesis from ceramide prevented ethanol-induced apoptosis in these cells. CDP-choline has also been shown to alleviate neurodegeneration and stroke damage in human trials [164, 251, 252]. Choline is a rich source for methyl groups required to synthesize phosphatidylcholine in the Kennedy pathway of glycerophospholipid biosynthesis (Fig. 3). We have published a model that integrates C1 (one carbon unit) with phospho- and sphingolipid metabolism, suggesting that stem cells such as neural crest-derived cells rely on continuous supply with methyl group donors to convert ceramide into sphingomyelin. In our model, this is mediated by the Kennedy pathway for de novo biosynthesis of phosphatidylcholine, which is the precursor for the generation of sphingomyelin from ceramide [135, 165] (Fig. 3). The proposed dependence of neural crest cells as a metabolically specialized compartment on the ongoing supply with methyl group donors to synthesize sphingomyelin is not obvious since one would assume that there is a sufficient amount of phosphatidylcholine coming from the bloodstream. On the other hand, our observations are consistent with previous studies showing that neuroepithelial cells in the neural tube are vulnerable toward interruption of sphingomyelin biosynthesis with the ceramide synthase inhibitor fumonisin B1 leading to neural tube defects [211].
Figure 3.
Metabolic compartmentalization of the Kennedy pathway for de novo glycerophospholipid biosynthesis and the sphingomyelin cycle in sphingolipid metabolism
Developmental niches such as the SVZ may induce a specialization of NSCs, e.g., through growth factor-dependent epigenetic regulation of gene expression, that is specific for the in vivo environment and results in a metabolic compartmentalization similar to that found between astrocytes and neurons. Figure 3 shows that this metabolic compartmentalization may very well determine the expression of bioactive lipids that have been discussed to regulate the differentiation of NSCs. Interestingly, the input metabolites such as serine or essential fatty acids are transported from astrocytes to neurons suggesting that the Kennedy pathway-to-sphingolipid metabolism integration comprises a metabolic compartment that is specifically activated in neurons (Fig. 3). Future studies will determine how similar supply with metabolites and metabolic compartmentalization may also affect bioactive lipid signaling in NSCs.
Acknowledgments
This work was supported by the NIH grant R01AG034389 to EB. Institutional support by the Institute of Molecular Medicine and Genetics, Georgia Health Sciences University, Augusta, GA (Director Dr. Lin Mei) is also acknowledged.
Abbreviations
- aPKC
atypical protein kinase C
- DAG
diacylglycerol
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- GSL
glycosphingolipid
- IGF
insulin-like growth factor
- IP3
inositoltrisphosphate
- LPA
lysophosphatidic acid
- LPL
lysophospholipid
- NSC
neural stem cell
- PAR-4
prostate apoptosis response 4
- PIP
phosphatidylinositolphosphate
- PLC
phospholipase C
- RA
retinoic acid
- S18
N-oleoyl serinol
- S1P
sphingosine-1-phosphate
- SVZ
subventricular zone
References
- 1.Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol. 1982;94:253–262. doi: 10.1083/jcb.94.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deschamps J, de Laaf R, Verrijzer P, de Gouw M, Destree O, Meijlink F. The mouse Hox2.3 homeobox-containing gene: regulation in differentiating pluripotent stem cells and expression pattern in embryos. Differentiation. 1987;35:21–30. doi: 10.1111/j.1432-0436.1987.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Yang M, Dawes J, Khillan JS. Suppression of ES cell differentiation by retinol (vitamin A) via the overexpression of Nanog. Differentiation. 2007;75:682–693. doi: 10.1111/j.1432-0436.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 5.Sun G, Shi Y. Nuclear receptors in stem cells and their therapeutic potential. Adv Drug Deliv Rev. 2010;62:1299–1306. doi: 10.1016/j.addr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MH, Cho YS, Han YM. Simvastatin suppresses self-renewal of mouse embryonic stem cells by inhibiting RhoA geranylgeranylation. Stem Cells. 2007;25:1654–1663. doi: 10.1634/stemcells.2006-0753. [DOI] [PubMed] [Google Scholar]
- 7.Pagkalos J, Cha JM, Kang Y, Heliotis M, Tsiridis E, Mantalaris A. Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J Bone Miner Res. 2010;25:2470–2478. doi: 10.1002/jbmr.163. [DOI] [PubMed] [Google Scholar]
- 8.Kishi Y, Takahashi J, Koyanagi M, Morizane A, Okamoto Y, Horiguchi S, Tashiro K, Honjo T, Fujii S, Hashimoto N. Estrogen promotes differentiation and survival of dopaminergic neurons derived from human neural stem cells. J Neurosci Res. 2005;79:279–286. doi: 10.1002/jnr.20362. [DOI] [PubMed] [Google Scholar]
- 9.Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol. 2011;231:72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labombarda F, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurol. 2011;231:135–146. doi: 10.1016/j.expneurol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 12.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 13.Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res. 2010;51:2082–2089. doi: 10.1194/jlr.M001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Zhang H, Litingtung Y, Chiang C. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc Natl Acad Sci U S A. 2006;103:6548–6553. doi: 10.1073/pnas.0600124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osakada F, Takahashi M. Neural Induction and Patterning in Mammalian Pluripotent Stem Cells. CNS Neurol Disord Drug Targets. 2011 doi: 10.2174/187152711795563958. [DOI] [PubMed] [Google Scholar]
- 17.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 21.Hla T, Lee MJ, Ancellin N, Thangada S, Liu CH, Kluk M, Chae SS, Wu MT. Sphingosine-1-phosphate signaling via the EDG-1 family of G-protein-coupled receptors. Ann N Y Acad Sci. 2000;905:16–24. doi: 10.1111/j.1749-6632.2000.tb06534.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin ME, Herr DR, Chun J. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130–138. doi: 10.1016/j.prostaglandins.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okudaira S, Yukiura H, Aoki J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 2010;92:698–706. doi: 10.1016/j.biochi.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92:949–966. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 25.Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 26.Ye X, Ishii I, Kingsbury MA, Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim Biophys Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- 27.Nakanaga K, Hama K, Aoki J. Autotaxin--an LPA producing enzyme with diverse functions. J Biochem. 2010;148:13–24. doi: 10.1093/jb/mvq052. [DOI] [PubMed] [Google Scholar]
- 28.Samadi N, Bekele R, Capatos D, Venkatraman G, Sariahmetoglu M, Brindley DN. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. 2011;93:61–70. doi: 10.1016/j.biochi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Hurst JH, Mumaw J, Machacek DW, Sturkie C, Callihan P, Stice SL, Hooks SB. Human neural progenitors express functional lysophospholipid receptors that regulate cell growth and morphology. BMC Neurosci. 2008;9:118. doi: 10.1186/1471-2202-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pebay A, Bonder CS, Pitson SM. Stem cell regulation by lysophospholipids. Prostaglandins Other Lipid Mediat. 2007;84:83–97. doi: 10.1016/j.prostaglandins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- 32.Pitson SM, Pebay A. Regulation of stem cell pluripotency and neural differentiation by lysophospholipids. Neurosignals. 2009;17:242–254. doi: 10.1159/000231891. [DOI] [PubMed] [Google Scholar]
- 33.Callihan P, Mumaw J, Machacek DW, Stice SL, Hooks SB. Regulation of stem cell pluripotency and differentiation by G protein coupled receptors. Pharmacol Ther. 2011;129:290–306. doi: 10.1016/j.pharmthera.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: biosynthesis, pharmacology, and therapeutic frontiers. Curr Top Med Chem. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 36.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 37.Szefel J, Piotrowska M, Kruszewski WJ, Jankun J, Lysiak-Szydlowska W, Skrzypczak-Jankun E. Eicosanoids in prevention and management of diseases. Curr Mol Med. 2011;11:13–25. doi: 10.2174/156652411794474374. [DOI] [PubMed] [Google Scholar]
- 38.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 39.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 40.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, Zon LI. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logan CM, Giordano A, Puca A, Cassone M. Prostaglandin E2: at the crossroads between stem cell development, inflammation and cancer. Cancer Biol Ther. 2007;6:1517–1520. doi: 10.4161/cbt.6.10.4750. [DOI] [PubMed] [Google Scholar]
- 42.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun SP, Lee MY, Ryu JM, Han HJ. Interaction between PGE2 and EGF receptor through MAPKs in mouse embryonic stem cell proliferation. Cell Mol Life Sci. 2009;66:1603–1616. doi: 10.1007/s00018-009-9076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostenis E. A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther. 2004;102:243–257. doi: 10.1016/j.pharmthera.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Layden BT, Newman M, Chen F, Fisher A, Lowe WL., Jr G protein coupled receptors in embryonic stem cells: a role for Gs-alpha signaling. PLoS One. 2010:e9105. doi: 10.1371/journal.pone.0009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilkenny DM, Rocheleau JV, Price J, Reich MB, Miller GG. c-Src regulation of fibroblast growth factor-induced proliferation in murine embryonic fibroblasts. J Biol Chem. 2003;278:17448–17454. doi: 10.1074/jbc.M209698200. [DOI] [PubMed] [Google Scholar]
- 47.Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, Benton HP, Trauger SA, Desponts C, Ding S, Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chillar A, So SP, Ruan CH, Shelat H, Geng YJ, Ruan KH. A profile of NSAID-targeted arachidonic acid metabolisms in human embryonic stem cells (hESCs): Implication of the negative effects of NSAIDs on heart tissue regeneration. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Xu XQ, Graichen R, Soo SY, Balakrishnan T, Rahmat SN, Sieh S, Tham SC, Freund C, Moore J, Mummery C, Colman A, Zweigerdt R, Davidson BP. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958–970. doi: 10.1111/j.1432-0436.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 50.Finkensieper A, Kieser S, Bekhite MM, Richter M, Mueller JP, Graebner R, Figulla HR, Sauer H, Wartenberg M. The 5-lipoxygenase pathway regulates vasculogenesis in differentiating mouse embryonic stem cells. Cardiovasc Res. 2010;86:37–44. doi: 10.1093/cvr/cvp385. [DOI] [PubMed] [Google Scholar]
- 51.Kim MH, Lee YJ, Kim MO, Kim JS, Han HJ. Effect of leukotriene D4 on mouse embryonic stem cell migration and proliferation: involvement of PI3K/Akt as well as GSK-3beta/beta-catenin signaling pathways. J Cell Biochem. 2010;111:686–698. doi: 10.1002/jcb.22755. [DOI] [PubMed] [Google Scholar]
- 52.Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma D, Zhang M, Larsen CP, Xu F, Hua W, Yamashima T, Mao Y, Zhou L. DHA promotes the neuronal differentiation of rat neural stem cells transfected with GPR40 gene. Brain Res. 2010;1330:1–8. doi: 10.1016/j.brainres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Oudin MJ, Gajendra S, Williams G, Hobbs C, Lalli G, Doherty P. Endocannabinoids regulate the migration of subventricular zone-derived neuroblasts in the postnatal brain. J Neurosci. 2011;31:4000–4011. doi: 10.1523/JNEUROSCI.5483-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galve-Roperh I, Aguado T, Rueda D, Velasco G, Guzman M. Endocannabinoids: a new family of lipid mediators involved in the regulation of neural cell development. Curr Pharm Des. 2006;12:2319–2325. doi: 10.2174/138161206777585139. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi T, Su TP. Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Subcell Biochem. 2010;51:381–398. doi: 10.1007/978-90-481-8622-8_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi T, Fujimoto M. Detergent-resistant microdomains determine the localization of sigma-1 receptors to the endoplasmic reticulum-mitochondria junction. Mol Pharmacol. 2010;77:517–528. doi: 10.1124/mol.109.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramachandran S, Chu UB, Mavlyutov TA, Pal A, Pyne S, Ruoho AE. The sigma1 receptor interacts with N-alkyl amines and endogenous sphingolipids. Eur J Pharmacol. 2009;609:19–26. doi: 10.1016/j.ejphar.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse. 2004;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frebel K, Wiese S. Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans. 2006;34:1287–1290. doi: 10.1042/BST0341287. [DOI] [PubMed] [Google Scholar]
- 62.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 63.Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem. 2007;282:6265–6273. doi: 10.1074/jbc.M610906200. [DOI] [PubMed] [Google Scholar]
- 64.Umehara H, Kimura T, Ohtsuka S, Nakamura T, Kitajima K, Ikawa M, Okabe M, Niwa H, Nakano T. Efficient derivation of embryonic stem cells by inhibition of glycogen synthase kinase-3. Stem Cells. 2007;25:2705–2711. doi: 10.1634/stemcells.2007-0086. [DOI] [PubMed] [Google Scholar]
- 65.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 66.Bhattacharya B, Miura T, Brandenberg R, Mejido J, Luo Y, Yang AX, Joshi BH, Irene G, Thies RS, Amit M, Lyons I, Condie BG, Iskovitz-Eldor J, Rao MS, Puri RK. Gene Expression in Human Embryonic Stem Cell Lines: Unique Molecular Signature. Blood. 2003;103:2956–2964. doi: 10.1182/blood-2003-09-3314. [DOI] [PubMed] [Google Scholar]
- 67.Kelly KF, Ng DY, Jayakumaran G, Wood GA, Koide H, Doble BW. beta-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell. 2011;8:214–227. doi: 10.1016/j.stem.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 69.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 70.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okita K, Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Curr Stem Cell Res Ther. 2006;1:103–111. doi: 10.2174/157488806775269061. [DOI] [PubMed] [Google Scholar]
- 72.Schuringa JJ, van der Schaaf S, Vellenga E, Eggen BJ, Kruijer W. LIF-induced STAT3 signaling in murine versus human embryonal carcinoma (EC) cells. Exp Cell Res. 2002;274:119–129. doi: 10.1006/excr.2001.5454. [DOI] [PubMed] [Google Scholar]
- 73.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 75.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 76.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, Clouthier SG, Wicha MS. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otaegi G, Yusta-Boyo MJ, Vergano-Vera E, Mendez-Gomez HR, Carrera AC, Abad JL, Gonzalez M, de la Rosa EJ, Vicario-Abejon C, de Pablo F. Modulation of the PI 3-kinase-Akt signalling pathway by IGF-I and PTEN regulates the differentiation of neural stem/precursor cells. J Cell Sci. 2006;119:2739–2748. doi: 10.1242/jcs.03012. [DOI] [PubMed] [Google Scholar]
- 78.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A. GSK-3betadependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene. 2000;19:537–545. doi: 10.1038/sj.onc.1203359. [DOI] [PubMed] [Google Scholar]
- 80.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 81.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Li X, Eswarakumar VP, Seger R, Lonai P. Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene. 2000;19:3750–3756. doi: 10.1038/sj.onc.1203726. [DOI] [PubMed] [Google Scholar]
- 83.Chen S, Do JT, Zhang Q, Yao S, Yan F, Peters EC, Scholer HR, Schultz PG, Ding S. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103:17266–17271. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- 85.Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyssiotis CA, Lairson LL, Boitano AE, Wurdak H, Zhu S, Schultz PG. Chemical control of stem cell fate and developmental potential. Angew Chem Int Ed Engl. 2011;50:200–242. doi: 10.1002/anie.201004284. [DOI] [PubMed] [Google Scholar]
- 87.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plo I, Bettaieb A, Payrastre B, Mansat-De Mas V, Bordier C, Rousse A, Kowalski-Chauvel A, Laurent G, Lautier D. The phosphoinositide 3-kinase/Akt pathway is activated by daunorubicin in human acute myeloid leukemia cell lines. FEBS Lett. 1999;452:150–154. doi: 10.1016/s0014-5793(99)00631-6. [DOI] [PubMed] [Google Scholar]
- 89.Buehr M, Smith A. Genesis of embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2003;358:1397–1402. doi: 10.1098/rstb.2003.1327. discussion 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding VM, Ling L, Natarajan S, Yap MG, Cool SM, Choo AB. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J Cell Physiol. 2010;225:417–428. doi: 10.1002/jcp.22214. [DOI] [PubMed] [Google Scholar]
- 91.Zhang P, Andrianakos R, Yang Y, Liu C, Lu W. Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J Biol Chem. 2010;285:9180–9189. doi: 10.1074/jbc.M109.077958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouhon IA, Kato H, Chandran S, Allen ND. Neural differentiation of mouse embryonic stem cells in chemically defined medium. Brain Res Bull. 2005;68:62–75. doi: 10.1016/j.brainresbull.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 93.Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 94.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–356. doi: 10.1002/iub.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao L, Spassieva SD, Jucius TJ, Shultz LD, Shick HE, Macklin WB, Hannun YA, Obeid LM, Ackerman SL. A deficiency of ceramide biosynthesis causes cerebellar purkinje cell neurodegeneration and lipofuscin accumulation. PLoS Genet. 2011:e1002063. doi: 10.1371/journal.pgen.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park H, Haynes CA, Nairn AV, Kulik M, Dalton S, Moremen K, Merrill AH., Jr Transcript profiling and lipidomic analysis of ceramide subspecies in mouse embryonic stem cells and embryoid bodies. J Lipid Res. 2010;51:480–489. doi: 10.1194/jlr.M000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, Erez-Roman R, Ben-David O, Levy M, Holzman D, Park H, Nyska A, Merrill AH, Jr, Futerman AH. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem. 2010;285:10911–10923. doi: 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brugger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH, Jr, Futerman AH. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J Biol Chem. 2010;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imgrund S, Hartmann D, Farwanah H, Eckhardt M, Sandhoff R, Degen J, Gieselmann V, Sandhoff K, Willecke K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. J Lipid Res. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 105.Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. Faseb J. 2008 doi: 10.1096/fj.08-120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Basu S, Bayoumy S, Zhang Y, Lozano J, Kolesnick R. BAD enables ceramide to signal apoptosis via Ras and Raf-1. J Biol Chem. 1998;273:30419–30426. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- 107.Yin X, Zafrullah M, Lee H, Haimovitz-Friedman A, Fuks Z, Kolesnick R. A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cell Physiol Biochem. 2009;24:219–230. doi: 10.1159/000233248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. doi: 10.1074/jbc.275.1.177. [DOI] [PubMed] [Google Scholar]
- 109.Krishnamurthy K, Wang G, Silva J, Condie BG, Bieberich E. Ceramide Regulates Atypical PKC{zeta}/{lambda}-mediated Cell Polarity in Primitive Ectoderm Cells: A NOVEL FUNCTION OF SPHINGOLIPIDS IN MORPHOGENESIS. J Biol Chem. 2007;282:3379–3390. doi: 10.1074/jbc.M607779200. [DOI] [PubMed] [Google Scholar]
- 110.Wang G, Krishnamurthy K, Umapathy NS, Verin AD, Bieberich E. The carboxyl-terminal domain of atypical protein kinase Czeta binds to ceramide and regulates junction formation in epithelial cells. J Biol Chem. 2009;284:14469–14475. doi: 10.1074/jbc.M808909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 112.Lozano J, Berra E, Municio MM, Diaz-Meco MT, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 113.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. Embo J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 115.Fox TE, Houck KL, O'Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 116.Wang YM, Seibenhener ML, Vandenplas ML, Wooten MW. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. J Neurosci Res. 1999;55:293–302. doi: 10.1002/(SICI)1097-4547(19990201)55:3<293::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 117.Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 118.Kovac J, Oster H, Leitges M. Expression of the atypical protein kinase C (aPKC) isoforms iota/lambda and zeta during mouse embryogenesis. Gene Expr Patterns. 2007;7:187–196. doi: 10.1016/j.modgep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 119.Cui S, Otten C, Rohr S, Abdelilah-Seyfried S, Link BA. Analysis of aPKClambda and aPKCzeta reveals multiple and redundant functions during vertebrate retinogenesis. Mol Cell Neurosci. 2007;34:431–444. doi: 10.1016/j.mcn.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koike C, Nishida A, Akimoto K, Nakaya MA, Noda T, Ohno S, Furukawa T. Function of atypical protein kinase C lambda in differentiating photoreceptors is required for proper lamination of mouse retina. J Neurosci. 2005;25:10290–10298. doi: 10.1523/JNEUROSCI.3657-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dutta D, Ray S, Home P, Larson M, Wolfe MW, Paul S. Self Renewal vs. Lineage Commitment of Embryonic Stem Cells: Protein Kinase C Signaling Shifts the Balance. Stem Cells. 2011 doi: 10.1002/stem.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, Cancelas JA. Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci U S A. 2011;108:9957–9962. doi: 10.1073/pnas.1103132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sells SF, Wood DP, Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 124.Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 125.Diaz-Meco MT, Lallena MJ, Monjas A, Frutos S, Moscat J. Inactivation of the inhibitory kappaB protein kinase/nuclear factor kappaB pathway by Par-4 expression potentiates tumor necrosis factor alpha-induced apoptosis. J Biol Chem. 1999;274:19606–19612. doi: 10.1074/jbc.274.28.19606. [DOI] [PubMed] [Google Scholar]
- 126.Bieberich E, MacKinnon S, Silva J, Noggle S, Condie BG. Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide. J Cell Biol. 2003;162:469–479. doi: 10.1083/jcb.200212067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J Biol Chem. 2001;276:44396–44404. doi: 10.1074/jbc.M107239200. [DOI] [PubMed] [Google Scholar]
- 128.Bieberich E, Silva J, Wang G, Krishnamurthy K, Condie BG. Selective apoptosis of pluripotent mouse and human stem cells by novel ceramide analogues prevents teratoma formation and enriches for neural precursors in ES cell-derived neural transplants. J Cell Biol. 2004;167:723–734. doi: 10.1083/jcb.200405144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang G, Silva J, Krishnamurthy K, Bieberich E. A novel isoform of prostate apoptosis response 4 (PAR-4) that co-distributes with F-actin and prevents apoptosis in neural stem cells. Apoptosis. 2006;11:315–325. doi: 10.1007/s10495-006-3979-8. [DOI] [PubMed] [Google Scholar]
- 130.Bieberich E. Lipid vesicle-mediated affinity chromatography using magnetic activated cell sorting (LIMACS): a novel method to analyze protein-lipid interaction. J Vis Exp. 2011 doi: 10.3791/2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Blitterswijk WJ. Hypothesis: ceramide conditionally activates atypical protein kinases C Raf-1 and KSR through binding to their cysteine-rich domains. Biochem J. 1998;331(Pt 2):679–680. [PMC free article] [PubMed] [Google Scholar]
- 132.Cho W, Stahelin RV. Membrane-protein interactions in cell signaling and membrane trafficking. Annu Rev Biophys Biomol Struct. 2005;34:119–151. doi: 10.1146/annurev.biophys.33.110502.133337. [DOI] [PubMed] [Google Scholar]
- 133.Stahelin RV, Wang J, Blatner NR, Rafter JD, Murray D, Cho W. The origin of C1A-C2 interdomain interactions in protein kinase Calpha. J Biol Chem. 2005;280:36452–36463. doi: 10.1074/jbc.M506224200. [DOI] [PubMed] [Google Scholar]
- 134.Gallegos LL, Newton AC. Spatiotemporal dynamics of lipid signaling: protein kinase C as a paradigm. IUBMB Life. 2008;60:782–789. doi: 10.1002/iub.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bieberich E. Ceramide signaling in cancer and stem cells. Future Lipidol. 2008;3:273–300. doi: 10.2217/17460875.3.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E. Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. J Neurochem. 2008;106:718–733. doi: 10.1111/j.1471-4159.2008.05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bieberich E. Ceramide in stem cell differentiation and embryo development: novel functions of a topological cell-signaling lipid and the concept of ceramide compartments. J Lipids. 2011;2011:610306. doi: 10.1155/2011/610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 139.Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 140.Wang G, Krishnamurthy K, Bieberich E. Regulation of primary cilia formation by ceramide. J Lipid Res. 2009;50:2103–2110. doi: 10.1194/jlr.M900097-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, Brakebusch C. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn. 2007;236:2767–2778. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- 142.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 143.Bieberich E. Smart drugs for smarter stem cells: making SENSe (sphingolipid-enhanced neural stem cells) of ceramide. Neurosignals. 2008;16:124–139. doi: 10.1159/000111558. [DOI] [PubMed] [Google Scholar]
- 144.Bieberich E. There is More to a Lipid than just Being a Fat: Sphingolipid-Guided Differentiation of Oligodendroglial Lineage from Embryonic Stem Cells. Neurochem Res. 2010;36:1601–1611. doi: 10.1007/s11064-010-0338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bieberich E. Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J. 2004;21:315–327. doi: 10.1023/B:GLYC.0000046274.35732.47. [DOI] [PubMed] [Google Scholar]
- 146.Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 147.Zhou H, Summers SA, Birnbaum MJ, Pittman RN. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 148.Bieberich E, Hu B, Silva J, MacKinnon S, Yu RK, Fillmore H, Broaddus WC, Ottenbrite RM. Synthesis and characterization of novel ceramide analogs for induction of apoptosis in human cancer cells. Cancer Lett. 2002;181:55–64. doi: 10.1016/s0304-3835(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 149.Herget T, Esdar C, Oehrlein SA, Heinrich M, Schutze S, Maelicke A, van Echten-Deckert G. Production of ceramides causes apoptosis during early neural differentiation in vitro. J Biol Chem. 2000;275:30344–30354. doi: 10.1074/jbc.M000714200. [DOI] [PubMed] [Google Scholar]
- 150.Coelho RP, Saini HS, Sato-Bigbee C. Sphingosine-1-phosphate and oligodendrocytes: from cell development to the treatment of multiple sclerosis. Prostaglandins Other Lipid Mediat. 2010;91:139–144. doi: 10.1016/j.prostaglandins.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 151.Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- 152.Saini HS, Coelho RP, Goparaju SK, Jolly PS, Maceyka M, Spiegel S, Sato-Bigbee C. Novel role of sphingosine kinase 1 as a mediator of neurotrophin-3 action in oligodendrocyte progenitors. J Neurochem. 2005;95:1298–1310. doi: 10.1111/j.1471-4159.2005.03451.x. [DOI] [PubMed] [Google Scholar]
- 153.Dottori M, Leung J, Turnley AM, Pebay A. Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells. Stem Cells. 2008;26:1146–1154. doi: 10.1634/stemcells.2007-1118. [DOI] [PubMed] [Google Scholar]
- 154.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- 157.Bieberich E. Replacement of insulin by LongR3-IGF-1 allows for the differentiation of ES cells into neuroprogenitors and insulin-secreting cells. Anal Biochem. 2005;346:185–187. doi: 10.1016/j.ab.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 158.Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Noble M, Smith J, Power J, Mayer-Proschel M. Redox state as a central modulator of precursor cell function. Ann N Y Acad Sci. 2003;991:251–271. doi: 10.1111/j.1749-6632.2003.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 160.Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- 161.Noble M. Implications for CNS repair of redox modulation of cell survival, division and differentiation. Curr Alzheimer Res. 2006;3:37–47. doi: 10.2174/156720506775697188. [DOI] [PubMed] [Google Scholar]
- 162.Noble M, Proschel C, Mayer-Proschel M. Getting a GRi)P on oligodendrocyte development. Dev Biol. 2004;265:33–52. doi: 10.1016/j.ydbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 163.Calkins MJ, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010;115:557–568. doi: 10.1093/toxsci/kfq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang G, Bieberich E. Prenatal alcohol exposure triggers ceramide-induced apoptosis in neural crest-derived tissues concurrent with defective cranial development. Cell Death Dis. 2010;1:e46. doi: 10.1038/cddis.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Numakawa T, Nakayama H, Suzuki S, Kubo T, Nara F, Numakawa Y, Yokomaku D, Araki T, Ishimoto T, Ogura A, Taguchi T. Nerve growth factor-induced glutamate release is via p75 receptor, ceramide, and Ca(2+) from ryanodine receptor in developing cerebellar neurons. J Biol Chem. 2003;278:41259–41269. doi: 10.1074/jbc.M304409200. [DOI] [PubMed] [Google Scholar]
- 167.Sharma C, Smith T, Li S, Schroepfer GJ, Jr, Needleman DH. Inhibition of Ca2+ release channel (ryanodine receptor) activity by sphingolipid bases: mechanism of action. Chem Phys Lipids. 2000;104:1–11. doi: 10.1016/s0009-3084(99)00106-1. [DOI] [PubMed] [Google Scholar]
- 168.Beech DJ, Bahnasi YM, Dedman AM, Al-Shawaf E. TRPC channel lipid specificity and mechanisms of lipid regulation. Cell Calcium. 2009;45:583–588. doi: 10.1016/j.ceca.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, Obeid LM, Gudz TI. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J Biol Chem. 2011;286:4644–4658. doi: 10.1074/jbc.M110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ledeen R, Wu G. GM1 in the nuclear envelope regulates nuclear calcium through association with a nuclear sodium-calcium exchanger. J Neurochem. 2007;103(Suppl 1):126–134. doi: 10.1111/j.1471-4159.2007.04722.x. [DOI] [PubMed] [Google Scholar]
- 172.Ledeen R, Wu G. New findings on nuclear gangliosides: overview on metabolism and function. J Neurochem. 2011;116:714–720. doi: 10.1111/j.1471-4159.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 173.Wu GS, Vaswani KK, Lu ZH, Ledeen RW. Gangliosides stimulate calcium flux in neuro-2A cells and require exogenous calcium for neuritogenesis. J Neurochem. 1990;55:484–491. doi: 10.1111/j.1471-4159.1990.tb04161.x. [DOI] [PubMed] [Google Scholar]
- 174.Wu G, Ledeen RW. Gangliosides as modulators of neuronal calcium. Prog Brain Res. 1994;101:101–112. doi: 10.1016/s0079-6123(08)61942-1. [DOI] [PubMed] [Google Scholar]
- 175.Wu G, Lu ZH, Ledeen RW. GM1 ganglioside in the nuclear membrane modulates nuclear calcium homeostasis during neurite outgrowth. J Neurochem. 1995;65:1419–1422. doi: 10.1046/j.1471-4159.1995.65031419.x. [DOI] [PubMed] [Google Scholar]
- 176.Xie X, Wu G, Lu ZH, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem. 2002;81:1185–1195. doi: 10.1046/j.1471-4159.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 177.Wu G, Lu ZH, Obukhov AG, Nowycky MC, Ledeen RW. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with alpha5beta1 integrin initiates neurite outgrowth. J Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:10829–10834. doi: 10.1073/pnas.0903408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 180.Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci. 2001;58:2053–2068. doi: 10.1007/PL00000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu RK, Suzuki Y, Yanagisawa M. Membrane glycolipids in stem cells. FEBS Lett. 2010;584:1694–1699. doi: 10.1016/j.febslet.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 183.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Balcarova-Stander J, Pfeiffer SE, Fuller SD, Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. Embo J. 1984;3:2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol. 1987;105:1623–1635. doi: 10.1083/jcb.105.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 188.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 189.Butters TD, Hughes RC. Solubilization and fractionation of glycoproteins and glycolipids of KB cell membranes. Biochem J. 1974;140:469–478. doi: 10.1042/bj1400469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gurd JW, Evans WH, Perkins HR. Chemical characterization of the proteins and glycoproteins of mouse liver plasma membranes solubilized by sequential extraction with aqueous and organic solvents. Biochem J. 1972;126:459–466. doi: 10.1042/bj1260459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barriere H, Chailley B, Chambard M, Selzner JP, Mauchamp J, Gabrion J. Cholesterol-rich microdomains in rat and porcine thyroid membranes involved in TSH-induced endocytotic processes. Acta Anat (Basel) 1988;132:205–215. doi: 10.1159/000146575. [DOI] [PubMed] [Google Scholar]
- 192.Fields RD, Black JA, Waxman SG. Filipin-cholesterol binding in CNS axons prior to myelination: evidence for microheterogeneity in premyelinated axolemma. Brain Res. 1987;404:21–32. doi: 10.1016/0006-8993(87)91351-5. [DOI] [PubMed] [Google Scholar]
- 193.Severs NJ, Robenek H. Detection of microdomains in biomembranes. An appraisal of recent developments in freeze-fracture cytochemistry. 1983;737:373–408. doi: 10.1016/0304-4157(83)90007-2. [DOI] [PubMed] [Google Scholar]
- 194.Bremer EG, Hakomori S. Gangliosides as receptor modulators. Adv Exp Med Biol. 1984;174:381–394. doi: 10.1007/978-1-4684-1200-0_32. [DOI] [PubMed] [Google Scholar]
- 195.Bremer EG, Hakomori S. GM3 ganglioside induces hamster fibroblast growth inhibition in chemically-defined medium: ganglioside may regulate growth factor receptor function. Biochem Biophys Res Commun. 1982;106:711–718. doi: 10.1016/0006-291x(82)91769-7. [DOI] [PubMed] [Google Scholar]
- 196.Hakomori Si SI. The glycosynapse. Proc Natl Acad Sci U S A. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shu L, Lee L, Chang Y, Holzman LB, Edwards CA, Shelden E, Shayman JA. Caveolar structure and protein sorting are maintained in NIH 3T3 cells independent of glycosphingolipid depletion. Arch Biochem Biophys. 2000;373:83–90. doi: 10.1006/abbi.1999.1553. [DOI] [PubMed] [Google Scholar]
- 198.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 199.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 200.Jasmin JF, Yang M, Iacovitti L, Lisanti MP. Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle. 2009;8:3978–3983. doi: 10.4161/cc.8.23.10206. [DOI] [PubMed] [Google Scholar]
- 201.Balbis A, Parmar A, Wang Y, Baquiran G, Posner BI. Compartmentalization of signaling-competent epidermal growth factor receptors in endosomes. Endocrinology. 2007;148:2944–2954. doi: 10.1210/en.2006-1674. [DOI] [PubMed] [Google Scholar]
- 202.Biedi C, Panetta D, Segat D, Cordera R, Maggi D. Specificity of insulin-like growth factor I and insulin on Shc phosphorylation and Grb2 recruitment in caveolae. Endocrinology. 2003;144:5497–5503. doi: 10.1210/en.2003-0417. [DOI] [PubMed] [Google Scholar]
- 203.Bryant MR, Marta CB, Kim FS, Bansal R. Phosphorylation and lipid raft association of fibroblast growth factor receptor-2 in oligodendrocytes. Glia. 2009;57:935–946. doi: 10.1002/glia.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gutierrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30:1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yanagisawa M, Nakamura K, Taga T. Glycosphingolipid synthesis inhibitor represses cytokine-induced activation of the Ras-MAPK pathway in embryonic neural precursor cells. J Biochem. 2005;138:285–291. doi: 10.1093/jb/mvi129. [DOI] [PubMed] [Google Scholar]
- 206.Miljan EA, Bremer EG. Regulation of growth factor receptors by gangliosides. Sci STKE. 2002:RE15. doi: 10.1126/stke.2002.160.re15. [DOI] [PubMed] [Google Scholar]
- 207.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 208.Resh MD. Membrane targeting of lipid modified signal transduction proteins. Subcell Biochem. 2004;37:217–232. doi: 10.1007/978-1-4757-5806-1_6. [DOI] [PubMed] [Google Scholar]
- 209.Roy S, Plowman S, Rotblat B, Prior IA, Muncke C, Grainger S, Parton RG, Henis YI, Kloog Y, Hancock JF. Individual palmitoyl residues serve distinct roles in H-ras trafficking, microlocalization, and signaling. Mol Cell Biol. 2005;25:6722–6733. doi: 10.1128/MCB.25.15.6722-6733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gelineau-van Waes J, Starr L, Maddox J, Aleman F, Voss KA, Wilberding J, Riley RT. Maternal fumonisin exposure and risk for neural tube defects: mechanisms in an in vivo mouse model. Birth Defects Res A Clin Mol Teratol. 2005;73:487–497. doi: 10.1002/bdra.20148. [DOI] [PubMed] [Google Scholar]
- 211.Marasas WF, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, Missmer SA, Cabrera J, Torres O, Gelderblom WC, Allegood J, Martinez C, Maddox J, Miller JD, Starr L, Sullards MC, Roman AV, Voss KA, Wang E, Merrill AH., Jr Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 212.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 213.Gulbins E, Dreschers S, Wilker B, Grassme H. Ceramide, membrane rafts and infections. J Mol Med. 2004;82:357–363. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- 214.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 215.Ngamukote S, Yanagisawa M, Ariga T, Ando S, Yu RK. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J Neurochem. 2007;103:2327–2341. doi: 10.1111/j.1471-4159.2007.04910.x. [DOI] [PubMed] [Google Scholar]
- 216.Suetake K, Liour SS, Tencomnao T, Yu RK. Expression of gangliosides in an immortalized neural progenitor/stem cell line. J Neurosci Res. 2003;74:769–776. doi: 10.1002/jnr.10802. [DOI] [PubMed] [Google Scholar]
- 217.Yu RK. Gangliosides: structure and analysis. Adv Exp Med Biol. 1984;174:39–53. doi: 10.1007/978-1-4684-1200-0_4. [DOI] [PubMed] [Google Scholar]
- 218.Yu RK. Development regulation of ganglioside metabolism. Prog Brain Res. 1994;101:31–44. doi: 10.1016/s0079-6123(08)61938-x. [DOI] [PubMed] [Google Scholar]
- 219.Yu RK, Macala LJ, Farooq M, Sbaschnig-Agler M, Norton WT, Ledeen RW. Ganglioside and lipid composition of bulk-isolated rat and bovine oligodendroglia. J Neurosci Res. 1989;23:136–141. doi: 10.1002/jnr.490230203. [DOI] [PubMed] [Google Scholar]
- 220.Yu RK, Macala LJ, Taki T, Weinfield HM, Yu FS. Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J Neurochem. 1988;50:1825–1829. doi: 10.1111/j.1471-4159.1988.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 221.Yu RK, Nakatani Y, Yanagisawa M. The role of glycosphingolipid metabolism in the developing brain. J Lipid Res. 2009;50(Suppl):S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 2002;1573:406–413. doi: 10.1016/s0304-4165(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 223.Bansal R, Winkler S, Bheddah S. Negative regulation of oligodendrocyte differentiation by galactosphingolipids. J Neurosci. 1999;19:7913–7924. doi: 10.1523/JNEUROSCI.19-18-07913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- 225.Lavie Y, Fiucci G, Czarny M, Liscovitch M. Changes in membrane microdomains and caveolae constituents in multidrug-resistant cancer cells. Lipids. 1999;34(Suppl):S57–S63. doi: 10.1007/BF02562229. [DOI] [PubMed] [Google Scholar]
- 226.Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 227.Spassieva S, Bieberich E. The Gut-To-Breast Connection - Interdependence Of Sterols And Sphingolipids In Multidrug Resistance And Breast Cancer Therapy. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152011797655168. in press. [DOI] [PubMed] [Google Scholar]
- 228.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, Sandhoff R, Sandhoff K, Proia RL. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Inokuchi J, Sugiura Y, Furukawa K. Reduced motor and sensory functions and emotional response in GM3-only mice: emergence from early stage of life and exacerbation with aging. Behav Brain Res. 2009;198:74–82. doi: 10.1016/j.bbr.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 230.Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat. 2002;200:249–258. doi: 10.1046/j.1469-7580.2002.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Enver T, Soneji S, Joshi C, Brown J, Iborra F, Orntoft T, Thykjaer T, Maltby E, Smith K, Abu Dawud R, Jones M, Matin M, Gokhale P, Draper J, Andrews PW. Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum Mol Genet. 2005;14:3129–3140. doi: 10.1093/hmg/ddi345. [DOI] [PubMed] [Google Scholar]
- 232.Fenderson BA, Radin N, Andrews PW. Differentiation antigens of human germ cell tumours: distribution of carbohydrate epitopes on glycolipids and glycoproteins analyzed using PDMP, an inhibitor of glycolipid synthesis. Eur Urol. 1993;23:30–36. doi: 10.1159/000474567. discussion 36–37. [DOI] [PubMed] [Google Scholar]
- 233.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 234.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zeng X, Miura T, Luo Y, Bhattacharya B, Condie B, Chen J, Ginis I, Lyons I, Mejido J, Puri RK, Rao MS, Freed WJ. Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cells. 2004;22:292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]
- 236.Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp Cell Res. 2006;312:2454–2464. doi: 10.1016/j.yexcr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 237.Rathjen J, Lake JA, Bettess MD, Washington JM, Chapman G, Rathjen PD. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112(Pt 5):601–612. doi: 10.1242/jcs.112.5.601. [DOI] [PubMed] [Google Scholar]
- 238.Liour SS, Kraemer SA, Dinkins MB, Su CY, Yanagisawa M, Yu RK. Further characterization of embryonic stem cell-derived radial glial cells. Glia. 2006;53:43–56. doi: 10.1002/glia.20257. [DOI] [PubMed] [Google Scholar]
- 239.Liang YJ, Kuo HH, Lin CH, Chen YY, Yang BC, Cheng YY, Yu AL, Khoo KH, Yu J. Switching of the core structures of glycosphingolipids from globo- and lacto- to ganglio-series upon human embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2010;107:22564–22569. doi: 10.1073/pnas.1007290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Steelant WF, Kawakami Y, Ito A, Handa K, Bruyneel EA, Mareel M, Hakomori S. Monosialyl-Gb5 organized with cSrc and FAK in GEM of human breast carcinoma MCF-7 cells defines their invasive properties. FEBS Lett. 2002;531:93–98. doi: 10.1016/s0014-5793(02)03484-1. [DOI] [PubMed] [Google Scholar]
- 241.Zeng G, Gao L, Freischutz B, Tokuda A, Yu RK. Developmental expression of rat brain GD3-and GT3-synthases. Ann N Y Acad Sci. 1998;845:430. doi: 10.1111/j.1749-6632.1998.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 242.Wu G, Lu ZH, Wang J, Wang Y, Xie X, Meyenhofer MF, Ledeen RW. Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: protection with LIGA 20, a membrane-permeant analog of GM1. J Neurosci. 2005;25:11014–11022. doi: 10.1523/JNEUROSCI.3635-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Xie X, Wu G, Lu ZH, Rohowsky-Kochan C, Ledeen RW. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction. Neurochem Res. 2004;29:2135–2146. doi: 10.1007/s11064-004-6887-8. [DOI] [PubMed] [Google Scholar]
- 244.Yamashita T, Wu YP, Sandhoff R, Werth N, Mizukami H, Ellis JM, Dupree JL, Geyer R, Sandhoff K, Proia RL. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci U S A. 2005;102:2725–2730. doi: 10.1073/pnas.0407785102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chiavegatto S, Sun J, Nelson RJ, Schnaar RL. A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Exp Neurol. 2000;166:227–234. doi: 10.1006/exnr.2000.7504. [DOI] [PubMed] [Google Scholar]
- 246.Dupree JL, Popko B. Genetic dissection of myelin galactolipid function. J Neurocytol. 1999;28:271–279. doi: 10.1023/a:1007049310758. [DOI] [PubMed] [Google Scholar]
- 247.Goldman JE, Hirano M, Yu RK, Seyfried TN. GD3 ganglioside is a glycolipid characteristic of immature neuroectodermal cells. J Neuroimmunol. 1984;7:179–192. doi: 10.1016/s0165-5728(84)80017-x. [DOI] [PubMed] [Google Scholar]
- 248.Nakatani Y, Yanagisawa M, Suzuki Y, Yu RK. Characterization of GD3 ganglioside as a novel biomarker of mouse neural stem cells. Glycobiology. 2010;20:78–86. doi: 10.1093/glycob/cwp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cakir T, Alsan S, Saybasili H, Akin A, Ulgen KO. Reconstruction and flux analysis of coupling between metabolic pathways of astrocytes and neurons: application to cerebral hypoxia. Theor Biol Med Model. 2007;4:48. doi: 10.1186/1742-4682-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pfrieger FW, Ungerer N. Cholesterol metabolism in neurons and astrocytes. Prog Lipid Res. 2011;50:357–371. doi: 10.1016/j.plipres.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 251.Clark WM. Efficacy of citicoline as an acute stroke treatment. Expert Opin Pharmacother. 2009;10:839–846. doi: 10.1517/17460440902835475. [DOI] [PubMed] [Google Scholar]
- 252.Takasaki K, Uchida K, Fujikawa R, Nogami A, Nakamura K, Kawasaki C, Yamaguchi K, Morita M, Morishita K, Kubota K, Katsurabayashi S, Mishima K, Fujiwara M, Iwasaki K. Neuroprotective effects of citidine-5-diphosphocholine on impaired spatial memory in a rat model of cerebrovascular dementia. J Pharmacol Sci. 2011;116:232–237. doi: 10.1254/jphs.11013fp. [DOI] [PubMed] [Google Scholar]