Abstract
Potocki-Lupski syndrome (PTLS; OMIM 610883) is a genomic syndrome that arises as a result of a duplication of 17p11.2. Although numerous cases of individuals with PTLS have been presented in the literature, its behavioral characterization is still ambiguous. We present a male child with a de novo dup(17)(p11.2p11.2) and he does not possess any autistic features, but is characterized by severe speech and language impairment. In the context of the analyses of this patient and other cases of PTLS, we argue that the central feature of the syndrome appears to be related to diminished speech and language capacity, rather than the specific social deficits central to autism.
Keywords: Language and speech impairment; Potocki-Lupski syndrome; 17p11.2, EFCBP1; inv(8)(q21.3-q24.1)
Introduction
Potocki-Lupski syndrome—arising as a result of a duplication of 17p11.2—has been associated with a wide range of congenital anomalies such as ophthalmic, cardiovascular, orthopedic, oral-pharyngeal, and renal abnormalities, microcephaly, distinct facial features, including pronounced nose, ears, and forehead and geometrical (triangular or square) faces, and a number of cognitive and behavioral indicators indicative of developmental delay. Intellectual functioning in described cases ranges from borderline to severe intellectual disability. Similarly, the range of behaviors is broad, with some reports citing autism-spectrum disorder (ASD) behaviors, obsessive-compulsive behaviors, hyperactivity and aggression the presence of some or all such behaviors. Arguably, the most common features of patients with PTLS are feeding difficulties and failure to thrive in infancy, and speech and language impairments [1, 2]. Here we report on a male child with de novo maternally inherited dup(17)(p11.2p11.2) identified by the Human 1M-Duo Bead array (Illumina). This patient has severe language difficulties without pronounced intellectual disability, autistic features or evidence of structural brain abnormalities.
Clinical Report
The patient was referred to the Child Study Center of Yale Medical School at 10:10 years of age. During an otherwise uneventful pregnancy, amniocentesis performed at 16 weeks indicated a paracentric inversion on the long arm of chromosome 8, which is also present in the patient’s father and paternal grandmother, both of whom are high-functioning individuals. The patient was a full-term baby boy, 2,890 grams at birth, delivered via an uncomplicated vaginal delivery. His APGAR scores were 7 at one minute, and 9 at five minutes. Immediately after birth, the patient reportedly had difficulty learning to nurse but readily drank from a dosing cup. Within four to five days he began nursing and was eventually weaned at 10 months. Although motor milestones (sitting independently at 9 months, standing at 12, walking at 13–15) were all met at the late end of normal limits, his fine motor skills remained challenged for a while. The patient’s first words (i.e., “moo,” “maa”) appeared at 18 months, but he did not say his first real words until 3 years of age, and did not start generating simple 3–5 word phrases until 4. Reportedly, his early speech was “very difficult to understand”. At the time of evaluation, a consistent misarticulation of the /r/ phoneme was observed. An oral-mechanism examination ruled out the presence of gross anomalies of oral structures and functioning, although several mild to moderate deviations were observed. The patient could follow spoken directions if they were repeated, which was especially necessary for multi-step directions. He began speech therapy at 2:8 years, which was reportedly “initially unproductive;” he was then taught sign language at 2:10 and started to sign as soon as he was exposed to it. Multiple developmental and psychological evaluations were performed. Table summarizes past diagnoses and the results of psychometric assessments (a range of percentiles is shown for a variety of assessments). The patient attended a regular public school in the USA, but received special education accommodations. His cognitive performance was remarkably uneven, ranging from extremely low to average; specifically his performance was consistently low in the verbal domain and higher in the visual-spatial domain. The patient had learned how to read and write and his mathematic skills were in the average range. Both the receptive and expressive skills of the patient remained low or extremely low, regardless of the intensive therapies he received. There is no indication of aggression, lack of sociability (or any autism-related features), or obsessive-compulsive behavior had been registered. Functional MRI studies (Supplementary Method and Figures) did not detect any pronounced structural or functional brain abnormalities, with the exception of, compared to his typical peers, the relatively low activation levels in the left transverse temporal gyrus (for speech perception tasks) and relatively high activation levels in the left fusiform gyrus (Supplementary Method and Figures).
Table.
The patient’s phenotype across ages and multiple domains
| Assessments and Diagnoses | Age | |||||
|---|---|---|---|---|---|---|
| 4:9 | 5:7 | 6:4 | 8:7 | 10:10 | 11:5 | |
| %-ile2 | %-ile | %-ile | ||||
| Diagnoses1 | E,D, | SLD | RD, | |||
| DD | DWE, | |||||
| LD, | ||||||
| ELD | ||||||
| Intellectual Functioning | 1–50 | 2–37 | 1–18 | |||
| Adaptive Functioning | 6–53 | |||||
| Speech and Language | 1–6 | 1–6 | 1–6 | |||
| Academic Functioning | 1–16 | 1–32 | ||||
Notes:
E = Encephalopathy, unspecified; D = Dyspraxia or lack of coordination; DD = Developmental Delay (delayed developmental milestones); SLD = Speech and Language Disability; RD = Reading Disorder; DWE = Disorder of Written Expression, LD= Learning Disorder Not Otherwise Specified (NOS); ELD = Expressive Language Disorder
Range of percentiles on a wide variety of psychological and psychoeducational assessments. As for Standard Scores (SS) for the patients two evaluations at Yale, they were 80 and 75 at the 9th and 5th percentile ranks on fluid and crystallized indexes, respectively, of the Kaufman Assessment Battery for Children, Second Edition. For reading, SS were 71, 67, and 68 at the 2nd 1st, and 2nd %-ile on the Word Recognition, Pseudoword Decoding, and Reading Comprehension subtests of the Kaufman Test of Educational Achievement. For writing, SS were 75 and 63 at the 5th and 1st %-ile on the Spelling and Written Expression subtests of the Kaufman Test of Educational Achievement. For mathematic skills SS were 93 and 82 at the 32nd and 12th %-ile on Computation and Concepts and Application subtests of the Kaufman Test of Educational Achievement.
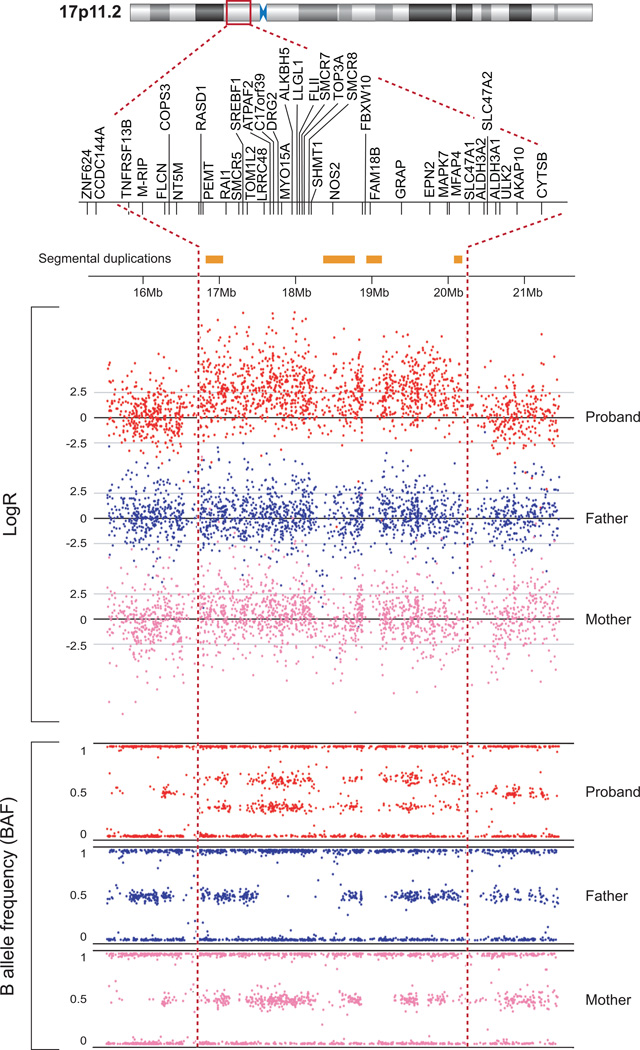
Figure.
Figure shows duplication at 17p11.2 at positions 16,497,803 and 20,292,768 bp of build NCBI36/hg18. The increase in the log R ratio values (log R ratio zero represents diploid copy number and increased log2 ratios represents duplicated regions) and the split in the B allele frequencies (BAF; allelic composition) plotted for each SNP.
Discussion
We presented here a case of PTLS which, both behaviorally and in terms of the specifics of his brain functioning, does not fit the categorization of ASD and permits the formulation of the hypothesis that the central feature of PTLS is in language (not social) dysfunction. Although it has been reported that 80% of PTLS patients show some autistic features [2, 3], there are reports of individuals with PTLS who demonstrate none of these features [1, 4]. Whereas the centrality of autism-associated features for the phenotype of PTLS has been questioned, what has not been questioned is the presence of speech and language impairments in this genomic disorder. However, the types and degrees of these impairments have been neither qualified nor quantified precisely. With the exception of a recent publication that details the behavioral phenotypes of 15 individuals with PTLS [3], the majority of existing reports provide only very general, clinical accounts of the speech and language challenges encountered by individuals with this syndrome. Of critical importance is that although the extent of intellectual disability and social functioning appears to be quite variable across patients with the syndrome, speech and language impairment so far emerges as a consistent finding. Moreover, there are reports of cases in which general cognitive functioning, and especially nonverbal functioning, is higher than the levels of speech and language functioning, suggesting that these difficulties in PTLS are not simply a consequence of global intellectual delay. Also of interest are reports of prominent sucking/feeding difficulties in individuals with PTLS; there is evidence connecting such difficulties, regardless of their etiologies, with speech and language disorders [5, 6].
In addition to the 17p11.2 duplication, the patient was known to carry a paracentric inversion of 8q21.3-q24.1 (Supplementary Method) and same rearrangement had previously been confirmed in both the unaffected father and the paternal grandmother. Fine mapping using FISH showed that the inversion disrupts the gene, EFCB1P/NECAB1 (EF hand calcium binding protein 1) [7] and syntrophin beta 1 (SNTB1), a dystrophin-associated protein [8]. The EFCBP1 has no expression in peripheral lymphocytes; this prevented a direct quantitative assessment of EFCBP1 mRNA in the patient. We indicated no change in the expression levels of SNTB1 in the proband. Nonetheless we cannot rule out the contribution of these genes to the phenotype without measuring their expression in the brain cells.
Phenotypic variability appears to be quite common in genomic syndromes, and a variety of hypotheses, ranging from a two hit model [9], to occult compound heterozygous mutations within deleted regions [10], to epigenetic effects and environmental contributors, are all of intense interest with regard to understanding the complex genotype-phenotype relationships we observed. This manuscript contributes to the field’s understanding of non-linear and complex relationships between the genome and behavior.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greco D, Romano C, Reitano S, Barone C, Benedetto DD, Castiglia L, et al. Three new patients with dup(17)(p11.2p11.2) without autism. Clin Genet. 2008;73:294–296. doi: 10.1111/j.1399-0004.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 2.Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, et al. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- 4.Treadwell-Deering DE, Powell MP, Potocki L. Cognitive and behavioral characterization of the Potocki-Lupski syndrome (duplication 17p11.2) J Dev Behav Pediatr. 2010;31:137–143. doi: 10.1097/DBP.0b013e3181cda67e. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- 6.McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Semin Speech Lang. 2006;27:300–309. doi: 10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- 7.Wu H, Li D, Shan Y, Wan B, Hexige S, Guo J, et al. EFCBP1/NECAB1, a brain-specifically expressed gene with highest abundance in temporal lobe, encodes a protein containing EF-hand and antibiotic biosynthesis monooxygenase domains. DNA Seq. 2007;18:73–79. doi: 10.1080/10425170500511271. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizawa K, Inaba K, Mannen H, Kikuchi T, Mizutani M, Tsuji S. Analyses of beta-1 syntrophin, syndecan 2 and gem GTPase as candidates for chicken muscular dystrophy. Exp Anim. 2003;52:391–386. doi: 10.1538/expanim.52.391. [DOI] [PubMed] [Google Scholar]
- 9.Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, Itsara A, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–209. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan WM, Traboulsi EI, Arthur B, Friedman N, Andrews C, Engle EC. Horizontal gaze palsy with progressive scoliosis can result from compound heterozygous mutations in ROBO3. J Med Genet. 2006;43:e11. doi: 10.1136/jmg.2005.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.