Abstract
Background
Structural brain abnormalities are consistently found in schizophrenia (Sz) and have been associated with the familial risk for the disorder. We aim to define the relative contributions of genetic and nongenetic factors to the association between structural brain abnormalities and Sz in a uniquely powered cohort (Schizophrenia Twins and Relatives consortium).
Methods
An international multicenter magnetic resonance imaging collaboration was set up to pool magnetic resonance imaging scans from twin pairs in Utrecht (The Netherlands), Helsinki (Finland), London (United Kingdom), and Jena (Germany). A sample of 684 subjects took part, consisting of monozygotic twins (n = 410, with 51 patients from concordant and 52 from discordant pairs) and dizygotic twins (n = 274, with 39 patients from discordant pairs). The additive genetic, common, and unique environmental contributions to the association between brain volumes and risk for Sz were estimated by structural equation modeling.
Results
The heritabilities of most brain volumes were significant and ranged between 52% (temporal cortical gray matter) and 76% (cerebrum). Heritability of cerebral gray matter did not reach significance (34%). Significant phenotypic correlations were found between Sz and reduced volumes of the cerebrum (−.22 [−.30/−.14]) and white matter (−.17 [−.25/−.09]) and increased volume of the third ventricle (.18 [.08/.28]). These were predominantly due to overlapping genetic effects (77%, 94%, and 83%, respectively).
Conclusions
Some of the genes that transmit the risk for Sz also influence cerebral (white matter) volume.
Key Words: Brain volumes, multicenter, phenotypic correlation, schizophrenia, sMRI, twins
Although the causes of schizophrenia (Sz) are not completely understood, the importance of genetic factors has been firmly established by family, twin, and adoption studies. There is evidence for a substantial genetic contribution to the etiology of Sz, with heritability estimates up to 85% (1,2). Over the last decade a large number of linkage and (genome-wide) association studies have been carried out (3–5). The genes thus far identified only explain a small part of the genetic risk for Sz, although many seem to be involved in neurodevelopmental processes (6–8). This suggests that some of the genetic risk leading to the development of Sz is expressed as abnormal brain development.
In fact, abnormal brain structure is one of the most robust biological features of Sz (9). Volume loss in the prefrontal lobes, thalamus, superior temporal cortex, and hippocampus have consistently been demonstrated with in vivo and postmortem approaches (10). It is well-known that brain volume (BV) is highly heritable (11,12) and that the brain abnormalities found in Sz cosegregate with the illness within families (13,14). Furthermore, twin probands have smaller whole BVs than their nonaffected co-twins, who in turn have smaller brains than healthy twins (15–17). Hulshoff Pol et al. (18) showed that smaller white matter volume reflects the expression of genetic risk, whereas less gray matter is related to environmental risk factors. Thus, family and twin studies to date suggest that some of the brain abnormalities in Sz can be attributed to the genes conferring risk for this disorder. Although several twin-studies have tried to quantify the relative contribution of genetic and nongenetic factors to these brain abnormalities, these studies have all been small (due to recruitment challenges) and lacked power, leading to unreliable estimates. Furthermore, for a phenotype or endophenotype to be useful in the search for disease-related genes, it should not only be heritable but also share genetic variance with the risk for the disorder. Few studies have tested for such pleiotropic effects. The STAR (Schizophrenia Twins and Relatives) consortium was established to address these methodological problems, combining brain imaging data from most of the available twin samples with Sz.
Although the need to pool twin samples to gain sufficient statistical power is not disputed, it is a great challenge to combine structural magnetic resonance imaging (MRI) data, given the variability of magnetic resonance scanners and acquisition protocols between sites. The ADNI (Alzheimer's Disease Neuroimaging Initiative) (19) and BIRN (Biomedical Informatics Research Network) studies (20,21) developed useful recommendations on how best to conduct multicenter imaging protocols; however, in the present multicenter study, tuning of the MRI acquisition protocols was not possible because scans of the twin pairs were already acquired. Therefore, a group of calibration subjects was scanned at all participating research sites, with the same acquisition protocols the twins were scanned with. The image processing pipeline algorithm was optimized by tuning two calibration factors that separated gray matter, white matter, and cerebrospinal fluid. This resulted in comparable between-site volumes for whole brain, cerebral gray and white matter volume, cerebellum, and third and lateral ventricles across most sites (22) (see also Methods and Materials). Consequently, we are confident that using the calibration factors from this study in the processing of the MRI twin data resulted in a reliable and unique powered sample. We investigated the relative contribution of genetic and environmental influences on the association between Sz liability and BV.
Methods and Materials
Subjects
The STAR consortium pooled all twin samples with MRI brain scans, collected at the Institute of Psychiatry, London (United Kingdom), University of Helsinki, Helsinki (Finland, in collaboration with University of California, Los Angeles), University of Jena, Jena (Germany), Universitätskliniek Heidelberg, Heidelberg (Germany), and the University Medical Center Utrecht, Utrecht (UMCU, The Netherlands), to increase power for variance component analyses. All available MRI scans were collated within the Department of Psychiatry at the UMCU and processed with our processing pipeline (see following). Previously a calibration and data compatibility study concluded that the Heidelberg scans could not be reliably pooled with the other sites, due to low interscanner reliability (intraclass coefficients) of the gray and white matter volumes (22).
High-quality MRI scans were available for 684 individuals, including 142 patients with Sz and 542 unaffected individuals (co-twins and control twins). Mean age in the total sample was 37.97 years (SD = 11.31). Table 1 lists the numbers of included individuals as a function of disease state, site, age, and gender.
Table 1.
Number of Twins, Mean Age, and Gender Distribution of the Samples/Site
| Helsinki |
Jena |
London |
Utrecht |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (yrs) | Gender (F/M) | n | Age (yrs) | Gender (F/M) | n | Age (yrs) | Gender (F/M) | n | Age (yrs) | Gender (F/M) | |
| MZ-Conc | 13 | 42.31 (8.44) | 6/7 | 38 | 35.26 (9.13) | 9/29 | ||||||
| MZ-Disc Pt | 14 | 47.93 (3.63) | 8/6 | 11 | 34.46 (11.07) | 6/5 | 14 | 30.71 (10.86) | 4/10 | 13 | 37.52 (11.18) | 6/7 |
| Co-Twin | 15 | 48.20 (5.36) | 9/6 | 11 | 34.45 (11.07) | 6/5 | 17 | 32.51 (12.92) | 7/10 | 13 | 37.55 (11.15) | 6/7 |
| MZ-HC | 48 | 47.77 (3.48) | 22/26 | 16 | 34.98 (12.62) | 8/8 | 53 | 36.43 (10.13) | 20/33 | 134 | 33.99 (10.93) | 66/68 |
| DZ-Disc Pt | 23 | 47.43 (5.03) | 11/12 | 3 | 43.88 (17.24) | 2/1 | 13 | 35.38 (11.04) | 6/7 | |||
| Co-Twin | 23 | 48.30 (5.57) | 12/11 | 3 | 43.88 (17.24) | 2/1 | 13 | 35.23 (10.58) | 6/7 | |||
| DZ-HC | 50 | 49.32 (4.68) | 29/21 | 4 | 37.00 (13.86) | 0/4 | 142 | 32.66 (10.35) | 81/61 | |||
N = 684. Age given in years (SD).
Conc, concordant; Disc, discordant; DZ, dizygotic; F, female; HC, healthy control subjects; M, male; MZ, monozygotic; Pt, patient.
Information on recruitment and psychiatric assessment for each site is described in Supplement 1.
MRI Processing
Scans were acquired on Philips (Utrecht, The Netherlands; Jena, Germany), GE (London, United Kingdom), and Siemens (Munich, Germany) systems. Differences in scan acquisition between sites concerned, among others, scan orientation (coronal or sagittal) and voxel dimensions (see Table S1 in Supplement 1). The London twins were scanned at St. Georges Hospital (n = 78) and at the Maudsley hospital (n = 54) on identical 1.5-T GE Signa scanners with slightly different acquisition protocols (16). Six twins were scanned on both scanners. Intraclass correlation coefficient (ICC) estimates ranged from .84 (cerebral gray matter) to .95 (lateral ventricles).
Image processing of the brain scans from Utrecht, London, and Jena was done on the neuroimaging computer network of the Department of Psychiatry in Utrecht. The reproducibility of the segmentation process on scans from the Utrecht scanner was established with ICC (23) and were .96 or higher for all structures (24–26). The T1-weighted images were first put into Talairach orientation (no scaling) (27). If a T2-weighted image was available (Utrecht and London), an intracranial volume was automatically segmented from this image. After registration to the T1-weighted image with a mutual information maximization algorithm (28), this segment served as a mask for further segmentation steps. If no T2-weighted image was available, an intracranial mask was manually segmented from the T1-weighted image.
The T1-weighted images were corrected for scanner radiofrequency-field nonuniformity (29). Total brain segmentations were done automatically, with mathematical morphology operations, on the basis of thresholds obtained from the steepest slope of the gray matter peak in intensity histograms of the intracranial region (i.e., the cerebrospinal fluid/gray matter separation threshold is the position of the steepest slope multiplied by a calibrated factor [.73 for scans from all sites]). This has been validated before (22,25).
Cerebellum and lateral and third ventricular segmentations were carried out semi-automatically on the basis of histogram analyses followed by mathematical morphology operations on the T1-weighted image. Anatomic knowledge-based selection principles were used for these segmentations. All segments were checked and manually corrected if necessary.
Separation of cerebral gray and white matter was done by applying a single threshold to the voxels of the total brain in the T1-weighted image. For each image, the threshold was obtained automatically from the T1-weighted intensity histogram (25). We have previously shown (25) that a scaling factor has to be calibrated, because of the dependence of the shapes of the gray and white matter distributions on the acquisition parameters. It was calculated for Utrecht scans on the basis of a comparison in 80 scans, where the gray/white separation had been determined manually twice by three raters (fgw = .960). For scans from London and Jena, the threshold factors for gray/white separation were optimized (22) (London: fgw = .980; Jena: fgw = .970).
To obtain the cerebral white matter segmentation of the image, a selection of all voxels in the cerebrum with intensities above the calibrated threshold was made. The gray matter segment was calculated from the difference between the cerebrum segment and cerebral white matter segment. Absolute volumes were estimated from the product of the number of voxels in each segment and the voxel volume.
In addition, frontal, parietal, temporal, and occipital lobes were manually demarcated on a model brain (30) that was selected earlier among 200 brain images of subjects between 16 and 70 years of age (31). Brain images were registered to the model brain through the Automatic Non-linear Image Matching and Anatomical Labeling (ANIMAL) algorithm (32) to remove global differences in size and shape of the individual brains. The inverse of the transformation process registered the manual segmentations of the model brain to the brain images of all subjects. The gray matter segments from the individual brain images were used to identify cortical gray matter for each individual lobe.
The Helsinki images were processed with a processing pipeline at the University of California at Los Angeles. In brief, a semi-automated method based on Montreal Neurological Institute tools was used to create brain-only masks, and trained operators subsequently outlined the brain parenchyma on each section, eliminating pixels corresponding to the skull and meninges. A radiofrequency bias field correction algorithm eliminated intensity drifts due to scanner field inhomogeneity (29). The Functional MRI of the Brain's Automated Segmentation Tool (FAST) (33) was used to classify tissue types into gray matter, white matter, and cerebrospinal fluid.
Because a calibration dataset was available from four subjects scanned in Helsinki and Utrecht (i.e., eight scans in total), we were able to estimate the reliability of pooling data from the two processing procedures. All eight scans were processed with both procedures, and ICCs were measured for cerebral (gray and white matter) volumes. These were all >.99 after including site as a covariate. Therefore, cerebral (gray and white matter) volumes from the Helsinki twin sample were included in the analysis.
Statistical Analysis
Polychoric Correlations
To analyze the Sz data, a liability threshold model was applied, which assumes that disease risk is distributed normally (with a variance of one and mean of zero) (34). A threshold separates the distribution into affected and unaffected individuals. The liability distribution is unobserved, and its variance can be attributed to genetic and nongenetic factors. Resemblance between twins on the liability distribution is summarized with tetrachoric (dichotomous traits) or polychoric correlations (traits with > 1 threshold). The twin data were analyzed as a function of zygosity (monozygotic [MZ] and dizygotic [DZ]) with Sz status (yes/no) and BVs as outcome variables. Brain volumes were categorized into five classes (with roughly equal numbers/class) after regressing out the effects of one site (i.e., Helsinki, because of the different processing procedure), gender, age, and intracranial volume.
Polychoric (twin) correlations between the underlying liabilities for Sz and BV were estimated as a function of zygosity. The correlations estimated are: cross-trait within-member (Szm1–BVm1), within-trait cross-members in pairs of MZ twins or DZ twins (Szm1–Szm2 or BVm1–BVm2), cross-trait cross-member in pairs of MZ twins or DZ twins (Szm1–BVm2 or BVm1–Szm2). Several constraints were imposed. The cross-trait within-member correlations were constrained to be equal across all individuals in the sample, yielding only one Sz–BV correlation. The cross-trait cross-member correlations were constrained to be equal within the MZ twin and DZ twin group separately, such that Szm1–BVm2 = Szm2–BVm1 (Table 2). The thresholds for BVs were constrained to be equal across groups.
Table 2.
Polychoric Correlations Within-Member Cross-Trait, Cross-Member Within-Trait, and Cross-Member Cross-Trait for Sz and MRI BVs
| BVs | Within-Member Cross-Trait (Szm1– BVm1 = Szm2 – BVm2) Whole Sample | Cross-Member Within-Trait (BVm1 – BVm2) |
Cross-Member Cross-Trait (Szm1 – BVm2 = Szm2 – BVm1) |
||
|---|---|---|---|---|---|
| MZ Pairs | DZ Pairs | MZ Pairs | DZ Pairs | ||
| Cerebrum | −.22 (−.30 to −.14)a | .77 (.69 to .82)a | .25 (.04 to .44)a | −.17 (−.25 to −.09)a | −.09 (−.22 to .04) |
| Cerebral Gray | −.08 (−.11 to .00) | .57 (.45 to .66)a | .40 (.20 to .56)a | −.02 (−.10 to .07) | .07 (−.06 to .20) |
| Cerebral White | −.17 (−.25 to −.09)a | .73 (.65 to .80)a | .41 (.23 to .55)a | −.16 (−.24 to −.07)a | −.14 (−.26 to −.01)a |
| Lateral Ventricles | .10 (.00 to .10) | .73 (.63 to .80)a | .37 (.13 to .56)a | .04 (−.07 to .14) | .00 (−.19 to .17) |
| 3rd Ventricle | .19 (.09 to .29)a | .75 (.67 to .82)a | .40 (.16 to .59)a | .16 (.05 to .26)a | .18 (.00 to .35) |
| Frontal Cortical Gray | −.03 (−.13 to .06) | .56 (.43 to .67)a | .34 (.10 to .54)a | .07 (−.03 to .17) | −.07 (−.12 to .25) |
| Temporal Cortical Gray | −.04 (−.14 to .06) | .56 (.42 to .67)a | .23 (−.01 to .43) | .02 (−.03 to .12) | .11 (−.09 to .29) |
| Parietal Cortical Gray | .03 (−.07 to .13) | .68 (.56 to .76)a | .32a (.10 to .51) | .07 (−.03 to .17) | .05 (−.13 to .23) |
| Occipital Cortical Gray | .02 (−.09 to .02) | .65 (.53 to .74)a | .52 (.31 to .67)a | .03 (−.08 to .07) | .20 (.02 to .36)a |
Values given are n (95% confidence interval), estimated from the combined sample of MZ and DZ twins. The cross-member within-trait correlation for Sz (Szm1-Szm2) is constrained to be .92 in MZ pairs and .515 in DZ pairs; the threshold fixed to give a 1% prevalence. The within-member cross-trait correlations are constrained such that Szm1-BVm1 = Szm2-BVm2, and the cross-member cross-trait correlations are constrained such that Szm1-BVm2 = Szm2-BVm1.
BV, brain volume; MRI, magnetic resonance imaging; subscript m, member of a pair; other abbreviations as in Table 1.
Confidence intervals not including zero indicate significance.
Interpretation of Correlations
Significant cross-trait within-member correlations might imply common etiological influences. The power to distinguish between different factors (e.g., genetic or environmental) causing the correlation is derived from the MZ and DZ cross-trait cross-member correlations of the MZ twin pairs and DZ twin pairs. Significant cross-trait cross-member correlations (e.g., Szm1–BVm2) imply that these common etiological influences are familial. Whether these familial influences are genetic or environmental in origin is indicated by the MZ/DZ ratio of these correlations. A 2:1 ratio is indicative of additive genetic effects, whereas a 1:1 ratio suggests the influences of (shared) common environment in inducing a correlation between the traits. Nonsignificant cross-trait cross-member correlations imply that the etiological influences on Sz and BV are due to specific unique environmental effects (for background information see Neale et al. [35]).
Genetic Model Fitting
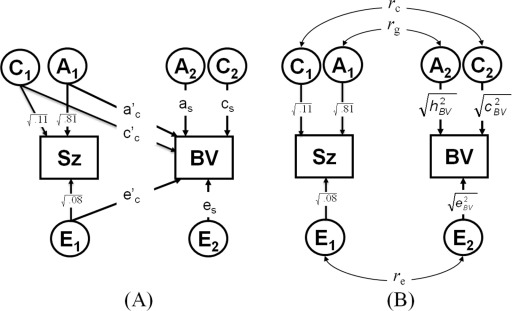
The aim of this study is to examine the extent of the correlation between Sz and BVs due to genetic overlap or to common environmental effects. Mx Statistical Modeling (36) was used for maximum likelihood genetic model fitting to directly estimate model parameters from the observed data. The genetic bivariate model is illustrated in Figure 1A. Additive genetic effects (A), common environmental (C) and unique environmental effects—including measurement error (E)—are specified such that factors A1, C1, and E1 influence both Sz (path ac, cc, ec) and BV (path a‘c, c‘c, e‘c), inducing a familial covariance that is either genetic (ac* a'c) or environmental (cc * c'c) and an individual specific environmental covariance (ec * e'c). Factors A2, C2, and E2 are specific to BV (as, cs, es). If there is no relevance in the specific order of the variables, the solution of this Cholesky decomposition can be standardized in a correlated-factors model (Figure 1B), where for example the paths from A1 to Sz and A2 to BV are the square roots of their heritabilities, and where the correlational path between A1 and A2 is the genetic correlation (rg). The part of the phenotypic correlation (rph), due to genetic effects can then be calculated by (√ h2SZ * rg * √ h2BV), the part due to C by (√ c2SZ * rc * √ c2BV) and the part due to E by (√ e2SZ * re * √ e2BV).
Figure 1.
(A) The applied bivariate model. Additive genetic effects: A; common environmental: C; and unique environmental effects: E are specified such that factors A1, C1, and E1 influence both schizophrenia (Sz; path ac, cc, ec) and brain volume (BV; path a'c, c'c, e'c), inducing a familial covariance that is either genetic (ac* a'c) or environmental (cc * c'c) and an individual specific environmental covariance (ec * e'c). Factors A2, C2, and E2 are specific to BV (as, cs, es). (B) The standardized correlated-factors solution, where the paths are the square roots of the heritabilities (h2), c2, and e2 and the double-headed arrows are the genetic c and e correlations (rg, rc,re). The proportion of the phenotypic correlation (rph) due to A effects can then be calculated by (√ h2SZ * rg * √ h2BV), the part due to C effects by (√ c2SZ * rc * √ c2BV), and the part due to E effects by (√ e2SZ * re * √ e2BV). For ascertainment correction, the fixed model parameters for schizophrenia are: h2 = .81, c2 = .11, e2 = .08.
Ascertainment Correction
The data are from twin pairs selected on the basis of the presence or absence of Sz rather than from a random population sample, requiring a correction for ascertainment. We corrected for ascertainment by constraining the genetic model parameters for Sz to constant values: point estimates (h2 = .81, c2 = .11, e2 = .08) from a recent meta-analysis (37) and the liability threshold to give a prevalence consistent with a lifetime risk of 1%. In contrast, the model parameters for brain structure (including liability thresholds) as well as the relationship with Sz are free parameters to be estimated from the data (Figure 1). This correction in the polychoric correlation scripts entails fixing the MZ and DZ/sib correlations for Sz to h2+c2 = .92 and to .5h2 + c2 = .515, respectively. This model has been described elsewhere in more detail (17) and applied to other Sz studies (38–40).
Results
Polychoric Correlations
Correlations between the underlying liabilities for Sz and each BV are presented in Table 2. First, the ratios of the MZ and DZ twin correlation indicate a significant contribution of genetic effects to the observed variation for each of the BVs (Figure 2). Second, significant cross-trait within-member correlations (column 1) were present for: 1) reduced cerebral and cerebral white matter volume, and 2) increased third ventricle volume. The significant MZ and DZ cross-member cross-trait correlations for each volume do support a genetically mediated association with risk for Sz. This is formally tested in the genetic model-fitting analyses.
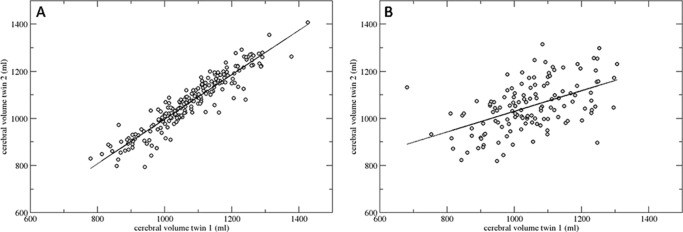
Figure 2.
Correlation between cerebral volume of Twin 1 with Twin 2 for (A) all monozygotic (n = 196) and (B) all dizygotic (n = 131) twin pairs, irrespective of illness.
Genetic Model Fitting
The results from the ACE model-fitting are presented in Table 3. Most of the BVs measured were significantly heritable and ranged between 52% (temporal cortical gray matter) and 76% (total cerebral volume). Heritability of cerebral gray matter (in particular, cortical frontal and occipital: 43% and 21%, respectively) did not reach significance (34%). Familial (common) environmental influences (c2) on the variation in each of the volumes were nonsignificant, except for cortical occipital gray matter volume (43%). The genetic (rg), shared-environmental (rc) and unique-environmental correlations (re) indicate that the source of the significant phenotypic overlap between risk for Sz and cerebral volume decrease was due to genetic and unique-environmental factors (including correlated measurement error). For gray matter (in particular, frontal and temporal cortex) volume and lateral ventricle volume, these were all due to nonfamilial factors (i.e., correlated unique-environmental factors).
Table 3.
Standardized Estimates of the Full Bivariate ACE Genetic Models for Sz and Each MRI BV
| h2BV | c2BV | e2BV | rg | rc | re | rph | |
|---|---|---|---|---|---|---|---|
| Cerebrum | .76 (.57/.82)a | .00 (0/.18) | .24 (.18/.32)a | −.21 (−.42/−.02)a | −.99 (−1/1) | −.37 (−.57/−.14)a | −.22 (−.30/−.14)a |
| Cerebral Gray | .34 (.00/.63) | .23 (0/.53) | .43 (.34/.55)a | −.33 (−.1/.16) | 1 (−1/1) | −.36 (−.55/−.15)a | −.08 (−.16/.00) |
| Cerebral White | .63 (.32/.79)a | .10 (0/.38) | .27 (.21/.36)a | −.08 (−.41/.13) | −.97 (−1/1) | −.09 (−.31/.15) | −.17 (−.25/−.09)a |
| Lateral Ventricles | .71 (.31/.80)a | .02 (0/.39) | .27 (.20/.37)a | .11 (−.27/.47) | −.99 (−1/1) | .41 (.13/.65)a | .10 (0/.19) |
| 3rd Ventricle | .64 (.29/.81)a | .11 (0/.40) | .25 (.18/.33)a | −.06 (−.21/.43) | 1 (−1/1) | .23 (−.07/.49) | .19 (.09/.28)a |
| Frontal Cortical Gray | .43 (.00/.67) | .13 (0/.53) | .44 (.33/.57)a | .01 (−1/1) | .51 (−1/1) | −.54 (−.74/−.29)a | −.03 (−.13/.06) |
| Temporal Cortical Gray | .52 (.13/.65)a | .02 (0/.36) | .45 (.34/.59)a | −.04 (−.56/.35) | .99 (−1/1) | −.34 (−.58/−.07)a | −.04 (−.14/.06) |
| Parietal Cortical Gray | .67 (.29/.76)a | 0 (0/.34) | .33 (.24/.44)a | .08 (−.27/.46) | .57 (−1/1) | −.26 (−.50/.02) | .03 (−.07/.13) |
| Occipital Cortical Gray | .21 (.00/.61) | .43 (.06/.64)a | .36 (.27/.48)a | −.48 (−1/.07) | 1 (1/1)a | −.08 (−.33/.18) | .01 (−.09/.11) |
Values given are n (95% confidence interval). h2, c2, e2 = standardized additive genetic, shared environmental, and nonshared environmental variance components; rg, rc, re = genetic, shared, and unshared environmental correlation; rph = total phenotypic correlation. Fixed (genetic) model for Sz used: h2=.81, c2=.11, e2=.08 and prevalence of 1%.
Abbreviations as in Tables 1 and 2.
Confidence intervals not including zero indicate significance.
Table S2 in Supplement 1 shows the more parsimonious AE models, where all nonsignificant common-environmental effects are ignored. There was a significant genetic correlation between reduced cerebral volume and risk for Sz (rg = −.21, 95% confidence interval [CI]: −.31 to −.10). The phenotypic correlation between reduced cerebral volume and Sz risk of −.22 (rph) could be broken down, with 77% due to common genetic factors (rph-a = −.17, 95% CI: −.25 to −.08), and 23% due to correlated subject-specific environmental factors (rph-e = −.05, 95% CI: −.08 to −.02).
Furthermore, for both reduced cerebral white matter and increased third ventricle volume, there was a significant phenotypic correlation with risk for Sz (rph = −.17, 95% CI: −.25 to −.09; rph = .18, 95% CI: .08 to .28, respectively). Genetic influences contribute significantly to the association between both structures and Sz liability. For cerebral white matter volume, overlapping A (genetic) factors = −.15 (95% CI: −.24 to −.07) accounting for approximately 94%. For increased third ventricle volume the part due to overlapping A factors was .15 (95% CI: .05 to .25) and an overlap of approximately 83%. For cerebral gray matter volume (in particular, cortical frontal and occipital) the influence of common environmental factors (C) could not be dropped from the model. A near-significant phenotypic correlation was found between gray matter and Sz (rph = −.08, 95% CI: −.16 to .00).
Discussion
In the largest twin study to date of Sz and BVs (n = 684 individuals) we examined the relative contributions of genetic and environmental influences on the association of BVs and Sz. In an international multicenter collaboration we pooled data from four research centers, creating a uniquely powered twin cohort. We found a small but significant association between Sz and a smaller cerebral volume, of which 77% can be explained by genetic factors that influence both the lower cerebral volume and (the risk for developing) Sz. Lower cerebral white matter volume as well as larger third ventricular volume also showed a significant association with the liability for Sz, which was largely explained by genetic factors. These accounted for significant portions of the phenotypic correlations (94% and 83%, respectively), indicating that smaller cerebral (white) matter and larger third ventricle are related to the genetic risk to develop Sz. Thus, our results suggest that the smaller total cerebral volume and particularly that of white matter and larger third ventricle volume is linked to Sz, mainly through genetic factors that are associated to the disorder. In contrast, a significant environmental correlation for cerebral gray matter (−.36; 95% CI: −.55 to −.15) and cortical frontal gray matter (−.54; 95% CI: −.74 to −.29) was found, without a significant phenotypic association between smaller cerebral or cortical frontal and occipital gray matter volume and liability to Sz. Interestingly, there are contrasting contributions (although not significant) of genetic and common environmental factors. In other words, a negative association was found between genetic factors and lower volume, whereas common environmental factors showed a positive association with larger gray matter volume. This is in line with a recent study in MZ twins concordant and discordant for Sz showing a heterogeneous pattern of prefrontal volume reduction in twins with Sz. Medial and orbital frontal cortex showed significant volumetric reductions in twins with Sz only if they were concordant but not discordant for the disorder, relative to control subjects. In contrast, inferior frontal cortex showed no statistically significant differences across groups, whereas superior frontal cortex was reduced in Sz patients, regardless of their concordance status (41).
That impaired brain development is important in the pathogenesis of Sz is not new (42,43). Evidence from both genetic and epidemiological studies that aberrant neurodevelopment is crucial in the risk for Sz is mounting. Association studies have identified common risk variants that are associated with Sz (e.g., neuregulin, dysbindin, Disrupted in Schizophrenia 1 [DISC1]). In addition, small structural changes (copy number variants) in the genome seem causative of Sz. Interestingly, these genes as well as the copy number variants seem to influence neurodevelopment, cell signaling, and synaptic functions (44). Moreover, Fatemi and Folom (45) imply that Neuregulin-I and DISC1 not only play a key role in brain development but are likely to be functionally convergent.
Epidemiological data show that deficits and delays in cognitive development (46–48), emotional problems, interpersonal difficulties, and impairments in neuromotor and receptive language (48) are present during childhood and adolescence in individuals who will later go on to develop Sz. Crucially, the risk for Sz is increased in those with a lower IQ score, in particular in the presence of impaired nonverbal reasoning (49). Furthermore, recent work has shown that there are significant common genetic influences acting on the covariance between IQ, schizotypy, abnormal social functioning, and schizophrenia (50). This suggests that the same genetic factors that impair intelligence, increase schizotypy, impede social development, and decrease BV in childhood also determine the liability to Sz (39,50).
Although we found a significant association between Sz and smaller cerebral volume, the correlation is relatively low, rph = −.22. This subtle effect might not be surprising, because it was not expected that the risk for Sz explains a large part of the variance in cerebral volume. A correlation of −.22 indicates that susceptibility for Sz explains 4.8% of the variance, which is small but by no means negligible. This is even lower for white matter volume (−.17). Importantly, our results show that this effect in cerebrum and cerebral white matter is largely (77% and 94%, respectively) explained by shared genetic rather than environmental or disease-related factors. The genetic contribution to association between white matter volume and Sz liability has been suggested previously with a classic repeated measures General Linear Model analyses in the Utrecht discordant twin sample (51). Of note, these data are also part of the current pooled sample.
Our findings must be viewed in light of several methodological limitations. First, this pooled twin sample is not a population-based sample of twins with Sz and healthy control twins from the United Kingdom, The Netherlands, and Germany, except for the twin sample from Helsinki. Second, this study found no significant shared environment contribution to the variation in BVs, which rules it out as a possible source of overlap with the disorder. However, by constraining the BV familial environmental paths to zero in the AE model for some volumes, its effects (even if very small) will automatically be apportioned to the genetic component, therefore inflating the genetic correlation between BV and disorder. Third, we cannot distinguish how much each site contributes to the explained variation in BV due to A or E. Fourth, in the present study we cannot account for biological gene × environment interaction effects, because in the classical twin model the effects of interaction and correlation between latent A, C, and E factors is assumed to be zero. Twin data on their own cannot resolve these issues. There are (rather complex) designs that enable ways of testing these effects (52), but that is beyond the scope of most twin studies.
Finally, the findings of our study are limited to global BV measures. The advantage of global BVs is their robustness and stability in measurements, as is indicated by the relatively high ICCs we found across sites. In contrast, the reliability of voxel-based and cortical thickness measurements seem to differ between brain areas (53). We can make no anatomically specific inferences about the location of the focal gray and white matter deficits and how they might be differently related to the genetic and environmental risk to develop Sz. Future studies should deploy more focally sensitive approaches to brain anatomy with voxel-based morphometry or cortical thickness measurements. In addition, associations with other candidate endophenotypic markers such as intellectual functioning (IQ) need to be investigated.
In conclusion, we found that genetic factors largely explain the modest but significant association between Sz and total cerebral and white matter volume decrease and increase in third ventricle volume. These findings indicate that some of the genes that increase the risk to develop Sz are likely to be involved in crucial neurodevelopmental processes in the brain. Thus, evidence from genetic, epidemiological, and—as indicated by the current results—neuroimaging studies seem to converge on the fact that the risk to develop Sz is the consequence of genetically mediated aberrant neurodevelopment.
Acknowledgments
Part of this study was funded by a National Alliance for Research on Schizophrenia and Depression Young Investigators Award (2004) to FR and a grant from the Dutch Brain Foundation (2004, 12.F04.11) awarded to RSK and a National Institutes of Health Grant MH52857 awarded to TDC. Part of the study in London was funded by a Wellcome Trust Research Training Fellowship (064971). The collection of the data in Germany was supported by the German Research Organization.
Dr. van Haren has received honoraria for education programs for AstraZeneca, Eli Lilly, and Janssen-Cilag. Dr. Weisbrod reports having received lecture fees from SCHUHFRIED GmbH, Austria. Dr. Cannon reports that, in the past 3 years, he has served as a consultant to Rules-Based Medicine on serum-based biomarker tests for schizophrenia. Dr. Hulshoff Pol has received honoraria for education programs for Ferris and Lundbeck. Dr. Kahn has received grants and honoraria for education programs or served as consultant for AstraZeneca, Bristal-Myers Squibb, Eli Lilly, Janssen-Cilag, Otsuka, and Roche. Dr. Picchioni has received travel and educational awards from Pfizer, Janssen-Cilag, and Eli Lily. Dr. Boomsma received a precompetitive grant from Pfizer. Dr. Murray has received honoraria for educational activities from Bristal-Myers Squibb, Janssen, A-Z, Lilly, Novartis, and Otsuka. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Authors NEMvH and FR contributed equally to this work.
Supplementary material cited in this article is available online.
Supplementary data
References
- 1.Kendler K.S., Pedersen N.L., Neale M.C., Mathe A.A. A pilot Swedish twin study of affective illness including hospital- and population-ascertained subsamples: Results of model fitting. Behav Genet. 1995;25:217–232. doi: 10.1007/BF02197180. [DOI] [PubMed] [Google Scholar]
- 2.Cardno A.G., Marshall E.J., Coid B., Macdonald A.M., Ribchester T.R., Davies N.J. Heritability estimates for psychotic disorders: The Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Woods S., Schosser A., McGuffin P. From age correction to genome-wide association. Acta Psychiatr Scand. 2009;120:355–362. doi: 10.1111/j.1600-0447.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- 4.Nothen M.M., Nieratschker V., Cichon S., Rietschel M. New findings in the genetics of major psychoses. Dialogues Clin Neurosci. 2010;12:85–93. doi: 10.31887/DCNS.2010.12.1/mnoethen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Donovan M.C., Craddock N.J., Owen M.J. Genetics of psychosis; insights from views across the genome. Hum Genet. 2009;126:3–12. doi: 10.1007/s00439-009-0703-0. [DOI] [PubMed] [Google Scholar]
- 6.Harrison P.J. Schizophrenia susceptibility genes and their neurodevelopmental implications: Focus on neuregulin 1. Novartis Found Symp. 2007;288:246–255. [PubMed] [Google Scholar]
- 7.Stevens H.E., Smith K.M., Rash B.G., Vaccarino F.M. Neural stem cell regulation, fibroblast growth factors, and the developmental origins of neuropsychiatric disorders. Front Neurosci. 2010;4:1–14. doi: 10.3389/fnins.2010.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsgodt K.H., Sun D., Jimenez A.M., Lutkenhoff E.S., Willhite R., van Erp T.G., Cannon T.D. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- 9.Wright I.C., Rabe-Hesketh S., Woodruff P.W., David A.S., Murray R.M., Bullmore E.T. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 10.Harrison P.J. The neuropathology of schizophrenia: A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 11.Peper J.S., Brouwer R.M., Boomsma D.I., Kahn R.S., Hulshoff Pol H.E. Genetic influences on human brain structure: A review of brain imaging studies in twins. Hum Brain Mapp. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaymaz N., van Os J. Heritability of structural brain traits an endophenotype approach to deconstruct schizophrenia. Int Rev Neurobiol. 2009;89:85–130. doi: 10.1016/S0074-7742(09)89005-3. [DOI] [PubMed] [Google Scholar]
- 13.Boos H.B., Aleman A., Cahn W., Hulshoff Pol H.E., Kahn R.S. Brain volumes in relatives of patients with schizophrenia: A meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 14.McDonald C., Grech A., Toulopoulou T., Schulze K., Chapple B., Sham P. Brain volumes in familial and non-familial schizophrenic probands and their unaffected relatives. Am J Med Genet. 2002;114:616–625. doi: 10.1002/ajmg.10604. [DOI] [PubMed] [Google Scholar]
- 15.Baare W.F., van Oel C.J., Hulshoff Pol H.E., Schnack H.G., Durston S., Sitskoorn M.M., Kahn R.S. Volumes of brain structures in twins discordant for schizophrenia. Arch Gen Psychiatry. 2001;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- 16.van Haren N.E., Picchioni M.M., McDonald C., Marshall N., Davis N., Ribchester T. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Rijsdijk F.V., van Haren N.E., Picchioni M.M., McDonald C., Toulopoulou T., Pol H.E. Brain MRI abnormalities in schizophrenia: Same genes or same environment? Psychol Med. 2005;35:1399–1409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- 18.Hulshoff Pol H.E., Brans R.G., van Haren N.E., Schnack H.G., Langen M., Baare W.F. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55:126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 19.Jack C.R., Jr, Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grethe J.S., Baru C., Gupta A., James M., Ludaescher B., Martone M.E. Biomedical informatics research network: building a national collaboratory to hasten the derivation of new understanding and treatment of disease. Stud Health Technol Inform. 2005;112:100–109. [PubMed] [Google Scholar]
- 21.Keator D.B., Grethe J.S., Marcus D., Ozyurt B., Gadde S., Murphy S. A national human neuroimaging collaboratory enabled by the Biomedical Informatics Research Network (BIRN) IEEE Trans Inf Technol Biomed. 2008;12:162–172. doi: 10.1109/TITB.2008.917893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnack H.G., van Haren N.E.M., Hulshoff Pol H.E., Picchioni M., Weisbrod M., Sauer H. Reliability of brain volumes from multicenter MRI acquisition: A calibration study. Hum Brain Mapp. 2004;22:312–320. doi: 10.1002/hbm.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartko J.J., Carpenter W.T., Jr On the methods and theory of reliability. J Nerv Ment Dis. 1976;163:307–317. doi: 10.1097/00005053-197611000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Hulshoff Pol H.E., Schnack H.G., Bertens M.G., van Haren N.E., van der Tweel I., Staal W.G. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- 25.Schnack H.G., Hulshoff Pol H.E., Baare W.F., Staal W.G., Viergever M.A., Kahn R.S. Automated separation of gray and white matter from MR images of the human brain. Neuroimage. 2001;13:230–237. doi: 10.1006/nimg.2000.0669. [DOI] [PubMed] [Google Scholar]
- 26.Schnack H.G., Hulshoff H.E., Baare W.F., Viergever M.A., Kahn R.S. Automatic segmentation of the ventricular system from MR images of the human brain. Neuroimage. 2001;14:95–104. doi: 10.1006/nimg.2001.0800. [DOI] [PubMed] [Google Scholar]
- 27.Talairach J., Tournoux P. Thieme; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- 28.Maes F., Collins D.L., Vandermeulen D., Marchal G., Suetens P. Multi-modality image registration by maximizing of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 29.Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 30.Palmen S.J., Hulshoff Pol H.E., Kemner C., Schnack H.G., Janssen J., Kahn R.S., van E.H. Larger brains in medication naive high-functioning subjects with pervasive developmental disorder. J Autism Dev Disord. 2004;34:603–613. doi: 10.1007/s10803-004-5282-2. [DOI] [PubMed] [Google Scholar]
- 31.Mandl R.C., Hulshoff Pol H.E., Collins D.L. Automatic volume measurement in schizophrenia: Non-linear or linear transformation? Neuroimage. 1999;9:112. [Google Scholar]
- 32.Collins D.L., Neelin P., Peters T.M., Evans A.C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 33.Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 34.Falconer D.S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 35.Neale M.C., Cardon L.R. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1992. Methodology for Genetic Studies of Twins and Families. [Google Scholar]
- 36.Neale M.C., Boker S.M., Zie G., Maes H.H. 5th edition. Department of Psychiatry; Richmond, Virginia: 1999. Mx: Statistical Modeling. [Google Scholar]
- 37.Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 38.Hall M.H., Rijsdijk F., Picchioni M., Schulze K., Ettinger U., Toulopoulou T. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164:804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- 39.Toulopoulou T., Picchioni M., Rijsdijk F., Hua-Hall M., Ettinger U., Sham P., Murray R. Substantial genetic overlap between neurocognition and schizophrenia: Genetic modeling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- 40.Rijsdijk F.V., Viding E., De Brito S., Forgiarini M., Mechelli A., Jones A.P., McCrory E. Heritable variations in gray matter concentration as a potential endophenotype for psychopathic traits. Arch Gen Psychiatry. 2010;67:406–413. doi: 10.1001/archgenpsychiatry.2010.20. [DOI] [PubMed] [Google Scholar]
- 41.Ettinger U., Schmechtig A., Toulopoulou T., Borg C., Orrells C., Owens S. Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr Bull. 2012;38:192–203. doi: 10.1093/schbul/sbq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray R.M., Lewis S.W. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberger D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 44.Gill M., Donohoe G., Corvin A. What have the genomics ever done for the psychoses? Psychol Med. 2010;40:529–540. doi: 10.1017/S0033291709991139. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi S.H., Folsom T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichenberg A., Weiser M., Rapp M.A., Rabinowitz J., Caspi A., Schmeidler J. Elaboration on premorbid intellectual performance in schizophrenia: Premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- 47.Reichenberg A., Caspi A., Harrington H., Houts R., Keefe R.S., Murray R.M. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: A 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon M., Caspi A., Moffitt T.E., Harrington H., Taylor A., Murray R.M., Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: Results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 49.Reichenberg A., Weiser M., Caspi A., Knobler H.Y., Lubin G., Harvey P.D. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006;28:193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- 50.Picchioni M.M., Walshe M., Toulopoulou T., McDonald C., Taylor M., Waters-Metenier S. Genetic modelling of childhood social development and personality in twins and siblings with schizophrenia. Psychol Med. 2010;40:1305–1316. doi: 10.1017/S0033291709991425. [DOI] [PubMed] [Google Scholar]
- 51.Hulshoff Pol H.E., Brans R.G., van Haren N.E., Schnack H.G., Langen M., Baare W.F. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55:126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 52.Rijsdijk F.V., Sham P.C. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 53.Schnack H.G., van Haren N.E., Brouwer R.M., van Baal G.C., Picchioni M., Weisbrod M. Mapping reliability in multicenter MRI: Voxel-based morphometry and cortical thickness. Hum Brain Mapp. 2010;31:1967–1982. doi: 10.1002/hbm.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.