Abstract
Facial beauty has both important social and biological implications. Research has shown that people tend to look longer at attractive than unattractive faces. However, little is known about whether an attractive face presented outside foveal vision can capture attention. The effect of facial attractiveness on covert attention was investigated in a spatial cuing task. Participants were asked to judge the orientation of a cued target presented to the left or right visual field while ignoring a task-irrelevant face image flashed in the opposite field. Relative to unattractive faces, the presence of attractive faces significantly lengthened task performance. The results suggest that facial beauty automatically competes with an ongoing cognitive task for spatial attention.
There is now substantial evidence that faces capture attention more than most common objects (e.g., Ro, Russell, & Lavie, 2001). Moreover, certain facial information such as anger and fear evokes attention more easily than neutral faces. Attention to such information is often rapid, unconscious, and mandatory (see Palermo & Rhodes, 2007, for a review). In this study, we examine whether the same characteristics are found in attention for facial beauty. This should offer important insights about how attentional systems prioritize and select meaningful biological information. The impact of beauty on social behavior has already been well documented in the literature (Langlois et al., 2000). However, unlike the study of attention to emotions, there has been little investigation into the relationship between facial attractiveness and attention.
Olson and Marshuetz (2005) have shown that facial beauty is appraised even when face images are presented for less than 20 ms and followed by backward masking. Their study suggests that facial attractiveness can be detected rapidly from transient and degraded visual information. Other studies have shown that participants tend to look longer at attractive than unattractive faces (Aharon et al., 2001) or take longer to decide on the attractiveness of attractive than unattractive faces (Kranz & Ishai, 2006). Recent literature has also suggested that neural responses to facial beauty are engaged automatically because they can be measured even when participants are performing an unrelated task rather than judging facial attractiveness explicitly (Aharon et al., 2001; O’Doherty et al., 2003; Winston et al., 2007). However, because attention in these studies was directly focused on the location where face images were presented, it is not clear whether appraisal for attractiveness can be achieved if spatial attention has already been directed elsewhere. Is the appraisal for beauty mandatory such that attractive faces can automatically compete with an ongoing task for attentional resources? Also, because faces were presented at fixation in these studies, it is not clear whether facial attractiveness can be detected outside the foveal vision.
To answer these questions, we employed a spatial endogenous cuing task, in which participants were asked to determine whether a laterally presented target letter was upright or inverted while ignoring a briefly flashed face on the opposite side of the display. The likely position of the forthcoming target was indicated by a central cue, which was used to induce covert attention (i.e., visual attention without orienting eye movements) to the cued location. We examined the presence of attractive faces on the task performance. Participants were told that the face was task irrelevant. We hypothesized that relative to an unattractive face, the presence of an attractive face would create a stronger interference with the task because attractive faces may automatically pull the attention away from the target.
To assess the effect of facial attractiveness on covert orienting, the face image and the target in each trial were presented simultaneously. Because the duration of a single eye fixation usually exceeds 200 ms (Rayner, 1983), the target and the face in this study were shown for 200 ms or shorter to suppress saccadic orienting to the stimuli. To make the task more difficult, the presentation time for these was reduced further from 200 ms in Experiment 1 to 100 ms in Experiment 2. If attractive faces capture spatial attention automatically, the effects should be relatively independent of task difficulty. Finally, we conducted Experiment 3 to replicate our main findings and to determine the role of eye movements.
Method
Participants
A total of 142 undergraduate and graduate students from the University of Hull participated in this study. Experiment 1 had 40 participants (30 females, 10 males), whose age ranged from 18 to 40 years (mean 23 ± 6.29 years). Experiment 2 had 43 participants (27 females, 16 males), whose age ranged from 18 to 45 years (mean 20 ± 3.92 years). Experiment 3 had 59 participants (35 females, 24 males), whose age ranged from 19 to 34 years (mean 21 ± 2.11 years). All participants had normal or corrected-to-normal vision.
Stimuli
The face database was obtained from the University of St. Andrews. It contains 702 frontal-view Caucasian faces whose external features (hair and clothing) were removed. All faces in the database were pre-rated by 19 raters (aged between 18 and 29 years, 12 females) for attractiveness on a 7-point scale. To contrast the effect of attractiveness, only the 82 most attractive and the 82 least attractive faces were used. The mean ratings for the two groups of faces were 4.11 and 2.23 respectively. These were significantly different from each other (p < .001). Both the attractive and the unattractive faces contained equal number of males and females. Four of these faces were reserved as the practice stimuli. The face size was normalized to 400 pixels from ear to ear, which subtended 16.6° of visual angle. Because face identification is highly sensitive to image contrast in peripheral presentation (Mäkelä, Näsänen, Rovamo, & Melmoth, 2001; Melmoth, Kukkonen, Mäkelä, & Rovamo, 2000), the luminance and contrast of the images were scaled to their means so that these low-level image properties could contribute little to any behavioral difference.
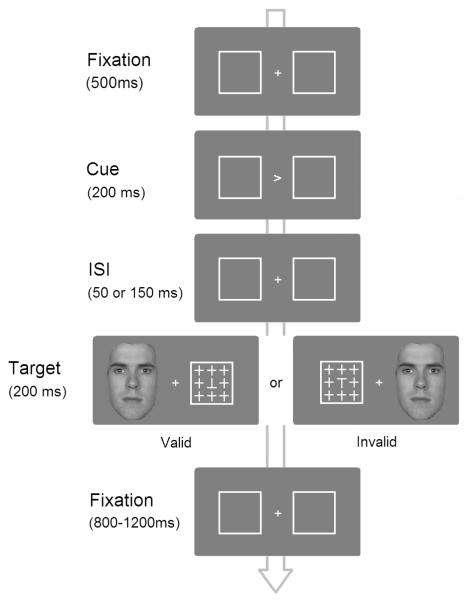
The cuing displays consisted of a central fixation point, a cue, and two 3.8° × 3.8° white boxes on a neutral gray background (see Figure 1). The distance between the center of the display and the outer edge of each box measured 5.5° of visual angle. The cue was a 1.5° white arrow. The target display consisted of a target letter ‘T’ embedded in the center of an array of 8 distractor crosses (see Figure 1). This configuration was the same from trial to trial. The size of the target and distractors was 1.2° × 1.2°. The letter ‘T’ was either shown upright or inverted. E-prime 1.1 was used to control the flow of the experiment and to collect response data.
Figure 1.
Illustration of the procedure used in the study. The target duration in Experiment 2 was changed from 200 to 100 ms.
Procedure
Participants were tested individually. An adjustable headrest was used to fix the participant’s viewing position, which was set 60 cm away from the computer monitor. The procedure for each trial of the experiments is illustrated in Figure 1. Each trial began with a central fixation cross and two peripheral boxes. The fixation cross was shown for 500 ms. It was then replaced by a 200 ms central cue, which pointed randomly to the right or left box. In 80% of trials (valid trials), the cue indicated the target location. In the remaining 20% trials (invalid trials), the cue pointed to the wrong location. The inter-stimulus interval (ISI) before the target presentation was 50 or 150 ms. The stimulus onset asynchrony (SOA), or the interval between the onset of the cue and the target, was thus 250 or 350 ms. SOAs around this range consistently produced relatively strong attention orienting effect in the literature where valid cues create faster response to a target than invalid cues (Funes & Lupiáñez, 2007). We used the two SOAs to determine the temporal window at which the effect of facial attractiveness on attentional orienting is maximal. In the experimental conditions, an attractive or unattractive face was shown simultaneously with the target for 200 ms (Experiments 1 and 3) or 100 ms (Experiment 2). Experiments 1 and 2 were identical except for the different target and face durations. In the baseline condition, the target and peripheral box were shown without a face for the same duration. We used this to evaluate the difference between the results of face-present and face-absent trials. The next frame showed the fixation point and peripheral boxes for a variable time ranging from 800 to 1200 ms to avoid easy predictions about the onset of the next trial. Participants were expected to respond within this time frame and the next trial started regardless of whether a response was recorded. Participants were told to ignore the faces while judging whether the target letter ‘T’ was upright or inverted presented in the opposite side. They were instructed to respond as quickly and accurately as possible by pressing one of the two keys on the keyboard. Participants were also reminded to maintain central fixation throughout the trials.
In Experiments 1 and 2, a total of 80 practice trials were run before the 720 experimental trials. Each of the 12 conditions (3 irrelevant stimuli × 2 visual fields × 2 SOAs) in the experimental trials had 48 valid and 12 invalid trials. Participants were given short breaks after every 240 trials. Experiment 3 was identical to Experiment 1 except that the long SOA (350ms) condition was excluded. This change was made because neither Experiment 1 nor 2 showed any effect of facial attractiveness at this SOA.
Because the interval between the cue and target onset was 250 ms or longer in this study, the results in these experiments could be affected by stimulus-driven eye movements even though the participants were told to maintain fixation. To test this possibility, Experiment 3 examined potential contributions of the eye movements by recording electrooculograms (EOGs) from five participants (see Hawkins et al., 1990, for more details about this method). The EOGs were recorded with two pairs of electrodes, amplified by a band pass of 0.1-100 Hz and digitized at a sampling rate of 250 Hz. The horizontal EOG was recorded from electrodes placed about 1.5 cm lateral to the left and right external canthi, and the vertical EOG was recorded with electrodes located above and under the left eye. Eye movement on a given trial was defined by deflections exceeding ±50 μv.
Results
The data were analyzed using repeated-measures analyses of variance (ANOVAs). The four variables were irrelevant stimuli (attractive, unattractive, or baseline), cue validity (valid vs. invalid), visual field (right vs. left), and SOA (250 vs. 350 ms). The overall accuracy results in all experiments were high (87-95%). Since no significant difference was found between the accuracy results of attractive and unattractive faces, we focus here mainly on the reaction time data. Analyses of reaction time were based on the data for the correct responses only. RT outliers were defined as 3 SD above/below the mean. We did not detect any outlier based on this criterion.
Experiment 1
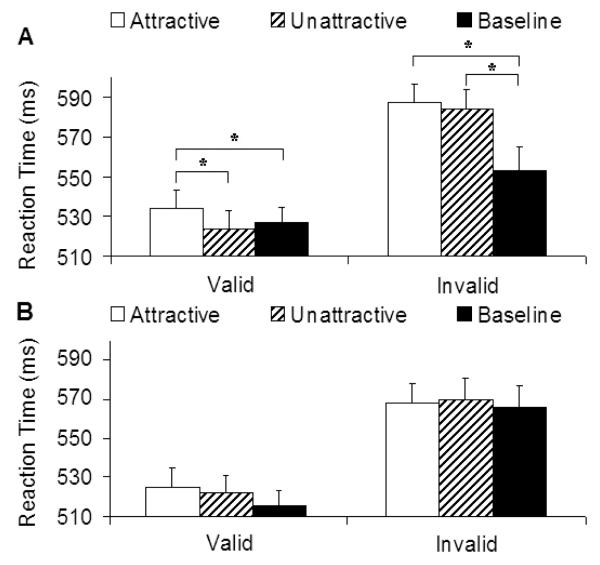
Results of reaction time data from Experiment 1 are shown in Figure 2. Initial analysis showed a significant main effect of visual field, F (1, 39) = 11.65, p < .005, where responses were faster when the target was presented in the left than the right visual field. There was also a main effect of irrelevant stimuli, F (2, 78) = 9.10, p < .005, where attractive and unattractive faces significantly lengthened response time than the baseline condition (ps < .01). However, since there were no significant interactions involving visual field and irrelevant stimuli (all ps > .32), the data from the two visual fields were combined in the subsequent analyses. This showed a significant three-way interaction among irrelevant stimuli, cue validity, and SOA, F (2, 78) = 6.04, p < .005.
Figure 2.
Mean reaction time in Experiment 1 as a function of irrelevant stimuli, SOA, and cue validity. Error bars represent standard errors. (A) SOA = 250 ms. (B) SOA = 350 ms.
To identify the source of this interaction, we conducted two separate simple main effects analyses for the two SOA conditions. This revealed that the effect of irrelevant stimuli occurred only at the shorter SOA (250 ms). As expected, valid cues produced faster reaction times than invalid cues, F (1, 39) = 34.39, p < .0001. We also found a significant interaction between irrelevant stimuli and cue validity, F (2, 78) = 7.54, p < .001. Separate ANOVAs were conducted for the invalid and valid cue conditions. A significant main effect of irrelevant stimuli was found in the valid cue condition, F (2, 78) = 8.18, p < .001. Consistent with our hypothesis, attractive faces delayed response times more than unattractive faces and the baseline, ts (39) = 4.21 and 2.75, ps < .001 and .01, respectively. No difference was found between unattractive and baseline conditions, t (39) = −1.10, p = .28. The effect implies that facial beauty can trigger processes that compete with an ongoing cognitive task for attentional resources. In the invalid cue condition, on the other hand, reaction times for attractive and unattractive faces were comparable, t (42) = 0.76, p = .45, although both were slower than the baseline, ts (42) = 3.43 and 3.19, ps < .001 and .005, respectively.
Experiment 2
Experiment 1 showed that attractive faces affect spatial attention more strongly than unattractive faces in the valid cue condition. Experiment 2 further investigated whether the effect could be replicated when the duration of target and face stimuli was reduced from 200 ms to 100 ms.
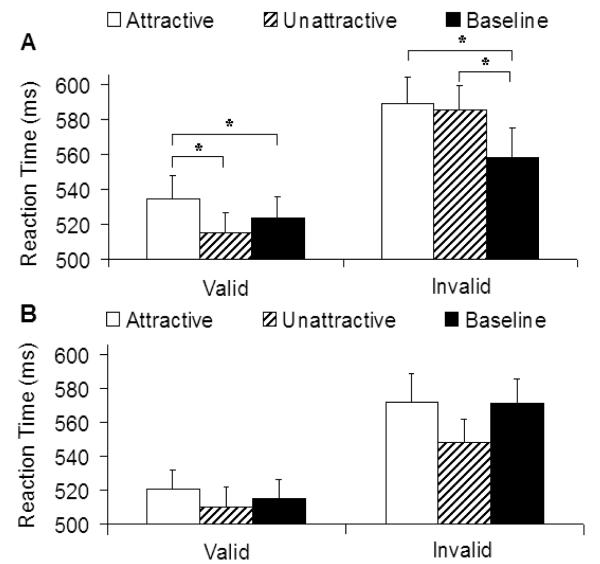
Analysis of the reaction time data showed that the main effect of SOA was significant, F (1, 42) = 5.71, p < .02, where responses were faster for the long SOA than the short SOA. There was also a main effect of irrelevant stimuli, F (2, 84) = 4.83, p < .01, where only attractive faces lengthened response time relative to the baseline condition (p < .005). ANOVA showed a significant four-way interaction, F (2, 84) = 6.82, p < .005. Separate ANOVAs for the two visual fields revealed that the effect of irrelevant stimuli was present when the face was shown to the left visual field, F (2, 84) = 5.26, p < .01, but not when they were presented in the right visual field (p = .47). Results from the left visual field are shown in Figure 3A. For comparison, results from the right visual field are shown in Figure 3B. Only short SOA results from this experiment are presented, because our subsequent analysis did not show effects of facial attractiveness in the long SOA condition.
Figure 3.
Mean reaction time in Experiment 2 as a function of irrelevant stimuli and cue validity (SOA = 250 ms). Error bars represent standard errors. (A) Face on the left and target on the right visual field. (B) Face on the right and target on the left visual field.
All interactions involving irrelevant stimuli for the right visual field (irrelevant stimuli × cue validity, irrelevant stimuli × SOA, and irrelevant stimuli × cue validity × SOA) were also not significant (ps > .1). The results of visual field in this experiment were thus different from Experiment 1, where the effect of facial attractiveness was present in both visual fields. Our main statistical analysis was therefore conducted on the condition where the face stimuli were presented in the left visual field. Results from the left visual field revealed a significant three-way interaction between irrelevant stimuli, cue validity and SOA, F (2, 84) = 6.15, p < .005. Separate ANOVAs were conducted for the two SOA conditions. Consistent with Experiment 1, the results of the long SOA showed no significant main effect of irrelevant stimuli or interaction between this and cue validity (ps > .19), and only the short SOA produced a significant interaction between irrelevant stimuli and cue validity, F (2, 84) = 6.16, p < .005. Also consistent with Experiment 1, the reaction time was significantly slower when an attractive face was shown with the target than when an unattractive face, t (42) = 3.74, p < .001, or baseline was shown with the target, t (42) = 2.13, p < .04. Again the effect was observed only when the cue was valid. When the cue was invalid, reaction times for the attractive and unattractive face conditions did not differ (p = .57), although both attractive and unattractive face conditions delayed response to the target relative to the baseline condition, ts (42) = 3.74 and 2.20, ps < .001 and .04, respectively.
Experiment 2 showed that the facial attractiveness effects found in Experiment 1 can survive a reduction of the presentation time for the face and target from 200 ms to 100 ms but only if the face stimuli are presented to the left visual field.
Experiment 3
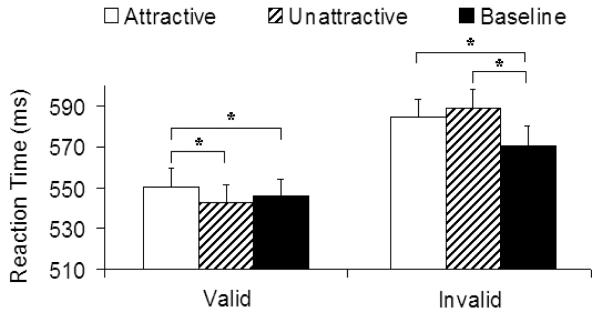
The RT results are shown in Figure 4. Because there were no significant interactions involving visual field and irrelevant stimuli (all ps > .42), the data from the two visual fields were combined in the subsequent analyses. There was a significant main effect of irrelevant stimuli, F (2, 116) = 5.16, p < .01. There was also a significant interaction between irrelevant stimuli and cue validity, F (2, 116) = 6.50, p < .005. Consistant with Experiments 1 and 2, the main effect of irrelevant stimuli was significant in the valid cue condition, F (2, 116) = 6.09, p < .005. The subsequent pairwise comparisons showed that attractive faces delayed the reaction times more than the unattractive face and baseline conditions, ts (58) = 3.96 and 2.02, ps < .0005 and .05, respectively. In the invalid condition, there was a significant main effect of irrelevant stimuli, F (2, 116) = 5.80, p < .005, where both attractive and untractive faces delayed the reaction times more than the baseline condition, ts (58) = 2.24 and 3.71, ps< .03 and .0005. However, there was no difference between the results of attractive and unattractive conditions (p = .37).
Figure 4.
Mean reaction time in Experiment 3 as a function of irrelevant stimuli and cue validity (SOA = 250 ms). Error bars represent standard errors.
Table 1 shows the proportion of trials on which an eye movement was made to the target or irrelevant stimuli following the onset of the central cue. There was no significant main effect of irrelevant stimuli, F (2, 8) = 0.18, p = .84, or interaction between irrelevant stimuli and cue validity, F (2, 8) = 2.52, p = .14. The results showed that eye movements occurred infrequently in all conditions, varying between 2% and 14% of trials across the five participants. The data were collapsed across cue validity because the overall eye movements to the cued and uncued positions did not differ from each other, F (1, 4) = 0.06, p = .82. Our results are consistent with Hawkins et al. (1990), who also found no systematic eye movements toward the cued location. The results suggest that the effect of facial attractiveness was not due to foveal fixation on the target or face stimuli regardless of whether the cue was valid or invalid.
Table 1.
Proportion of trials on which an eye movement was made to target or irrelevant stimuli following the onset of central cue
| Target | AF | Target | UF | Target | Baseline | |
|---|---|---|---|---|---|---|
| Participant 1 | .058 | .072 | .051 | .072 | .038 | .038 |
| Participant 2 | .033 | .039 | .044 | .053 | .033 | .042 |
| Participant 3 | .020 | .008 | .008 | .014 | .019 | .014 |
| Participant 4 | .053 | .049 | .042 | .044 | .069 | .050 |
| Participant 5 | .081 | .060 | .063 | .068 | .065 | .072 |
Legend:
AF = attractive face
UF = unattractive face
The accuracy results in Experiments 1-3 did not show significant difference between the attractive and unattractive faces (ps > .1). The interactions involving irrelevant stimuli and other conditions (cue validity, SOA, and visual field) were also not significant (all ps > .1).
Discussion
Our results showed that the presence of attractive faces in these experiments had a detrimental effect on the speed of judgment for the target orientation. The participants’ voluntary allocation of covert attention to the target induced by the central cue was more attenuated by an attractive face than a less attractive one even though it was task irrelevant. The speedy detection of facial beauty is consistent with previous findings (Olson & Marshuetz, 2005; Locher, Unger, Sociedade, & Wahl, 1993).
When the presentation time for the target and the face image was reduced from 200 ms (Experiment 1) to 100 ms (Experiment 2), the effects of facial attractiveness on spatial attention were only found when the face image was presented to the left visual field. This result suggests a right hemisphere advantage for processing facial beauty. It may echo the right hemisphere’s dominance in processing facial and emotional information (e.g. Kanwisher, McDermott, & Chun, 1997; van Strien, & Valstar, 2004). Future imaging studies should help localize the sites in the right hemisphere that have led to the difference between the results of right and left visual field presentations.
In all three experiments, the effect of facial attractiveness was found only when the central cue was valid. When the cue was invalid, there was no difference between the results of attractive and unattractive faces. This may be due to the fact that attention in the invalid cue trials was already directed to the face rather than to the target. Participants had to reorient attention to the target after this. The effect of attractive faces may rely on a shift of spatial attention that was oriented elsewhere. The experiments also revealed that the facial attractiveness effect was primarily associated with the short SOA (250 ms). It is not entirely clear why the same effect was not found in the long SOA (350 ms). It is possible, however, that the participants were better prepared after a long SOA to focus more robustly on the target. Indeed, the overall reaction times were faster in this SOA condition.
The attentional bias for attractive faces found in this study signals their biological significance. Researchers have suggested that the preference for attractive faces is deeply rooted in evolution (Langlois et al., 2000; Rhodes, 2006). This Darwinian approach helps to explain why attractive faces could receive more attention. Some researchers have shown that attractive faces carry important information about mate quality (Johnston, 2006). There has been evidence that although males and females both rate beautiful male and female faces as attractive, their reward circuitry and related brain regions are more strongly activated by the faces of opposite gender (Aharon et al., 2001, Ishai, 2007). However, whether the current finding is modulated by face gender remains to be seen. Due to limited number of trials and male participants, the current design makes it difficult to perform this analysis. This issue will be subject to future investigations.
In summary, our study shows that facial beauty is a powerful stimulus that competes with other visual information for spatial attention. The findings imply that the effect of facial attractiveness extends beyond explicit social behavior and has profound impact right from the entry point of cognitive processing.
Acknowledgments
This research was supported by grants from The Royal Society, Marie Curie Incoming International Fellowship, and Natural Science Foundation of China (Project 30700229). We thank Professor David Perrett for offering the face stimuli, Tim Alexander and the reviewers for comments on an earlier version of the manuscript, and Malathy Rengamani and Bryony Hughes for data collection.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Funes MJ, Lupiáñez J. Separate mechanisms recruited by exogenous and endogenous spatial cues: Evidence from a spatial stroop paradigm. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:348–362. doi: 10.1037/0096-1523.33.2.348. [DOI] [PubMed] [Google Scholar]
- Hawkins HL, Hillyard SA, Luck SJ, Mouloua M, Downing CJ, Woodward DP. Visual attention modulates signal detectability. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:802–811. doi: 10.1037//0096-1523.16.4.802. [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. International Journal of Psychophysiology. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Johnston VS. Mate choice decisions: The role of facial beauty. Trends in Cognitive Sciences. 2006;10:9–13. doi: 10.1016/j.tics.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The Fusiform Face Area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Langlois JH, Kalakanis LE, Rubenstein AJ, Larson AD, Hallam MJ, Smoot MT. Maxims or myths of beauty: A meta-analytic and theoretical review. Psychological Bulletin. 2000;126:390–423. doi: 10.1037/0033-2909.126.3.390. [DOI] [PubMed] [Google Scholar]
- Locher P, Unger R, Sociedade P, Wahl J. At first glance: Accessibility of the physical attractiveness stereotype. Sex Roles. 1993;28:729–743. [Google Scholar]
- Mäkelä P, Näsänen R, Rovamo J, Melmoth D. Identification of facial images in peripheral vision. Vision Research. 2001;41:599–610. doi: 10.1016/s0042-6989(00)00259-5. [DOI] [PubMed] [Google Scholar]
- Melmoth DR, Kukkonen HT, Mäkelä PK, Rovamo JM. The effect of contrast and size scaling on face perception in foveal and extrafoveal vision. Investigative Ophthalmology and Visual Science. 2000;41:2811–2819. [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Olson IR, Marshuetz C. Facial attractiveness is appraised in a glance. Emotion. 2005;5:498–502. doi: 10.1037/1528-3542.5.4.498. [DOI] [PubMed] [Google Scholar]
- Rayner K. Eye movements in reading: Perceptual and language processes. Academic Press; New York: 1983. [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review Psychology. 2006;57:119–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Ro T, Russell C, Lavie N. Changing faces: A detection advantage in the flicker paradigm. Psychological Science. 2001;12:94–99. doi: 10.1111/1467-9280.00317. [DOI] [PubMed] [Google Scholar]
- Van Strien JW, Valstar LH. The lateralized emotional stroop task: left visual field interference in women. Emotion. 2004;4:403–409. doi: 10.1037/1528-3542.4.4.403. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]