Abstract
The precise polarization and orientation of developing neurons is essential for the correct wiring of the brain. In pyramidal excitatory neurons, polarization begins with the sprouting of opposite neurites, which later define directed migration and axo-dendritic domains. We here show that endogenous N-cadherin concentrates at one pole of the newborn neuron, from where the first neurite subsequently emerges. Ectopic N-cadherin is sufficient to favour the place of appearance of the first neurite. The Golgi and centrosome move towards this newly formed morphological pole in a second step, which is regulated by PI3K and the actin/microtubule cytoskeleton. Moreover, loss of function experiments in vivo showed that developing neurons with a non-functional N-cadherin misorient their cell axis. These results show that polarization of N-cadherin in the immediate post-mitotic stage is an early and crucial mechanism in neuronal polarity.
Keywords: cell axis orientation, centrosome, cortical development, N-cadherin, neuronal polarity
Introduction
Neuronal polarization starts with the breakage of membrane symmetry to entail the growth of the first neurite, which determines the position of the second neurite from the opposite pole (de Anda et al, 2008). This bipolar architecture supports migration (Hatanaka and Murakami, 2002) and subsequent axonal and dendritic growth (Noctor et al, 2004), and thus brain organizations primarily depends on the mechanisms that regulate the earliest polarized events directing the neuronal axis of growth.
The first growth axis may be defined through spatial constraints, either by the asymmetric distribution of extrinsic environmental cues (non-cell-autonomous) or by the cell-autonomous polarization of growth-supporting cellular components. Experimental evidence suggests both mechanisms: gradients of secreted molecules, for example, reelin or Semaphorin 3A, have been shown to influence neuronal positioning and axon-dendrite orientation, both in vivo and in vitro (Polleux et al, 1998, 2000; Tissir and Goffinet, 2003; Jossin and Cooper, 2011). On the other hand, isolated neurons in vitro can establish their first growth axis cell-autonomously, in the absence of asymmetric extracellular cues (Dotti et al, 1988; Powell et al, 1997), and the growth of the first neurite was attributed to the polarized organization of intracellular growth-supporting organelles, such as the Golgi apparatus and the centrosome (Zmuda and Rivas, 1998; de Anda et al, 2005). These evidences have led to the hypothesis that the centrosome position itself determines the orientation of the neuronal polarity axis, in a process similar to that shown in Caenorhabditis elegans oocytes, where the centrosome triggers the concentration of polarity molecules in its vicinity (Cowan and Hyman, 2004).
Much is known on the regulation of axon growth from an already formed neurite (Wiggin et al, 2005), but neuronal polarization begins earlier, possibly even before the appearance of the first neurite, which is a poorly studied process so far. The formation of the first neurite is a fundamental step for the establishment of the migration axis and therefore for brain organization and function. We therefore investigated the contribution of both cell-extrinsic and cell-intrinsic signals towards the selection of this growth site in developing pyramidal neurons. Our work unravels one of the earliest signals that determine the orientation of the neuronal growth axis and polarity.
Results
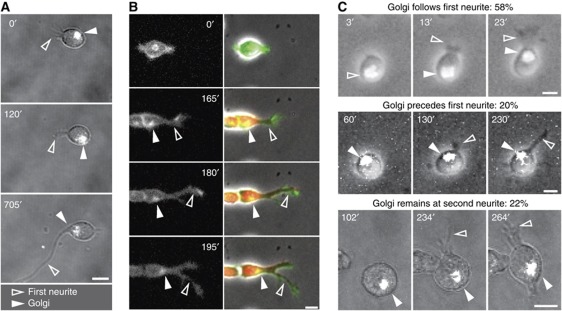
It has been suggested that asymmetric microtubule polymerization and microtubule-mediated membrane delivery can define the site of first neurite outgrowth by simple proximity, in a quantitative process (de Anda et al, 2005, 2008). It is possible however that organelle asymmetry is not the true initiator of polarity, but the consequence of a polarized membrane-signalling event. To test this, we performed a careful evaluation of the behaviour of the Golgi apparatus and the centrosome in relation to changes in cell shape as neurons undertake differentiation. Hippocampal embryonic neurons were transfected with tagged organelle-specific probes labelling the centrosome (centrin1–GFP) or the Golgi (GT–GFP or GT–TAG-RFP; palmitoylated–GFP; Supplementary Figure S1A) immediately after cell dissociation and kept in suspension for 2 h to avoid the initiation of differentiation before imaging. This preplating step also provided time for the synthesis of the organelle marking proteins and to regenerate surface molecules, which may have been shed by the trypsin treatment used for dissociation (Takeichi and Nakagawa, 2001). Cells were then transferred to poly-L-lysine (PLL)-coated coverslips and immediately imaged at high frequency. This study revealed that, shortly after plating, Golgi and centrosome are highly motile until they become fixed to the pole of the cell that undergoes a morphological deformation, comprising a small neurite (Figure 1). Since Golgi and centrosome always co-localize in our cultured neurons (Supplementary Figure S1B) (Dotti and Banker, 1991), we monitored only the position of either organelle, in order to minimize stress to the cells. Temporal analysis revealed that, in 16 of 29 cells (55%) Golgi and centrosome moved to the origin of the first neurite after the morphological change had occurred (Figure 1A; Supplementary Figure S1C). To ascertain that this was not the consequence of neurite regeneration, following mechanical dissociation, the same experiment was performed in neurons generated in vitro from neuronal precursors, which undergo bona fide first neurite initiation. Also, in this case, the Golgi in a large proportion of cells was recruited to the site of first neurite only after this had formed (Figure 1B; Supplementary Figure S1D). The same conclusion is supported also by the analysis of cells reassembling the Golgi after Brefeldin A (BFA) treatment (Figure 1C). BFA led to the fragmentation and delocalization of the Golgi, and following BFA washout the Golgi reassembled within 2–10 min. This experimental design gave us the possibility to study in much more detail the temporal relationship of de novo Golgi assembly, Golgi localization and first neurite formation. We followed 40 cells at high time resolution concomitantly imaging the reconstitution of the fluorescent Golgi and the formation of the first neurite. The first neurite formed 10 min to 2 h after Golgi reassembly at a site unrelated to the position of the reassembled Golgi, and the Golgi was recruited to the site of the first neurite after this had formed in 58% of the observed neurons (preceded the neurite in 20%, remained at second neurite in 22%; Figure 1C). Similar observations were made monitoring the centrosome position after BFA washout: the centrosome followed the first neurite in 53% of the neurons (preceded the neurite in 35%, remained at second neurite in 12%; 17 cells). This first series of results on one hand confirms the existence of a tight spatial correlation between cytoplasmic and neuronal morphological polarization, as previously reported (Lefcort and Bentley, 1989; de Anda et al, 2005), and demonstrates on the other hand, for the first time, that cytoplasmic polarity follows morphological polarization.
Figure 1.
First neurite formation precedes Golgi and centrosome translocation. (A–C) Time-lapse analysis of Golgi movements in polarizing neurons. (A) Hippocampal neurons transfected with the N-terminus of galactosyltransferase tagged with GFP (GT–GFP) to visualize the Golgi (white in grey image). (B) Mouse embryonic 12.5 cortical neurons transfected with a palmitoylated version of EGFP to visualize cell membrane morphology and Golgi positioning (see Supplementary Figure S1A). Cherry fluorescent protein expressed by the neuron-specific α-tubulin promoter (red in merged images) is used to identify terminally differentiated neurons. (C) Examples of neurons expressing GT–GFP with a de novo assembled Golgi after BFA treatment and washout. Golgi: filled arrowheads. First neurite: open arrowheads. All scale bars=5 μm.
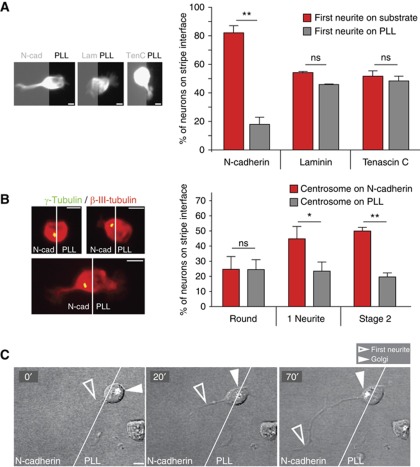
Our finding that neurite outgrowth precedes Golgi and centrosome translocation indicates that an event initiated at the membrane promotes local growth and subsequent recruitment of cell organelles to the growth site. Neurite outgrowth requires adhesion to substrates or to other cells, so that it is very likely that a spatially coded adhesion event triggers a localized deformation of the plasma membrane and a subsequent Golgi/centrosome translocation to this place, similar to cytotoxic T cells contacting their target cells (Stinchcombe et al, 2006). In order to test this hypothesis, we focused on N-cadherin and laminin, because of their abundance in the CNS (Venstrom and Reichardt, 1993) and their well-known role in neurite growth (Esch et al, 1999). The controlled environment in our in vitro system enables us to locally stimulate the cell periphery at random places with a defined substrate, and thus investigate the relevance of different adhesion-mediated signalling pathways. Dissociated hippocampal neurons were seeded on coverslips coated with alternating stripes of N-cadherin or PLL and the location of the first neurite was analysed. Neurons whose cell bodies were situated at the interface of the two substrates were examined for symmetry breaks. Analysis of fixed samples revealed that the first neurite formed preferentially from the pole facing N-cadherin (88±2.7%; Figure 2A; Supplementary Figure S2A). Given our previous observation that morphological deformation preceded Golgi/centrosome polarization, we analysed if localized N-cadherin signalling coincided with organelle positioning. Indeed, in the majority of neurons, the centrosome and Golgi stacks were located at the N-cadherin contacting pole after first neurite formation (Figure 2B; Supplementary Figure S2B). Moreover, time-lapse microscopy directly demonstrated that growth from the N-cadherin contacting side preceded the polarization of these organelles to the growing pole (Figure 2C). We made the same observation in neurons newly generated in vitro, after seeding neuronal precursors isolated from the cortical ventricular zone (VZ)/subventricular zone (SVZ) of E14 mice and also in neurons derived from the cortex of E16 mice (Supplementary Figure S2C and D). N-cadherin-mediated organelle polarization is also supported by our observation that the centrosome is relocated to cell–cell contact sites in living neurons (Supplementary Figure S2E and F), which are highly enriched in N-cadherin (Supplementary Figure S2G).
Figure 2.
N-cadherin determines the site of first neurite growth and subsequently recruits Golgi and centrosome. (A) Examples of neurons immunolabelled with anti-βIII-tubulin developing on coverslips coated with alternating stripes of the indicated substrate and PLL. Quantification of neurons with the soma on the interface of the two substrates shows that neurons grow the first neurite preferentially at the N-cadherin facing pole (experimental n=4). Asymmetric contact with either laminin (n=4) or Tenascin C (n=3) does not trigger outgrowth of the first neurite (⩾9 neurons/experiment). (B) Examples of centrosome position in hippocampal neurons immunolabelled with a neuron-specific antibody (anti-βIII-tubulin) and with an antibody recognizing the centrosome (anti-γ-tubulin) with their soma on the PLL/N-cadherin interface. The centrosome position of neurons was quantified at different developmental stages (round neurons n=5, one neurite n=7, stage 2 neurons n=9, ⩾8 cells/experiment). The centrosome is recruited towards the N-cadherin substrate. (A, B) t-Test, *P<0.05, **P<0.001. (C) Time-lapse sequence of a neuron developing on the interface of N-cadherin and PLL transfected with GT–EGFP: after the outgrowth of the first neurite (open arrowhead) on the N-cadherin stripe, the Golgi (filled arrowhead) is recruited to this site. All scale bars=5 μm.
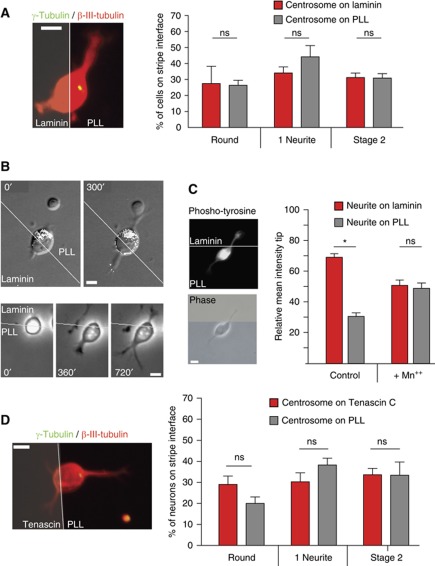
To check if polarity is specifically regulated by N-cadherin and to exclude that the observed effects were a simple consequence of challenging neurons with a substrate more adhesive than PLL, we repeated the analysis on neurons lying on a PLL–laminin interface. Different from the N-cadherin challenge, neurons presented a randomly oriented Golgi, centrosome and first neurite, towards either laminin or PLL (Figures 2A and 3A and B), albeit the strong laminin-induced integrin signalling (Figure 3C), as indicated by the local accumulation of tyrosine-phosphorylated proteins (Robles and Gomez, 2006) at the laminin contact site. This result implies that the site of polarization is not determined by adhesive strength, but depends on adhesive specificity. As a further control, neurons were challenged with Tenascin C, again in alternating stripes with PLL. Once more, Golgi/centrosome positioning and first neurite formation was not influenced by asymmetric Tenascin C signals (Figures 2A and 3D).
Figure 3.
Golgi and centrosome recruitment is substrate specific. (A) Hippocampal neurons growing on coverslips coated with alternating stripes of laminin and PLL were immunolabelled with a neuron-specific antibody (anti-βIII-tubulin) and with an antibody recognizing the centrosome (anti-γ-tubulin). The centrosome position was quantified at different developmental stages (experimental n=5 in round neurons, n=7 in one neurite neurons, n=9 in stage 2 neurons; ⩾8 cells/experiment). (B) Time-lapse sequence of a hippocampal neuron developing on the interface of Laminin and PLL transfected with GT–EGFP: neither the outgrowth of the first neurite (lower panel) nor the position of the Golgi (upper panel, Golgi is shown in white in the grey image) is influenced by laminin. (C) Laminin induces local integrin signalling at early time points evident by a spatially restricted increase in phospho-tyrosine signal. Manganese treatment inhibits integrin signalling and as a consequence local tyrosine-phosphorylation, demonstrating that this is a specific effect. (D) Hippocampal neurons were plated on coverslips coated with alternating stripes of Tenascin C and PLL, fixed after different times and immunolabelled with a neuron-specific antibody (βIII-tubulin) and antibodies recognizing the centrosome (anti-γ-tubulin, filled arrowheads). The position of the centrosome in neurons being in contact with both substrates with respect to Tenascin C at different developmental stages was monitored (experimental n=3 in round neurons, n=4 one neurite, n=5 stage 2 neurons, ⩾8 cells/experiment, two-tailed paired t-test).
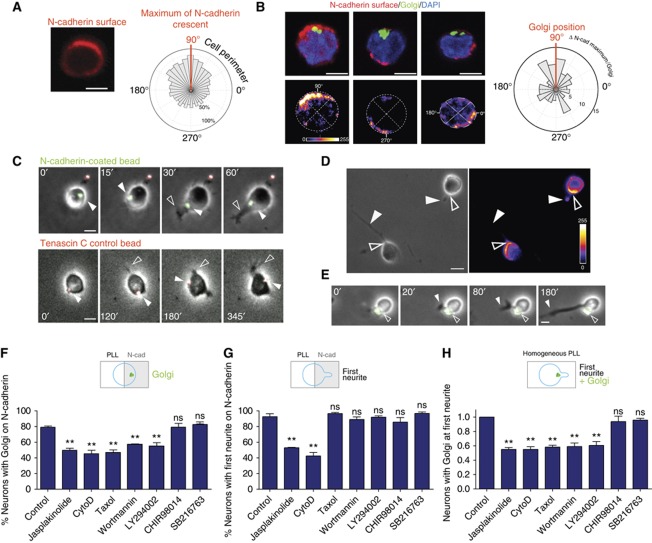
If an N-cadherin pathway controls the first events in hippocampal neuron polarization, it was to expect a polarized N-cadherin distribution in cells grown in a homogeneous environment. To test this, the surface distribution of N-cadherin was detected using an antibody that recognizes an extracellular epitope of N-cadherin. This analysis clearly showed that N-cadherin was enriched in one pole of neurons before neurite outgrowth started (Figure 4A). The N-cadherin enrichment did not always co-localize with the Golgi (Figure 4B) in concordance with our observations that this organelle is recruited by the N-cadherin signal after first neurite formation (Figure 2). Subsequently, we tested whether first neurite growth occurs from the same pole where N-cadherin clusters. In order to detect extracellular N-cadherin in living neurons at the very first developmental steps, hippocampal neurons were grown in the presence of low concentrations of latex microspheres coated with the extracellular N-cadherin domain (able to bind to cellular N-cadherin in living cells) and a green fluorescent substrate. Those beads were used in order to detect sites of high surface expression of N-cadherin in developing neurons. As specificity control served beads coated with Tenascin C or PLL and a red fluorescent substrate. Figure 4C (upper panel) shows an example in which an N-cadherin bead was associated with the surface of a round cell and slowly moved from the initial point of association to the pole from which later on the first neurite grew. In contrast, control beads coated with Tenascin C (Figure 4C, lower panel) moved randomly on the neuronal surface. Staining for the endogenous protein revealed that the N-cadherin beads were accumulated at the same place where cells showed the highest level of endogenous protein (Figure 4D and E), that is, the base of the growing first neurite, again proving the validity of our approach. Control beads by contrast were randomly distributed and not bound to specific sites of the neurons (Figure 4C, lower panel). The specificity of this approach is indicated by the fact that the majority of N-cadherin beads (85%; 34 out of 42 cells) but not the control beads stably remained at or were recruited to the N-cadherin enriched site from which the first neurite grew (Figure 4E). Together with the stripe data, these results demonstrate that N-cadherin marks and influences the site of neuronal first asymmetry.
Figure 4.
N-cadherin accumulates in one pole and mediates centrosome repositioning via PI3K and actin. (A) Labelling of surface N-cadherin and alignment of maxima of N-cadherin surface fluorescence in round neurons shows the presence of an N-cadherin accumulation (circular graph represents the mean fluorescence at the cell surface respect the maximum: experimental n=3, 15 cells/experiment). (B) This N-cadherin accumulation is not always correlated with the Golgi pole as shown by a frequency distribution of the angles between the Golgi and N-cadherin maxima in individual neurons in the circular frequency plot. (C) Beads (1 μm diameter) coated with N-cadherin (green) are recruited to the site from which the first neurite is growing (upper panel), while Tenascin C coated control beads (red) are not recruited to any specific site of the cell surface (lower panel). (D) N-cadherin is still concentrated at the site from which the first neurite grows, here demonstrated by the polarized surface distribution of N-cadherin and (E): the stable attachment of N-cadherin-coated beads. All scale bars=5 μm. (F, G) Hippocampal neurons grown in the presence of specific inhibitors on coverslips coated with alternating stripes of N-cadherin and PLL were fixed after different times and immunolabelled with an antibody recognizing the centrosome or the Golgi and the neuron-specific antibody anti-βIII-tubulin. (F) Fraction of neurons recruiting the Golgi/centrosome towards N-cadherin. (G) Fraction of neurons orienting the first neurite towards N-cadherin. (H) Hippocampal neurons were grown on homogeneous PLL in the presence of toxins, fixed and immunolabelled as in (F, G). Fraction of neurons grown only on PLL in which the first bud is located at the Golgi/centrosome pole. (F–H) ⩾10 neurons/experiment. Experimental n=3. ANOVA followed by Dunnett's test versus control, **P<0.01.
To determine whether first morphological deformation and intracellular polarization are mechanistically linked, we incubated neurons seeded on N-cadherin—PLL stripes with drugs interfering with signalling cascades shown to be involved in the regulation of polarity. The use of different specific inhibitors for PI3K (LY294002 and Wortmannin) and GSK3 (SB216763 and CHIR98014) revealed that the movement of Golgi/centrosome to the N-cadherin site depends on PI3K but not on GSK3 signalling (Figure 4F). Moreover, an intact actin and microtubule cytoskeleton is required, since translocation to the N-cadherin stripe was inhibited by either the actin destabilizing drug cytochalasin D, the F-actin stabilizing drug Jasplakinolide or the microtubule stabilizing drug taxol (Figure 4F). To test whether this occurs in neurons growing on a homogeneous substrate, newly developing neurons were incubated in the presence of the same drugs. This experiment confirmed that organelle translocation to the first neurite depends on the same molecules required for N-cadherin-oriented organelle recruitment (Figure 4H). On the other hand, cytochalasin D or Jasplakinolide, but not drugs decreasing microtubule dynamics or PI(3,4,5)P3 formation, prevented the preferential formation of the first neurite on N-cadherin stripes (Figure 4G). These results suggest that the very first apparent step of polarization (membrane deformation) is mainly actin-driven while the second (organelle translocation) requires actin, microtubules and PI3K. Therefore, these processes, although being mechanistically linked, can be considered as separate events.
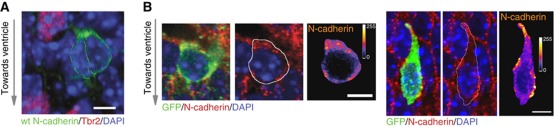
To gain insight into the role of N-cadherin in neuronal polarity in vivo, we analysed the distribution of GFP N-cadherin in embryonic neurons after in utero electroporation (Figure 5A). Freshly divided, still attached to each other and not yet differentiated pairs of GFP-positive neurons in the SVZ presented a strong polarization of the N-cadherin–GFP at that pole which showed a small deformation of the membrane (Figure 5A; Supplementary Figure S3A). To ascertain that this is not due to overexpression, we analysed the distribution of endogenous N-cadherin in newly born neurons in the cortex. In order to identify freshly generated neurons and to be able to clearly follow their perimeter, we used mice expressing a membrane-bound GFP under the neuron-specific βIII-tubulin promoter, which is activated early after neurogenesis (Attardo et al, 2008). In E14.5 and E15.5 embryos, N-cadherin appeared enriched at the apical surface in radial glia cells, which are present along and facing the lateral ventricles (Kadowaki et al, 2007). It is known that after asymmetric cell division, this apical membrane remains largely in the daughter radial glia cell (Kosodo et al, 2004). However, analysis of N-cadherin distribution at high magnification revealed the presence of the protein also in neurons in the upper cell layers, in the SVZ, the intermediate zone (IZ) and the cortical plate (CP). Interestingly, in these regions N-cadherin is present in distinct patches (Figure 5B; Supplementary Figure S3B). The analysis of 30 individual undifferentiated neurons in the VZ and SVZ revealed a clear accumulation of N-cadherin to one pole of the cells (Figure 5B; Supplementary Figure S3B). In 66% of the cases, this accumulation was evident in one or two of the poles oriented in the radial direction and only in 33% of the cells located orthogonally.
Figure 5.
N-cadherin distribution in vivo. (A) A pair of neurons just generated in the SVZ (note the adjacent Tbr2-positive basal progenitors) show a crescent of N-cadherin GFP towards the CP. (B) Paraffin sections from Tubb3–mGFP mice were labelled with N-cadherin. Two examples are shown: the left picture shows the co-labelling of mGFP (new neuron), N-cadherin and DAPI (nucleus). The middle picture shows N-cadherin and DAPI with the outline of the GFP-positive cell drawn and the right picture the intensity of N-cadherin only in this GFP positive selected neuron. Scale bars=5 μm.
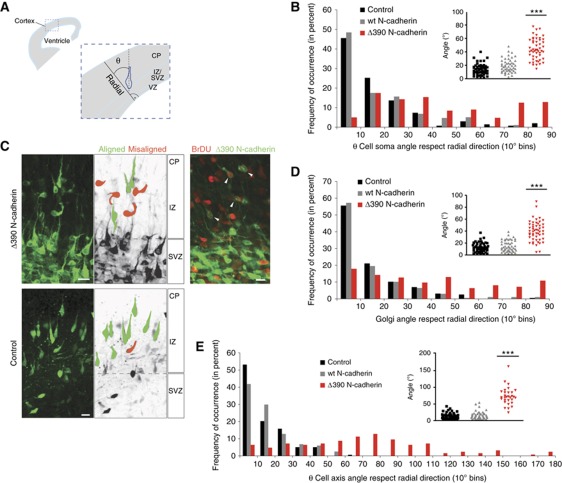
As our results in vitro indicated that N-cadherin plays a role in the spatial selection of the first neurite, and since the second neurite originates by default from the opposite site (de Anda et al, 2008; Menchon et al, 2011), it is likely that, in vivo, N-cadherin defines the orientation of the polarity axis and that the lack of a functional N-cadherin would result in neuronal cell axis misalignment. Neurons developing in a conditional N-cadherin knockout mice indeed displayed a disoriented cell axis but is was not clear whether this was due to a disruption of radial glia cell morphology (Kadowaki et al, 2007). Therefore, in order to study cell-autonomous effects, we interfered with endogenous N-cadherin with a dominant negative N-cadherin (N-cadherinΔ390), which lacks a large part of the extracellular domain (Fujimori and Takeichi, 1993; Shoval et al, 2007). Supplementary Figure 3C shows lack of reorientation of the centrosome towards a local N-cadherin substrate in neurons in vitro transfected with these mutants, indicating the validity of these constructs for loss-of-function experiments. Mutant N-cadherin was transfected in E14.5 embryos by in utero electroporation. We then analysed the orientation of transfected neurons in the lower CP upper IZ just after their exit from the SVZ, and when they are just about to radially migrate towards the upper layers of the CP (Gotz and Huttner, 2005; Kriegstein et al, 2006). A large proportion of newly generated neurons is present in this zone (Figure 6B and C) probably derived from basal progenitors (Kowalczyk et al, 2009). GFP-transfected control neurons were, as expected, aligned with their bipolar axis in the radial direction, as well as neurons transfected with wt N-cadherin–GFP (Figure 6B and C). However, the bipolar cell axis of neurons transfected with Δ390N-cadherin–GFP significantly deviated from this radial direction (Figure 6B and C). Birthdating experiments using BrDU and EdU co-labelling revealed that the analysed neurons are in a large percentage younger than 11h (ca. 36–40% of transfected neurons were BrDU positive after an 11 h pulse) (Figure 6C). This suggests that N-cadherin-mediated cell–cell interactions are important shortly after cell division for the site selection of the initial polarity (first neurite formation) and hence for the orientation of the bipolar axis. The Golgi (and in turn the centrosome) position was as well misaligned in neurons transfected with Δ390N-cadherin (Figure 6D). In accordance with the findings that centrosome positioning later on is important for radial directed migration (Solecki et al, 2004), we found that neurons, transfected with dominant negative N-cadherin, migrated shorter distances in the CP region (Supplementary Figure S3D) and exited less frequently the SVZ (Supplementary Figure S3E) as compared with GFP or wt N-cadherin-transfected neurons supporting the need of proper axis establishment for directed migration. To further assess the acute role of N-cadherin in the establishment of polarity, we analysed the axis orientation of neurons expressing dominant negative N-cadherin which developed on top of vibratome-cut wild-type cortical coronal sections. As previously described (Polleux et al, 1998), control neurons that attached in the CP area grew their first neurites in a direction determined by their position with respect to the slice architecture, that is, a radial direction in the CP zone but randomly in the IZ (Supplementary Figure S3F), an alignment that replicates exactly the orientation of endogenous neurons in those slices, suggesting that overlaid neurons follow the same endogenous polarity cues. Neurons transfected with GFP or wt N-cadherin–GFP and seeded on the CP area extended their neurites along the radial axis, while neurons transfected with the dominant negative construct deviated from this axis considerably, and were found in nearly random orientation (Figure 6E). Hence, N-cadherin-mediated signalling regulates initial morphological polarization and, by virtue of it, the establishment of a polarized axis of growth.
Figure 6.
N-cadherin orients the cell axis and influences polarity in vivo. (A) Schematic representation of our measurement of cell axis orientation in the developing cortex. Radial direction is perpendicular to the VZ. The angle deviating from radial direction (θ=0) has been quantified in all experiments and displayed as frequency distributions. (B) In utero electroporation of the lateral ventricles of E14.5 mice with GFP control vectors, wt N-cadherin–GFP or dominant negative Δ390N-cadherin–GFP. Neurons exiting the SVZ are analysed. (C) Representative z-stack projections of neurons analysed in (B). The right upper panel shows neurons transfected with Δ390N-cadherin–GFP (E14.5–E16.5), which were pulse labelled for 11 h with BrDU. (D) Golgi orientation with respect to the radial direction of the same neurons as in (B). (E) Quantification of embryonic cortical neurons transfected with wt N-cadherin–GFP, dominant negative Δ390 N-cadherin–GFP or dt-Tomato (internal control) were grown on coronal cortical sections. (B, D, E) All insets: population distribution (***P<0.001, Kruskal–Wallis with Dunn's multiple comparison test control and wt versus Δ390). Scale bars=10 μm.
Taken together, our data suggest an important instructive role of N-cadherin in the establishment of early polarity in neurons developing in vitro and in vivo.
Discussion
We here showed that the centrosome and the Golgi move towards the site of the first morphological polarization following a signal from the plasma membrane, clearly demonstrating that they are not the inducing event in neuronal polarization. Although this contradicted our own (de Anda et al, 2005) and other (Lefcort and Bentley, 1989; Zmuda and Rivas, 1998) data, which indicated that Golgi/centrosome position is instructive for first neurite growth, the current data are in line with findings in Drosophila and zebrafish, which showed that the position of the centrosome does not predict the site of polarization (Basto et al, 2006; Pollarolo et al, 2011; Randlett et al, 2011). The reason for the discrepancy between our own previous work (de Anda et al, 2005) and the current results seems to reside in the high temporal resolution time-lapse studies performed here, which allowed for a finer analysis of this intracellular organelle dynamics during morphological differentiation. A different question is whether Golgi and centrosome function plays a role in later steps in neuronal differentiation. It has been widely demonstrated that Golgi and centrosome recurrently localize at the base of the first neurite or at the leading edge of migrating neuron, suggesting a crucial role for axonal growth and neuronal migration (Solecki et al, 2009; de Anda et al, 2010). Yet, recent work in neurons with mechanically ablated centrosomes showed that this organelle is not necessary for axonal growth (Stiess et al, 2010). However, polarized membrane trafficking occurs normally in neurons lacking centrioles (Pollarolo et al, 2011), indicating that intracellular polarized membrane and microtubule asymmetry is inherent during neuronal polarization, irrespective of the mechanism by which this asymmetry is produced.
In addition to the above point, our data suggest the existence of a ‘signpost’ for polarized growth, which we identified as the cell adhesion molecule N-cadherin (Figures 2 and 3). Local stimulation with extrinsic N-cadherin was sufficient for the specification of the site from which the first neurite would grow (Figure 2A and C) and where Golgi and centrosome are recruited (Figure 2B and C). In a search for pathways, which mediate those effects, we found that the N-cadherin-triggered first neurite formation is mainly actin-driven while the organelle translocation requires actin, microtubules and PI3K but not GSK3 activity (Figure 4F and G). This observation is supported by numerous other evidences: cell–cell contacts related to adherens junctions locally recruit dynein in an actin-driven process which then, via β-catenin, recruits microtubules (Ligon et al, 2001). In addition at the leading edge of migrating neurons, the forward actin flow driven by myosin II has a direct influence on soma and centrosome translocation (He et al, 2010). Cadherin signalling is also able to alter microtubule organization by stabilizing microtubule ends (Chausovsky et al, 2000). PI3K is known to influence the efficiency of N-cadherin interaction with β-catenin (Zhang et al, 2010) to regulate the stability of microtubules and F-actin and to contribute to asymmetric PIP3 distribution. Yet, different from what was shown for axon formation (Zhou et al, 2004), PI3K does not seem to act via GSK3-inhibition (Figure 4F).
Neurons in vitro can establish their polarity in the absence of asymmetric extracellular cues and therefore the importance of N-cadherin signalling was questionable. We have two lines of evidence that also in vitro N-cadherin is used as a spatial cue. Indirect evidence is provided by experiments showing that the same pathways are needed to recruit the centrosome and Golgi to the first neurite in isolated neurons (Figure 4H) as shown for neurons challenged with extracellular N-cadherin (Figure 4F). Second, we could directly show an accumulation of membrane-bound N-cadherin on one pole of the neuron (Figure 4A and B) and in a second step demonstrate that this is the place from which later the first neurite grows (Figure 4C). Thus, we propose that in vitro cells are ready to receive extracellular signals in order to cluster their N-cadherin to one pole or alternatively, in the absence of those directing signals, use stochastic mechanisms (Menchon et al, 2011) to polarize N-cadherin.
Importantly, we also detected a polarized distribution of N-cadherin in freshly generated neurons in vivo (Figure 5). We argued that if N-cadherin is able to provide the initial signal for the orientation of the first neurite, and taking into account that the second neurite grows from the opposite site, it was then logical that N-cadherin should be important for neuronal axis alignment in vivo. In two different experiments, we demonstrated that this is the case, showing that neurons with a defective N-cadherin are not able anymore to properly establish their radial alignment of the cell axis (Figure 6), which later on leads to migration defects (Supplementary Figure S3D). A cadherin-landmark at the neuronal cell surface was also described in polarizing sensory neurons of the drosophila notum (Pollarolo et al, 2011), which indicates that the mechanisms of polarization are highly conserved among species and neuronal populations.
There are two other recent reports demonstrating migration defects due to defective N-cadherin signalling, both proposing a role of N-cadherin during glia-independent migration (multipolar migration or somal translocation) (Franco et al, 2011; Jossin and Cooper, 2011). They both suggest that Reelin signals via Rap1 in order to regulate N-cadherin expression levels. Our demonstration of the instructive role of N-cadherin on the very first steps of neuronal polarity and its role in the orientation of the neuronal cell axis indicates that, in addition, N-cadherin plays an essential role in an earlier event in cortical development: neuronal first polarization (different from the polarization that occurs after all neurites have formed). In further studies it will be important to understand how those different levels of control of cortical development by N-cadherin are interconnected. Our results are in line with the recent finding that the zebrafish homologue of N-cadherin, cadherin 2, is important for the directional chain migration of cerebellar granule neurons and for the localization of the centrosome in front of the nucleus in migrating cells (Rieger et al, 2009). A cell-autonomous feed-forward signalling by lateral clustering of N-cadherin is very likely to strengthen cell–cell interactions (Nelson, 2008). In future studies, it will be interesting to understand whether N-cadherin molecules expressed by the surrounding radial glia cells or by adjacent neurons constitute the signals that align migrating neurons. Also ours and other data (Kadowaki et al, 2007; Jossin and Cooper, 2011) suggest that axon formation is not prevented, which suggest a pure instructive role of N-cadherin. It will be crucial to reveal how plastic these N-cadherin-mediated polarity changes are during development, and how early polarity signals are passed on to later stages, especially considering the fact that in the mammalian cortex neurons migrate and encounter changes in environmental cues until they reach their final position. Moreover, it is quite likely that in order to achieve the complexity observed in the cortical architecture other adhesion molecules are needed.
In conclusion, our data demonstrate that a localized N-cadherin signalling constitutes an early landmark for the orientation of polarized outgrowth of neurons of the cortex and hippocampus, defining the site of breakage of morphological symmetry and triggering the recruitment of intracellular organelles to support further growth. However, the oriented migration of axons in zebrafish retinal neurons involves laminin signalling (Randlett et al, 2011), suggesting that different types of adhesion signals may define the site of polarization in different neuronal populations. Future work will elucidate this aspect. In any event, our data unveil one critical mechanism involved in the initial asymmetry of neurons, a process of fundamental impact for the subsequent development of the entire central nervous system.
Materials and methods
Constructs
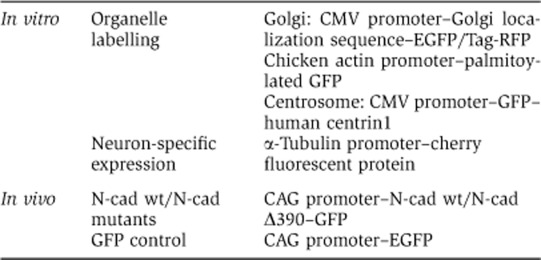
The Golgi was fluorescently marked by tagging a Golgi localization sequence (the N-terminal 81 aa of human galactosyltransferase (Llopis et al, 1998) at the N-terminus with either EGFP or Tag-RFP (kind gifts of O Griesbeck, Max Planck Institute of Neurobiology, Martinsried, Germany) or by using a palmitoylated version of EGFP, labelling intra- and extracellular membranes including the Golgi (Supplementary Figure S1A). The palmitoylated EGFP is an EGFP N-terminally tagged by the first 20 aa of GAP-43 expressed under the control of the chicken actin promoter. The centrosome was visualized by transfecting neurons with GFP-tagged human centrin1 (Piel et al, 2000). GFP-tagged N-Cadherin mutants cloned in pCAGG vector were kindly provided by C Kalcheim, Hebrew University-Hadassah Medical School, Jerusalem, Israel (Shoval et al, 2007). The fluorescent protein dt-Tomato was kindly provided by RY Tsien (HHMI—UCSD, La Jolla, USA). The pCAG-Venus vector was kindly provided by F Calderon de Anda (MIT Boston, USA). PCAG–GFP was from Addgene. The plasmid expressing cherry under the control of the α-tubulin promoter was a kind gift of SAM Shariati (KUL, Leuven, Belgium).
Overview of the used constructs:
Primary cultures
Rat embryonic hippocampal neurons were prepared (Banker and Goslin, 1988) and plated at a density of 2500 cells/cm2 on PLL-coated coverslips. Neurons were transfected in suspension using nucleofection (Amaxa). Transfected neurons were kept in suspension before plating for 2 h to allow the expression of the transgene and at the same time avoid the initiation of differentiation. Moreover that provides time to regenerate surface molecules (N-cadherin, integrins etc.), which may have been shed by the trypsin treatment used for dissociation of the neuronal tissue (Takeichi and Nakagawa, 2001).
Precursor cultures were obtained from E12.5 mouse cortices. Cortices were dissociated with Papain (Sigma; 10 U/ml), the tissue mechanically triturated and the cells were plated at a density of 2500–250 000 cells/cm2 on PLL (Sigma)-coated coverslips in DMEM with 1 mM Na-Pyruvate, Gln, N2, B27 (all Invitrogen), 1 mM N-acetyl-cysteine (Sigma) and 10 ng/ml bFGF. Neurons were transfected in suspension using nucleofection (Amaxa).
Culturing of neurons on patterned substrata
Coverslips with alternating stripes of Laminin (100 μg/ml; Sigma), Tenascin C (25 μg/ml; Chemicon) or N-Cadherin (10 μg/ml; R&D Systems, N-terminal extracellular domain) and PLL were prepared using silicon matrices (S Lang, MPI for Developmental Biology, Tübingen). PLL-coated coverslips were placed on top of the dry matrices and the channels filled with the substrates and a labelled secondary antibody Alexa 568) in PBS. After 2 h incubation at 37°C, channels were washed with PBS and neurons plated immediately. The development of individual neurons was observed on CELLocate coverslips (Eppendorf) or by time-lapse microscopy. Cytochalasin D (1 μM; Sigma), Jasplakinolide (1 μM; Invitrogen), Taxol (5 μM; Tocris), the PI3Kinase inhibitors LY294002 (50 μM; Promega) or Wortmannin (200 nM; Echelon Biosciences), or the GSK3 inhibitors SB216763 (40 μM; Tocris) or CHIR 98014 (500 nM; Axon Medchem) were added during the plating.
Slice overlay assay
The overlay assay was performed as described in detail in Polleux and Ghosh (2002). Cortices from E14.5; E15.5; or E16.5 mice were dissociated with Papain (Sigma; 10 U/ml), the tissue was mechanically triturated and the cells were transfected in suspension by nucleofection (Amaxa). Cells were kept for 2–3 h in suspension in slice medium and subsequently plated at a density of 2 million/ml on top of cortical coronal vibratome-cut slices from mice of the same age. Red fluorescent Td-tomato-transfected control cells were seeded together with the green fluorescent experimental neurons (transfected with wt N-cadherin–GFP or Δ390 N-cadherin–GFP) on the same slices, and served as indicator of zones where the alignment is radial (internal control). Quantification of slice overlay was performed using the open source ImageJ software (Rasband, WS, ImageJ, US National Institutes of Health, Bethesda, MD, USA). Briefly, images were manually aligned in accordance with the anatomy of coronal slices, and the angle of the radial axis of the slice was arbitrarily set to 0°. The orientation of the neurites was evaluated in ImageJ with the ‘fit ellipse’ measure tool for single neurons, normalized to the slice reference and plotted as frequency distribution.
Bead coating
Polybead® microspheres (diameter, 1 μm; Polysciences) were precoated for 1 h at 37°C with PLL and washed. Control beads were coated in borate buffer for 1 h at 37°C with anti-human Alexa 568 antibody in combination with Tenascin C (25 μg/ml; Chemicon) and ‘detection beads’ with anti-human Alexa 488 antibody in combination with N-cadherin N-terminal extracellular domain (10 μg/ml; R&D Systems). Following passive adsorption, microspheres were washed in borate buffer and resuspended in medium for imaging.
Live cell imaging
Neurons were transfected after cell dissociation using nucleofection (Amaxa) and kept in suspension for 2 h after transfection. Cells were then transferred to PLL-coated coverslips and immediately imaged at high frequency in closed stainless steel chambers. The chambers were tightly closed to avoid pH changes. Neuronal development and distribution of intracellular organelles and fluorescent beads (phase and fluorescent channels) was observed using a Nikon confocal microscope equipped with a heated stage in time frames of 2–10 min using the perfect focus system. Alternatively, epi-fluorescent images were taken using a CellR Olympus microscope equipped with a heated stage.
In utero electroporation
All animal experiments were approved by the Ethics Committee of the KU Leuven. Plasmids were injected in the lateral ventricles of E14.5 mouse embryos. Pregnant Swiss mice (E14.5) were anaesthetized by intramuscular injections of 88 μg ketamine and 132 μg xylazine per gram of body weight, uterine horns were exposed and the plasmids (1–2 μg/μl) mixed with Fast Green (Sigma) was microinjected in the lateral ventricles of embryos. Five current pulses (50 ms pulse/950 ms interval) were delivered across the head of the embryos (36 V) targeting the dorsal-medial part of the cortex. EdU was injected intra-peritoneal at a concentration of 3.3 mg/kg, and BrDU at 33 mg/kg body weight and BrDU additionally added at 0.8 mg/ml to the drinking water. Embryos were perfused with PBS and 4% paraformaldehyde (PFA), the brains dissected and postfixed for 6–10 h in 4% PFA at 4°C. Vibratome sections (100 μm) were collected and prepared for immunolabelling. Quantification of cell alignment in utero was performed as in the case of slice overlay experiments. The orientation of the Golgi was also evaluated with the ‘fit ellipse’ measure tool in single neurons, and all angular values were normalized to the slice reference and plotted as frequency distribution.
Immunocytochemistry
Neurons were fixed with 4% PFA (with 1.44 M sucrose, 1 M MgCl2, 100 mM EGTA) at 37°C for 10 min or for 3 min followed by fixation for 3 min in MeOH at −20°C. Cells were permeabilized for 3 min in 0.1% Triton X-100/PBS. After blocking in 2% FBS, 2% BSA, and 0.2% fish gelatin in PBS, neurons were incubated with the primary antibody (see Supplementary data) for 1 h at room temperature or at 4°C overnight. Secondary Alexa-conjugated antibodies (Invitrogen) were added for 45 min after washing in PBS.
Organotypic cultures were fixed with 4% PFA at 37°C for 30 min, permeabilized and blocked at 4°C overnight in PBS/0.3% Triton X-100/3% BSA/3% goat, incubated with the primary antibody at 4°C overnight and the secondary Alexa-conjugated antibodies for 3 h at room temperature.
Vibratome 100 mm sections of embryonic brains were permeabilized and blocked at 4°C for 2 h in PBS/0.3% Triton X-100/3% BSA/3% goat, incubated with the primary antibody at 4°C overnight and the secondary Alexa-conjugated antibodies for 3 h at room temperature. For BrDU detection, slices were pretreated with 1 M HCl (10 min 4°C) and 2 M HCl (10 min RT and 20 min 37°C) with subsequent washes in 0.1 M borate buffer. EdU-positive cells were identified using the Click-iT® EdU Alexa Fluor® 647 Imaging Kit (Invitrogen).
Paraffin-embedded sections: embryonic brains were fixed in 4%PFA followed by progressive alcohol-assisted dehydration and paraffin embedding. In all, 6 μm thick sections were processed for immunohistochemistry using an automated platform (Ventana Ultra, Ventana Medical Systems, Roche; details of procedures can be obtained at request).
The following primary antibodies were used: anti-pericentrin and anti-βIII-tubulin from rabbit (Covance); anti-GM130, anti-β-catenin, anti-p120-catenin and anti-N-cadherin (against intracellular domain) from BD Transduction Laboratories; anti-γ-tubulin, anti-α-catenin, anti-βIII-tubulin and anti-N-cadherin (Sigma); anti-α-tubulin (Calbiochem); anti-tau-1 and anti-NeuN (Chemicon); anti-P-tyrosine (Upstate); anti-GFP (rabbit: Invitrogen; chicken: Aves labs), Ki67 (Novacastra), Tbr2 (Abcam). Nuclei were visualized using the Hoechst compound (Invitrogen). N-cadherin on the surface of neurons was detected by incubating neurons after fixation without permeabilization with an antibody recognizing a surface epitope of N-cadherin (clone GC-4; Sigma).
Supplementary Material
Acknowledgments
We thank C Kalcheim, O Griesbeck, F Calderon de Anda and RY Tsien for providing material; C Haffner and F Calderon de Anda for technical help and A Attardo for providing the tub-mGFP mouse line. This work was made possible by the type 3 large-infrastructure support InfraMouse by the Flanders Hercules Foundation (ZW09-03). This work was partially supported by the Flanders Fund for Scientific Research (FWO G 0.666.10N), the Federal Office for Scientific Affairs (IUAP p6/43) and Flemish Government Methusalem Grant to CGD. EFF has been in part supported by an EMBO fellowship (ASTF: 369.00-2007). FV was supported by the Italian Institute of Technology (Satellite Unit in Molecular Neuroscience).
Author contributions: The overall study was conceived and supervised by CGD and AG. AG and EFF designed, performed and analysed most of the experiments. SM supported the life imaging experiments. KV performed some of the experiments with dissociated neurons. ES performed the stainings on paraffin sections. WBH provided the tubulin GFP mice. AG, EFF, FV and CGD wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Attardo A, Calegari F, Haubensak W, Wilsch-Brauninger M, Huttner WB (2008) Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS One 3: e2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Goslin K (1988) Developments in neuronal cell culture. Nature 336: 185–186 [DOI] [PubMed] [Google Scholar]
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW (2006) Flies without centrioles. Cell 125: 1375–1386 [DOI] [PubMed] [Google Scholar]
- Chausovsky A, Bershadsky AD, Borisy GG (2000) Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol 2: 797–804 [DOI] [PubMed] [Google Scholar]
- Cowan CR, Hyman AA (2004) Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 431: 92–96 [DOI] [PubMed] [Google Scholar]
- de Anda F, Gartner A, Tsai LH, Dotti CG (2008) Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci 121: 178–185 [DOI] [PubMed] [Google Scholar]
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH (2010) Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci 30: 10391–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda FC, Pollarolo G, Da Silva JS, Camoletto PG, Feiguin F, Dotti CG (2005) Centrosome localization determines neuronal polarity. Nature 436: 704–708 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Banker G (1991) Intracellular organization of hippocampal neurons during the development of neuronal polarity. J Cell Sci Suppl 15: 75–84 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G (1999) Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci 19: 6417–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69: 482–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M (1993) Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell 4: 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788 [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Murakami F (2002) In vitro analysis of the origin, migratory behavior, and maturation of cortical pyramidal cells. J Comp Neurol 454: 1–14 [DOI] [PubMed] [Google Scholar]
- He M, Zhang ZH, Guan CB, Xia D, Yuan XB (2010) Leading tip drives soma translocation via forward F-actin flow during neuronal migration. J Neurosci 30: 10885–10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Cooper JA (2011) Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 14: 697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M (2007) N-cadherin mediates cortical organization in the mouse brain. Dev Biol 304: 22–33 [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB (2004) Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. EMBO J 23: 2314–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF (2009) Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex 19: 2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V (2006) Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci 7: 883–890 [DOI] [PubMed] [Google Scholar]
- Lefcort F, Bentley D (1989) Organization of cytoskeletal elements and organelles preceding growth cone emergence from an identified neuron in situ. J Cell Biol 108: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur EL (2001) Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 3: 913–917 [DOI] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY (1998) Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci USA 95: 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchon SA, Gartner A, Roman P, Dotti CG (2011) Neuronal (bi)polarity as a self-organized process enhanced by growing membrane. PLoS One 6: e24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ (2008) Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36: 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7: 136–144 [DOI] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M (2000) The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol 149: 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollarolo G, Schulz JG, Munck S, Dotti CG (2011) Cytokinesis remnants define first neuronal asymmetry in vivo. Nat Neurosci 14: 1525–1533 [DOI] [PubMed] [Google Scholar]
- Polleux F, Ghosh A (2002) The slice overlay assay: a versatile tool to study the influence of extracellular signals on neuronal development. Sci STKE 2002: PL9. [DOI] [PubMed] [Google Scholar]
- Polleux F, Giger RJ, Ginty DD, Kolodkin AL, Ghosh A (1998) Patterning of cortical efferent projections by semaphorin-neuropilin interactions. Science 282: 1904–1906 [DOI] [PubMed] [Google Scholar]
- Polleux F, Morrow T, Ghosh A (2000) Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature 404: 567–573 [DOI] [PubMed] [Google Scholar]
- Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME (1997) Development of polarity in cerebellar granule neurons. J Neurobiol 32: 223–236 [DOI] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA (2011) The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron 70: 266–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger S, Senghaas N, Walch A, Koster RW (2009) Cadherin-2 controls directional chain migration of cerebellar granule neurons. PLoS Biol 7: e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM (2006) Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci 9: 1274–1283 [DOI] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C (2007) Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 134: 491–501 [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME (2004) Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci 7: 1195–1203 [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Trivedi N, Govek EE, Kerekes RA, Gleason SS, Hatten ME (2009) Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron 63: 63–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F (2010) Axon extension occurs independently of centrosomal microtubule nucleation. Science 327: 704–707 [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM (2006) Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443: 462–465 [DOI] [PubMed] [Google Scholar]
- Takeichi M, Nakagawa S (2001) Cadherin-dependent cell-cell adhesion. Curr Protoc Cell Biol 9: Unit: 9.3.1–9.3.15 [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM (2003) Reelin and brain development. Nat Rev Neurosci 4: 496–505 [DOI] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF (1993) Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 7: 996–1003 [DOI] [PubMed] [Google Scholar]
- Wiggin GR, Fawcett JP, Pawson T (2005) Polarity proteins in axon specification and synaptogenesis. Dev Cell 8: 803–816 [DOI] [PubMed] [Google Scholar]
- Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A (2010) Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell 18: 472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42: 897–912 [DOI] [PubMed] [Google Scholar]
- Zmuda JF, Rivas RJ (1998) The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskeleton 41: 18–38 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.