Abstract
Visceral adipose tissue (VAT) is associated with adverse health effects including cardiovascular disease and type 2 diabetes. We developed a dual-energy X-ray absorptiometry (DXA) measurement of visceral adipose tissue (DXA-VAT) as a low cost and low radiation alternative to computed tomography (CT). DXA-VAT was compared to VAT assessed using CT by an expert reader (E-VAT). In addition, the same CT slice was also read by a clinical radiographer (C-VAT) and a best-fit anthropomorphic and demographic VAT model (A-VAT) was developed. Whole body DXA, CT at L4–L5, and anthropometry were measured on 272 black and white South African women (age 29 ± 8 years, BMI 28 ± 7 kg/m2, waist circumference (WC) 89 ± 16 cm). Approximately one-half of the dataset (n = 141) was randomly selected and used as a training set for the development of DXA-VAT and A-VAT, which were then used to estimate VAT on the remaining 131 women in a blinded fashion. DXA-VAT (r = 0.93, standard error of the estimate (SEE) = 16 cm2) and C-VAT (r = 0.93, SEE = 16 cm2) were strongly correlated to E-VAT. These correlations with E-VAT were significantly stronger (P < 0.001) than the correlations of individual anthropometry measurements and the A-VAT model (WC + age, r = 0.79, SEE = 27 cm2). The inclusion of anthropometric and demographic measurements did not substantially improve the correlation between DXA-VAT and E-VAT. DXA-VAT performed as well as a clinical read of VAT from a CT scan and better than anthropomorphic and demographic models.
Introduction
Visceral adipose tissue (VAT) is considered to be more closely associated with obesity related diseases, such as cardiovascular disease and type 2 diabetes than other indexes of obesity, such as BMI, waist circumference (WC) or waist-to-height ratio (WHtR) (1).
Computed tomography (CT) and magnetic resonance imaging (MRI) are considered “gold standards” for the measurement of VAT, but the radiation exposure associated with CT, the time required for MRI acquisition and image analysis, and the cost of both techniques have limited their use in both research and clinical medicine.
Extensive research has focused on determining alternative methods to estimate VAT. Dual-energy X-ray absorptiometry (DXA) has been considered one such candidate, as DXA can accurately and precisely measure whole body and regional distribution of fat and lean tissue (2,3). In addition, the X-ray radiation associated with a DXA scan is very low (4) (equivalent to about 1 day of natural background radiation) and the cost is relatively modest. Thus, several studies (5,6,7) have examined the ability of DXA to measure VAT by placing various subregions on whole body DXA images. However, these studies have shown that DXA-derived VAT is typically no better than using WC. Because the DXA measurement is a two-dimensional projection, placing subregions on a DXA image integrates both the VAT and the subcutaneous adipose tissue (SAT) in the region. We hypothesized that a more sophisticated DXA measurement would significantly improve the ability to predict VAT compared with anthropomorphic and demographic measurements such as WC, WhtR, BMI, weight, height, age, and ethnicity.
Methods and Procedures
Subjects
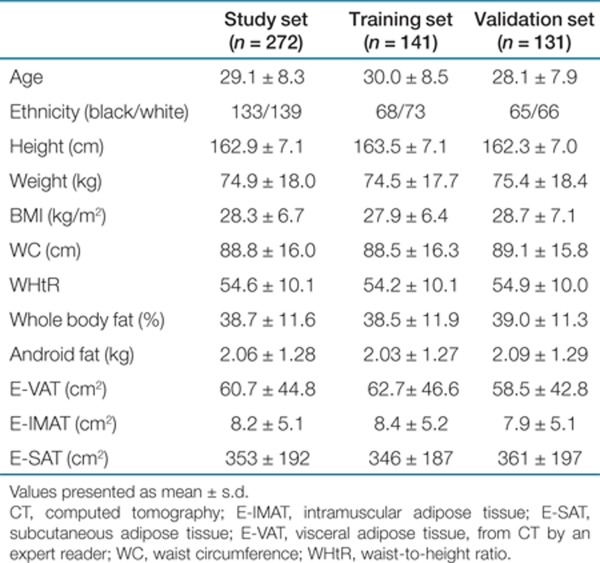
Two hundred and seventy-two women (age: 18–49 years, BMI: 17.7–45.8 kg/m2), approximately one-half self-described white (n = 139) and one-half self-described black (n = 133) were recruited from a community in Cape Town, South Africa. Inclusion criteria were age 18–45 years, no known metabolic disease, and not currently lactating, pregnant, or postmenopausal. From this study set, 141 women were randomly selected as a training set, while the remaining 131 women were reserved as a validation set and the developers of DXA-VAT were blinded to the CT VAT results of these 131 women. Comparing the age and body composition variables (Table 1) between the training and validation set with a two-tailed t-test, no statistically significant differences (P <0.05) were seen.
Table 1. Characteristics of the study subjects.
Measurements
Height (cm) and weight (kg) were measured with subjects wearing light clothing and no shoes. WC was measured at the level of the umbilicus. WC measurements of ten subjects were not recorded, and so these values were excluded from the anthropomorphic analysis.
Whole body DXA was performed using a Hologic Discovery W configured with software version 12.1 (Hologic, Bedford, MA). The DXA field of view was 195 × 65 cm and the DXA table weight limit was 204 kg. A single 10-mm CT slice was taken at the level of L4–L5 lumbar vertebra using a Toshiba Xpress Helical Scanner (Toshiba Medical Systems, Tokyo, Japan).
Analysis
To determine an expert reading of VAT (E-VAT), the images were analyzed under the direction and supervision of one of the authors (M.P.), the Director of the Image Reading Center of the Obesity Research Center, Columbia University who has coauthored many publications where CT and MRI were used for VAT assessment. Briefly, following a standardized protocol regularly used by this research center, a single, trained analyst used image analysis software (SliceOmatic V4.2, TomoVision, Montreal, Quebec, Canada) to measure E-VAT, subcutaneous adipose tissue by an expert reader (E-SAT), and intramuscular adipose tissue by an expert reader (E-IMAT). Thresholding methods were applied to identify the adipose tissue, with the threshold set for −30 to −190 Hounsfield units (HU), then manual delineation, using tools provided by the software, was used to separate SAT, VAT, and IMAT. Finally, pixels within the threshold that were not anatomically one of the three adipose tissue depots were removed. The coefficient of variation for repeated measurements of the same scan on consecutive days by this analyst is 1.7% for E-SAT, 2.3% for E-VAT, and 5.9% for E-IMAT.
For the clinical read of the CT slice, clinical radiographer (C-VAT) and C-SAT were measured by a licensed radiographer (requires a 3 year diploma) with extensive CT experience but without prior experience in visceral fat measurement using the general purpose slice analysis software provided by the CT manufacturer. The methods as presented in Smith et al. (8) for VAT and SAT measurements were followed. However, the additional step of separating SAT into superficial subcutaneous adipose tissue (SSAT) and the deep subcutaneous adipose tissue (DSAT) that Smith et al. describe was omitted and IMAT was not separately measured.
After correlating E-VAT and C-VAT, it was observed that two C-VAT measurements were significant outliers and the C-VAT measurements were therefore excluded from further analysis (the E-VAT measurements were retained).
All standard DXA measurements (excluding DXA-VAT) were analyzed using Hologic APEX 3.1 software (Hologic) according to standard procedures set forth in the users guide for the DXA instrument. The APEX software reports total body and regional fat mass and %fat results. A standard region of the APEX software is the android region, which overlies the abdomen and is described in the body composition user's guide (9). The inferior line of the android region is just at the superior edge of the iliac crest while the superior line is 20% of the distance between the iliac crest and the inferior edge of the subject's chin.
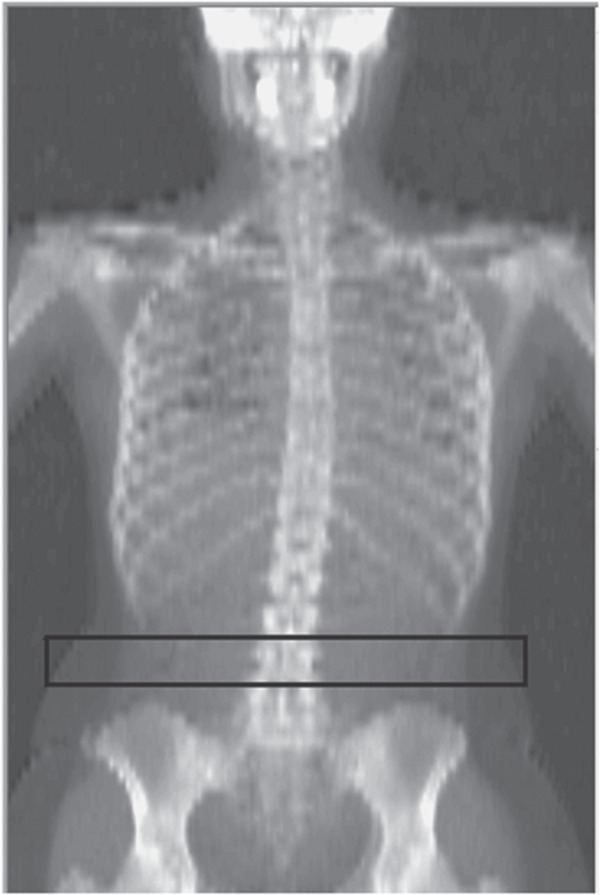
DXA-VAT was measured in a 5 cm wide region placed across the entire abdomen just above the iliac crest at a level that approximately coincided with the 4th lumbar vertebrae (Figure 1) on the whole body DXA scan. This region was selected to avoid any possible interference from iliac crest bone pixels (which might affect the DXA-VAT results) while still being low enough to approximately coincide with the region where VAT was measured by CT.
Figure 1.
DXA-VAT region of measurement. The pure subcutaneous fat lateral to the abdominal muscles can be seen in the image. DXA-VAT, dual-energy X-ray absorptiometry-visceral adipose tissue.
DXA is a two-dimensional projection method, so within the abdominal cavity, DXA measures both the visceral fat and the subcutaneous fat. However, on each side of the abdominal cavity DXA can directly measure the subcutaneous fat. The location of the abdominal cavity can be seen in the DXA image as a change in gray scale from darker to lighter as the tissue becomes a lower %fat due to the muscles of the abdominal wall (Figure 1). Using appropriate modeling, which is described in detail elsewhere (10,11), the amount of subcutaneous fat over the visceral cavity can be estimated from the DXA measurement of the subcutaneous fat on each side of the abdominal cavity. This estimate of the subcutaneous fat overlying the abdominal cavity added to the subcutaneous fat measured by DXA, can be subtracted from the total abdominal fat DXA measured (subcutaneous plus visceral fat) to give DXA-VAT. Stated simply, the lateral abdominal subcutaneous fat seen in the DXA image is used to estimate the anterior and posterior subcutaneous abdominal fat, allowing the visceral fat (DXA-VAT) to be estimated from the total abdominal fat measured.
Half (n = 141) of the DXA scans and anthropomorphic measurements were used as a training set to determine the DXA-VAT model parameters that maximized correlation with E-VAT. Subsequently, DXA-VAT was then calculated for the DXA scans in the validation set (n = 131) while the developers of DXA-VAT were still blinded to all CT measurements of the subjects in the validation set. After the data was unblinded and CT measurements made available, it was observed that a software error had caused two DXA-VAT measurements in the validation set to have grossly incorrect region of interests, not solely over the abdomen as intended but extending to the bottom of the scan table. The software error was corrected and these two DXA-VAT measurements were re-reported and the corrected results were used in the analysis. It was also verified that the software error did not change any of the other DXA-VAT results.
Statistical analysis
Linear regression was used to compare DXA-VAT, C-VAT, A-VAT, as well as individual anthropomorphic parameters (e.g., weight, WC, etc.) and standard DXA measures (e.g., %fat, total android fat, etc.) to E-VAT. Anthropomorphic and demographic VAT model (A-VAT) was created using multiple stepwise regression (both forward and backward) on the training set with the anthropomorphic and demographic variables of age, ethnicity, weight, height, WC, BMI, and WHtR. Limits of agreement between E-VAT and DXA-VAT, C-VAT and A-VAT were determined using the technique of Bland and Altman (12). Statistical analysis was performed using JMP V 5.1.2 (SAS Institute, Cary, NC).
Results
E-VAT correlated with age (r = 0.48, standard error of the estimate (SEE) = 39 cm2), weight (r = 0.70, SEE = 32 cm2), BMI (r = 0.69, SEE = 33 cm2), WC (r = 0.75 SEE = 29 cm2), and WHtR (r = 0.73, SEE = 31 cm2); all correlations were highly statistically significant (P < 0.0001). The best A-VAT determined using the training set and multiple stepwise regression was WC + age (r = 0.79, SEE = 27 cm2); after WC and age no other anthropomorphic or demographic variable entered the model at P < 0.10. The result was the same whether stepwise forward or stepwise backward multiple regression was performed.
E-VAT also correlated with the standard DXA measurements of total body fat (r = 0.78, SEE = 27 cm2), total body %fat (r = 0.68, SEE = 31 cm2), android fat (r = 0.79, SEE = 26 cm2), and android %fat (r = 0.70, SEE = 30 cm2); all correlations were highly statistically significant (P < 0.0001).
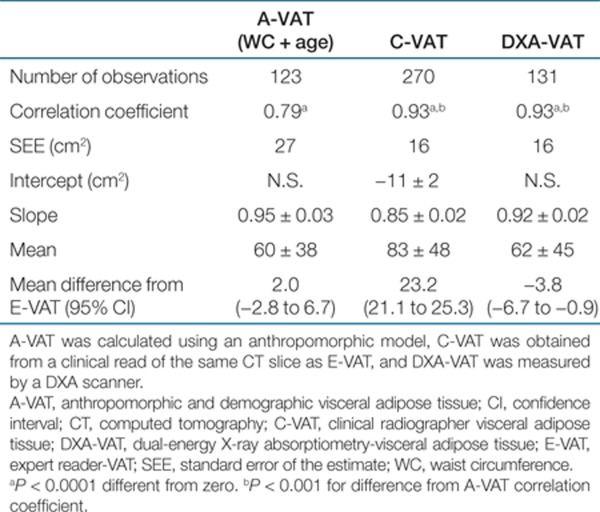
DXA-VAT and C-VAT were both highly correlated with E-VAT (Table 2 and Figure 2) with no detectable difference between the strength of the correlation between DXA-VAT or C-VAT vs. E-VAT (P = 0.79, i.e., the correlations reported in Table 2 for DXA-VAT and C-VAT were not statistically different from each other). In addition, both the associations between DXA-VAT and C-VAT vs. E-VAT were significantly (P < 0.001) stronger than that of A-VAT vs. E-VAT or any of the standard DXA measures vs. E-VAT (P < 0.001).
Table 2. Linear regression estimates between an expert read of VAT (E-VAT) and the three other VAT measures.
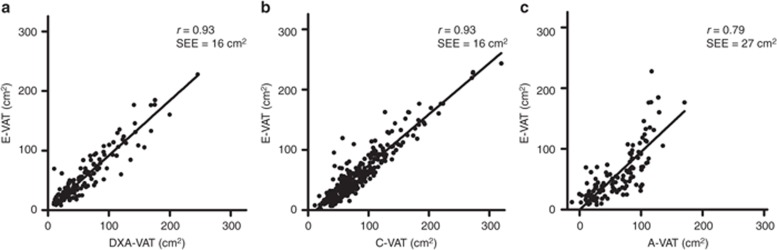
Figure 2.
Correlations between (a) E-VAT and DXA-VAT,(b) C-VAT, and (c) A-VAT. Only the validation set data are plotted for DXA-VAT and A-VAT, whereas the complete study set are plotted for C-VAT. A-VAT, anthropomorphic and demographic VAT model; C-VAT, clinical radiographer-VAT; DXA-VAT, dual-energy X-ray absorptiometry-visceral adipose tissue; E-VAT, expert reader-VAT; SEE, standard error of the estimate; VAT, visceral adipose tissue.
Since the measurement of C-VAT did not separately measure the IMAT but included IMAT with the VAT measurement, we also compared the correlation of C-VAT to the sum of E-VAT plus E-IMAT. The resulting correlation was unchanged (r = 0.93).
When the variables of age, ethnicity, weight, height, WC, BMI, and WHtR were added to DXA-VAT in forward stepwise regression, the only variable that added significantly to the model was the demographic variable age (P = 0.01). However, including age in the model with DXA-VAT only provided a modest improvement in the r value (increase of 0.005), a clinically insignificant difference. No other anthropomorphic measure provided a statistically significant improvement at the P < 0.05 level.
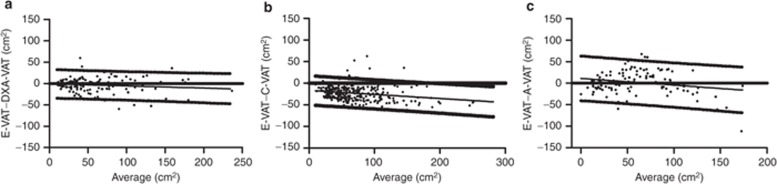
Bland–Altman plots of the limits of agreement between E-VAT vs. DXA-VAT and C-VAT vs. A-VAT are presented in Figure 3.
Figure 3.
Bland–Altman plots and linear regression estimate, with 95% prediction bands, between (a) E-VAT and DXA-VAT, (b) C-VAT, and (c) A-VAT. A-VAT, anthropomorphic and demographic VAT model; C-VAT, clinical radiographer-VAT; DXA-VAT, dual-energy X-ray absorptiometry-visceral adipose tissue; E-VAT, expert reader-VAT; VAT, visceral adipose tissue.
Discussion
The main findings of this study were that the newly derived DXA-VAT measurement was significantly better at predicting CT VAT than standard DXA measurements or the best combination of all the anthropomorphic measurements, and importantly, performed as well as a clinical read of VAT from a CT scan.
Similar to what previous work has shown (5,6,7), we found that the standard DXA-derived total body and regional measurements of fat mass and %fat were no better at predicting VAT measured by CT than anthropomorphic measurements such as BMI and WC.
This study also employed the use of a central, expert read of the CT data to determine E-VAT and this was compared to a clinical VAT read. The interobserver SEE of 16 cm2 that we report between C-VAT and E-VAT is consistent with the results of the study done by Potretzke et al. (13). The Potretzke et al. study used two software programs designed for VAT assessment and reported an SEE of 12 cm2 when these two programs were used by a single expert reader employing a similar methodology to set the HU thresholds in the CT images. Potretzke et al. then investigated interobserver variation between three experienced readers on a set of seven CT images. The greatest average difference between the three readers was 46 ± 19 cm2. In addition, Potretzke et al. reported on a comparison between an experienced reader and an inexperienced reader on 13 images, using an objective methodology to set HU thresholds. They found an average difference between the experienced and inexperienced readers of 14 ± 6 cm2. While Potretzke et al. did not report an SEE for the combination of these investigated variables in CT VAT reads on a large set of images as we do in this study, the magnitude of the individual effects in their study are consistent with the SEE we report. This observation concerning the variation in CT VAT is relevant when CT VAT studies between differing sites and research centers reported in the literature are compared.
The individual variation (represented by the SEE) of DXA-VAT compared with an expert read of CT VAT was of the same size as the variation present when the CT VAT was measured by a less experienced reader, an approach employed by many VAT studies, using different software and methodology. Both DXA-VAT and E-VAT were highly correlated (r = 0.93) and linearly related over a wide range of VAT. Moreover, the newly developed DXA-VAT measurement significantly outperformed A-VAT, the best anthropomorphic and demographic model, for the prediction of E-VAT as evidenced by DXA-VAT's higher correlation coefficient and smaller SEE. DXA-VAT predicted 86% (r2) of the variation of E-VAT, while A-VAT predicted only 62%, and this difference in correlation coefficients was significant at P < 0.001.
There are a number of important differences between VAT measured with CT and DXA-VAT. CT VAT is done with thresholds and each voxel has a binary assignment as either fat or not fat. Many voxels contain both fat and lean tissue, and are not wholly one or the other. For example, if CT were used to measure the fat content of finely ground beef containing 20% fat, it would find no fat at all, as the individual HU of the voxels would be above the HU threshold for fat.
In contrast, DXA does not use a thresholding algorithm but measures the %fat of each pixel in the image of the projected image of the object. Thus in the previous example DXA would accurately measure the ground beef as containing 20% fat. However, DXA is limited by its projectional nature and consequently visceral fat cannot be directly measured by DXA because subcutaneous fat lies above and below it. This subcutaneous fat must be modeled from the subcutaneous fat measurable laterally on the subject, which introduces errors in the estimate of VAT. However, though DXA-VAT has model dependence, there was no dependence on ethnicity, BMI, weight or WC when these parameters were included in a multiple logistic regression model with DXA-VAT for the prediction of E-VAT, indicating that DXA-VAT's prediction of E-VAT is independent of these variables. The study was large enough to detect a statistically significant (P < 0.01) dependence on age, but the size of the effect was so small that the addition of age would have left the reported correlation unchanged at r = 0.93 due to rounding to the second decimal.
Another difference between E-VAT and DXA-VAT was the region of measurement at L4 for DXA-VAT did not match exactly the region of interest utilized by the CT (L4–L5) slice. If the same region of interest was utilized for both measurements, it is possible that the correlations would have been higher. While DXA-VAT as currently implemented cannot be measured at the L4–L5 region, it may be able to measure regions at higher vertebral levels. Recent studies suggest that the L4–L5 region or umbilicus region may not be the most predictive of morbidity (14,15,16). These studies indicate that the L3 or L2 region correlate as well or better than the L4–L5 region with total visceral adiposity and markers of metabolic syndrome. Thus, there may well be advantages to using a higher VAT region instead of the traditional VAT location utilized by CT; however, this hypothesis will require further studies involving health outcomes.
All of the differences discussed above between CT VAT and DXA-VAT contribute to the SEE of the measurement and a low SEE is desired for agreement of classification of individuals as having high VAT or low VAT.
A limitation of this study related to agreement of classification of individuals is that this study did not contain a set of repeat DXA and CT measurements with complete repositioning of the subject between scans (17) to measure the precision inherent in the two techniques for VAT measurement. Another major limitation of this study is that the population did not include women over the age of 49 years or men. The newly developed DXA-VAT method will need to be fully investigated in these populations to see if the high correlations observed here can be reproduced and to determine whether gender or older age affects the DXA-VAT model.
Strengths of the study include the inclusion of a substantial number of both black and white women, a large range of BMI's, and CT reads of VAT using the same data by both a clinical site and an expert at an obesity research center.
Future studies should also focus on the precision of replicate DXA-VAT measurements done with complete repositioning of the patient and the development of DXA-VAT reference data from sources such as the National Health And Nutrition Examination Survey (NHANES), similar to that undertaken for other DXA body composition measures (18). In addition, the predictive ability of DXA-VAT could be examined retrospectively utilizing the many large studies with outcome data which included DXA whole body measurements at baseline (19,20,21).
In conclusion, we found a strong linear relationship between DXA-VAT and E-VAT with correlation significantly higher than could be obtained with the best anthropomorphic and demographic model. If these findings are supported by similar results in other populations, DXA-VAT may become a useful alternative to CT and MRI for the estimation of VAT in both clinical and research settings.
Acknowledgments
We thank Trevino Larry for transfer of CT scan data. Jack Bergman, Naomi Fenton of Symington Radiology and Linda Bewerunge are thanked for performing the CT and DXA scans, respectively. This study was funded by the South African Medical Research Council, the International Atomic Energy Agency, the National Research Foundation of South Africa, and the University of Cape Town.
T.L.K. and K.E.W. are employees of Hologic, Inc., the DXA manufacturer used in this study and own stock in the company. After unblinding the data, all authors had access to all results and analyses. The other authors declared no conflict of interest.
REFERENCES
- Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the 'beef'. Int J Obes (Lond) 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Tylavsky FA, Baer DJ.et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults Am J Clin Nutr 2005811018–1025. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang Z, Lohman T.et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women J Nutr 20071372775–2780. [DOI] [PubMed] [Google Scholar]
- Blake GM, Naeem M, Boutros M. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone. 2006;38:935–942. doi: 10.1016/j.bone.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Lee K, Lee S, Kim YJ, Kim YJ. Waist circumference, dual-energy X-ray absortiometrically measured abdominal adiposity, and computed tomographically derived intra-abdominal fat area on detecting metabolic risk factors in obese women. Nutrition. 2008;24:625–631. doi: 10.1016/j.nut.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Micklesfield LK, Evans J, Norris SA.et al. Dual-energy X-ray absorptiometry and anthropometric estimates of visceral fat in Black and White South African Women Obesity (Silver Spring) 201018619–624. [DOI] [PubMed] [Google Scholar]
- Gradmark AM, Rydh A, Renström F.et al. Computed tomography-based validation of abdominal adiposity measurements from ultrasonography, dual-energy X-ray absorptiometry and anthropometry Br J Nutr 2010104582–588. [DOI] [PubMed] [Google Scholar]
- Smith SR, Lovejoy JC, Greenway F.et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity Metabolism 200150425–435. [DOI] [PubMed] [Google Scholar]
- Body Composition User Guide, Document No. MAN-02354 Revision 001. (Hologic, Inc., 2010
- Kelly TL, Wilson KE, Ruth CR.Estimating visceral fat by dual-energy X-ray absorptiometry. US patent application number US2010–0234719 (Hologic, Inc.,2010
- Kelly TL, Wilson KE, Ruth CR.Visceral fat measurement. US patent application number US2011–0235881 (Hologic, Inc.,2011
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Potretzke AM, Schmitz KH, Jensen MD. Preventing overestimation of pixels in computed tomography assessment of visceral fat. Obes Res. 2004;12:1698–1701. doi: 10.1038/oby.2004.210. [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Chen J.et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks Int J Obes (Lond) 200731763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z.et al. Visceral adipose tissue: relations between single-slice areas and total volume Am J Clin Nutr 200480271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TS, Kelly IE, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1997;21:1161–1166. doi: 10.1038/sj.ijo.0800530. [DOI] [PubMed] [Google Scholar]
- Glüer CC, Blake G, Lu Y.et al. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques Osteoporos Int 19955262–270. [DOI] [PubMed] [Google Scholar]
- Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Beck TJ, Wu G.et al. Ethnic differences in femur geometry in the women's health initiative observational study Osteoporos Int 2011221377–1388. [DOI] [PubMed] [Google Scholar]
- Meng X, Zhu K, Devine A.et al. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women J Bone Miner Res 2009241827–1834. [DOI] [PubMed] [Google Scholar]
- Strotmeyer ES, Cauley JA, Schwartz AV.et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study J Bone Miner Res 2004191084–1091. [DOI] [PubMed] [Google Scholar]