Abstract
The posttranslational regulation of mammalian clock proteins has been assigned a time-keeping function, but seems to have more essential roles. Here we show that c-Jun N-terminal kinase (JNK), identified by inhibitor screening of BMAL1 phosphorylation at Ser 520/Thr 527/Ser 592, confers dynamic regulation on the clock. Knockdown of JNK1 and JNK2 abrogates BMAL1 phosphorylation and lengthens circadian period in fibroblasts. Mice deficient for neuron-specific isoform JNK3 have altered behavioural rhythms, with longer free-running period and compromised phase shifts to light. The locomotor rhythms are insensitive to intensity variance of constant light, deviating from Aschoff's rule. Thus, JNK regulates a core characteristic of the circadian clock by controlling the oscillation speed and the phase in response to light.
Keywords: JNK, BMAL1 phosphorylation, suprachiasmatic nucleus, behavioural rhythm
Introduction
In mammals, a master circadian clock governing behavioural rhythms is located in the hypothalamic suprachiasmatic nucleus (SCN), while the peripheral tissues and even cultured fibroblasts harbour self-sustained molecular clocks [1, 2]. In these cells, clock genes and their products form transcriptional/translational feedback loops, in which BMAL1 and CLOCK transactivate a series of genes including Per and Cry through E-box elements, and translated PER and CRY proteins suppress the function of BMAL1–CLOCK complex [3]. In the molecular cycling, the clock proteins are regulated by posttranslational modifications such as phosphorylation, in terms of activity, stability, localization and interaction [4]. Extrapolation from the clock system of cyanobacteria suggests more dynamic and essential contribution of protein phosphorylation in mammalian clockwork [5].
We previously found that circadian phosphorylation of BMAL1–CLOCK complex regulates its transactivation ability [6]. Molecularly, BMAL1 phosphorylation is catalysed by CKI [7], CKII [8] and GSK3 [9], while our in vitro study showed that BMAL1 is phosphorylated by extracellular signal-regulated kinase (ERK), a member of mitogen-activated protein kinase (MAPK) family [10]. In the SCN of Syrian hamsters, the three members of MAPK family, that is, ERK, p38 kinase and JNK, are activated not only in a circadian manner but also in response to light [11]. Among them, JNKs (JNK1–3) are stimulated by a variety of environmental signals such as hyperosmotic stimuli and ultraviolet radiation [12], but their role(s) in the mammalian clockwork has been enigmatic. Here we demonstrate that JNKs transmit light signals to BMAL1–CLOCK complex and control the oscillation speed and the phase response of the master clock governing the behavioural rhythms.
Results And Discussion
JNK phosphorylates BMAL1–CLOCK complex
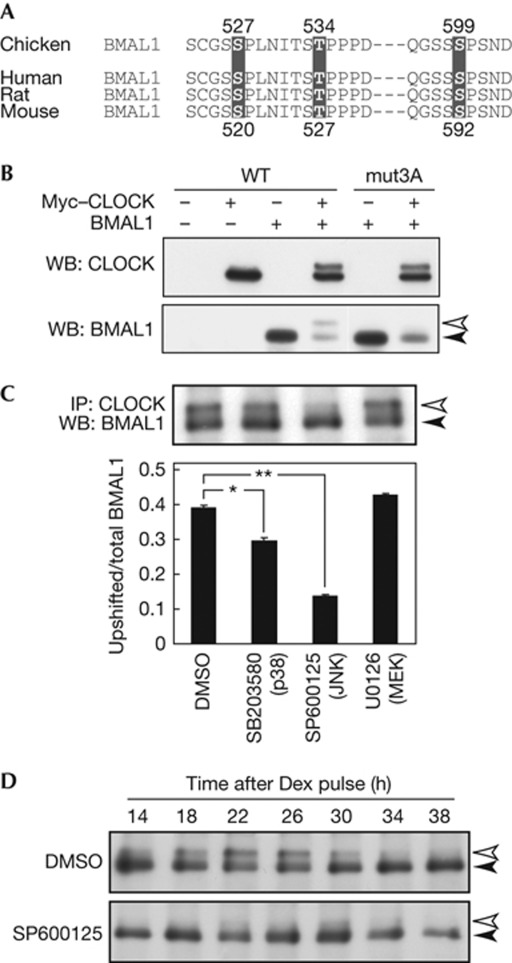
In vitro experiments previously showed that ERK2 phosphorylates chicken BMAL1 at Ser 527, Thr 534 and Ser 599 [10], which are conserved among BMAL1 proteins from other species; for example, Ser 520, Thr 527 and Ser 592 in mice (Fig 1A). As reported [6], BMAL1 phosphorylation can be characterized by its CLOCK dependency accompanying a decrease of BMAL1 level in NIH3T3 cells (Fig 1B). We found that the CLOCK-dependent phosphorylation of mouse BMAL1 was markedly reduced when the three phosphorylatable residues were mutated to Ala (mut3A; Fig 1B). The reduced phosphorylation of mut3A–BMAL1 is not due to impaired interaction with CLOCK, because (i) mut3A–BMAL1 also stimulated CLOCK phosphorylation and (ii) mut3A–BMAL1 level was reduced by coexpression of CLOCK as was observed for wild-type (WT) BMAL1 (Fig 1B).
Figure 1.
Effects of c-Jun N-terminal kinase (JNK) inhibitor SP600125 on BMAL1 phosphorylation. (A) The protein sequence around in vitro phosphorylation sites of chicken BMAL1 was aligned with the corresponding regions of mouse, rat and human BMAL1. (B) NIH3T3 cells were transfected with Myc–CLOCK/pSG5 and BMAL1/pcDNA3.1, and the cell lysates were subjected to immunoblot analysis. Ser 520, Thr 527 and Ser 592 in mouse BMAL1 were mutated to Ala (mut3A). (C) NIH3T3 cells were treated for 24 h with indicated inhibitors (20 μM). Data are means with s.e.m. (n=3). Single and double asterisks indicate P<0.05 and P<0.01, respectively (Student's t-test, versus DMSO). (D) NIH3T3 cells were treated with dexamethasone (Dex) to synchronize the cellular rhythm. After 2-h incubation, the medium was changed to normal culture medium with 20 μM SP600125 or DMSO (control) and this time point was defined as time 0. The cells were collected at indicated time points. (C,D) The total protein extracts of the cells were immunoprecipitated with anti-CLOCK mAb, followed by immunoblot analysis with anti-BMAL1 mAb. DMSO, dimethylsulphoxide; IP, immunoprecipitation; mAb, monoclonal antibody; WB, western blot; WT, wild type.
To explore contribution of MEK–ERK pathway to the phosphorylation, NIH3T3 cells were treated for 24 h with U0126, an inhibitor of MEK1/2 upstream of ERK1/2. We found no significant effect of U0126 on phosphorylation of endogenous BMAL1, whereas it was reduced dramatically by treatment with SP600125, an inhibitor of phosphorylation-dependent activation of JNK (Fig 1C). Importantly, the phosphorylation rhythm of BMAL1 in NIH3T3 cells [6] was abrogated by chronic treatment with SP600125 (Fig 1D).
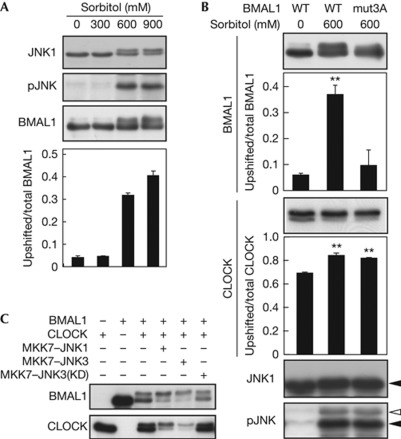
Then we examined whether BMAL1–CLOCK complex is phosphorylated by activation of JNK. A 30-min treatment of HEK293T cells with 600 mM sorbitol, a hyperosmotic stimulus that activates JNK [12] (Fig 2A, supplementary Fig S1A,B online), elevated phosphorylation of coexpressed BMAL1 (Fig 2A), while the phosphorylation was markedly attenuated when mut3A was introduced to BMAL1 (Fig 2B). Still, the protein band of mut3A–BMAL1 was weakly upshifted by JNK (supplementary Fig S1C online) potentially due to additional phosphorylation of BMAL1. The BMAL1 mutation had no effect on sorbitol-enhanced CLOCK phosphorylation (Fig 2B), which was inhibited by SP600125 treatment (supplementary Fig S1D online), suggesting that activated JNK mediates phosphorylation of BMAL1–CLOCK complex. More directly, expression of MKK7–JNK1 or MKK7–JNK3, a fusion with upstream kinase MKK7 that specifically activates JNKs [12], stimulated phosphorylation of BMAL1 and CLOCK coexpressed in NIH3T3 cells (Fig 2C). Such stimulation was not observed with MKK7–JNK3(Lys 93Ala), a fusion with a kinase-dead mutant of JNK3 (Fig 2C). An in vitro kinase assay demonstrated that preactivated JNK1, JNK2 or JNK3 phosphorylated glutathione S-transferase (GST)–BMAL1, but not GST (supplementary Fig S2A online), as did ERK1/2 and p38 kinase. These kinases also phosphorylated GST–CLOCK(364–537) harbouring Ser/Pro-rich domain that includes in vivo phosphorylation site Ser 427 [6]. These data indicate that the activated JNK isoforms directly phosphorylate BMAL1–CLOCK complex.
Figure 2.
c-Jun N-terminal kinase (JNK)-stimulated phosphorylation of BMAL1 and CLOCK proteins. (A,B) HEK293T cells were transfected with Myc–CLOCK/pSG5, BMAL1/pcDNA3.1 and JNK1/pSRα. Ser 520, Thr 527 and Ser 592 in mouse BMAL1 were mutated to Ala (mut3A). The cells were collected 30 min after the sorbitol stimulation (indicated concentrations) and were subjected to immunoblot analysis. Data are means with s.e.m. (n=3). Double asterisks indicate P<0.01 (Student's t-test, versus WT 0 mM). (C) NIH3T3 cells were transfected with Myc–CLOCK/pSG5, BMAL1/pcDNA3.1 and MKK7–JNKs/pCI. Total amount of DNA was adjusted by adding empty plasmids. The transfected cells were collected and subjected to immunoblot analysis. Lys 93 in JNK3 was mutated to Ala for a kinase-dead (KD) mutant, MKK7–JNK3(KD). WT, wild type.
JNK inhibition lengthens cellular rhythms
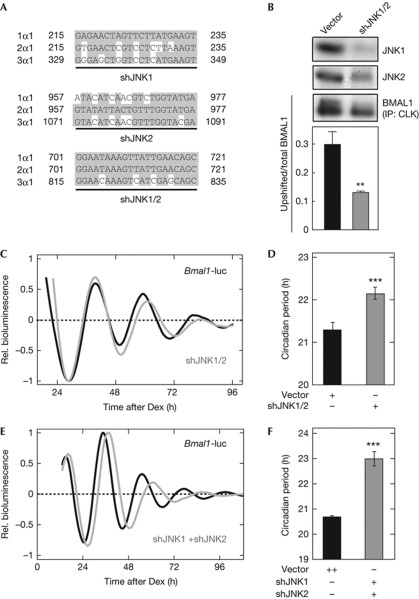
SP600125 treatment not only suppressed the circadian phosphorylation of BMAL1 (Fig 1D) but also lengthened the period of the cellular rhythms in in vivo systems such as NIH3T3, Rat-1, mouse embryonic fibroblasts and SCN explants (supplementary Fig S3A–D online), consistently with a previous study [13]. Although SP600125 is widely used to inhibit JNK activation [14], this drug might affect activities of other protein kinases [15]. The period-lengthening effect of SP600125 seemed to have an impact on CKI, because CKI inhibitor such as IC261 also lengthened the cellular rhythms [16, 17] (supplementary Fig S3B online). On the other hand, BMAL1 phosphorylation was not reduced in the presence of IC261 (supplementary Fig S3E online), supporting contribution of JNK signalling to BMAL1 phosphorylation. We investigated a role of JNKs for BMAL1 phosphorylation and cellular rhythms by performing knockdown experiments in NIH3T3 fibroblasts, which express Jnk1 and Jnk2 but not Jnk3 (supplementary Fig S4A–F online). JNK1 and JNK2 each have four splice variants, for which we designed short hairpin RNA (shRNA) constructs targeting all the variants (Fig 3A, supplementary Fig S4G online). Expression level of transfected JNK1 in NIH3T3 cells was markedly reduced by coexpression of shJNK1 or shJNK1/2. Similarly, JNK2 level was largely decreased by shJNK2 or shJNK1/2 (supplementary Fig S4H online). Importantly, transient transfection of shJNK1/2 reduced not only endogenous protein level of JNK1 and JNK2, but also phosphorylation level of endogenous BMAL1 in NIH3T3 cells (Fig 3B). Furthermore, we found significant lengthening of the circadian period when JNK1 and JNK2 were simultaneously knocked down either by shJNK1/2 (Fig 3C,D) or by co-transfection of shJNK1 and shJNK2 (Fig 3E,F). It is most probable that JNKs have a main regulatory role for the oscillation speed of the cellular clock by phosphorylating circadian component(s) including BMAL1.
Figure 3.
Effects of knockdown of c-Jun N-terminal kinase (JNK)1 and JNK2 on BMAL1 phosphorylation and cellular rhythms. (A) Two shRNA constructs, shJNK1 and shJNK2, respectively, target JNK1 and JNK2, while shJNK1/2 targets both JNK1 and JNK2. Nucleotide numbers starting from initiation codon are shown. (B) NIH3T3 cells were transfected with shJNK1/2 or the empty vector. After 48-h incubation, the cells were synchronized by 2-h dexamethasone (Dex) pulse. The cells were collected 24 h after the synchronization, and were subjected to immunoprecipitation (IP) and immunoblot analysis. Data are means with s.e.m. (n=4; **P<0.01, Student's t-test, versus empty vector). (C) Cellular rhythms were recorded from the NIH3T3 cells transfected by 0.5 μg of Bmal1us0.3/pGL4 with 0.5 μg of shJNK1/2 (grey line) or the empty vector pBS-mU6 (black line). (D) The circadian periods with and without shJNK1/2 were 22.15±0.14 and 21.31±0.16 h, respectively. Data are means with s.e.m. (n=10; ***P<0.001, Student's t-test, versus empty vector). (E) NIH3T3 cells were transfected by 0.5 μg of Bmal1us0.3/pGL4 in combination with 0.5 μg each of shJNK1/pBS-mU6 and shJNK2/pBS-mU6 (grey line) or 1 μg of the empty vector pBS-mU6 (black line). Cellular rhythms were synchronized by 2-h Dex treatment, and the time point of the medium change to the recording medium was defined as time 0. Shown were the detrended curves of bioluminescence rhythms. (F) Circadian periods of the rhythms with and without shJNK1 and shJNK2 were 22.99±0.28 and 20.68±0.05 h, respectively. Data are means with s.e.m. (n=4; ***P<0.001, Student's t-test, versus empty vector).
Altered behavioural rhythms in JNK-deficient mice
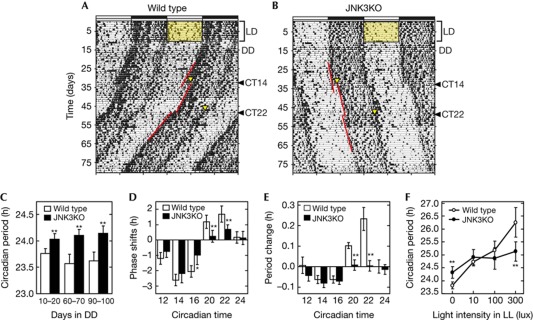
Among the three Jnk isoforms, Jnk1 and Jnk2 are ubiquitously expressed throughout the body, and mice lacking both Jnk1 and Jnk2 are embryonic lethal [18]. On the other hand, Jnk3 is expressed almost exclusively in the nervous system [19], and Jnk3-deficient mice are fertile with no apparent abnormalities in their development [20]. We found that Jnk3-deficient mice showed robust behavioural rhythms under light–dark (LD) cycles (Fig 4B, supplementary Fig S5A online). When transferred to constant darkness (DD), Jnk3-deficient mice exhibited significantly longer free-running period (24.1±0.1 h) than WT controls (23.6±0.2 h) at days 90 to 100 in DD (Fig 4C). A more striking effect was observed when the mutant mice were exposed to 30-min light pulse at subjective night. A remarkable reduction of the phase-shift, particularly the phase-advance, was observed when the light pulse was given at circadian time (CT) 20 and 22 (Fig 4D). We also found that the free-running period of Jnk3-deficient mice was unaltered even after receiving the light-pulse at the two time points (Fig 4E). Such a light pulse, on the other hand, caused a noticeable phase-dependent period-shortening in WT mice (Fig 4E), known as ‘aftereffect’ [21]. The aftereffect has an adaptive significance, because the light pulse modulates the circadian period towards a direction that reduces the phase-shift. Under 24-h LD cycles, shortening or lengthening of the circadian period could reduce the daily phase-shifts in animals having the circadian period longer or shorter than 24 h, respectively. Such an advantageous modulation, however, is missing in Jnk3-deficient mice, indicating that JNK also contributes to the frequency modulation of the circadian clock in a 24-h LD cycle.
Figure 4.
Behavioural rhythms and light-induced phase and period responses in Jnk3-deficient mice. Double-plotted spontaneous locomotor activity rhythms of representative wild-type (A) and Jnk3-deficient (B) mice. Horizontal black and white zones above each actograph indicate the dark and light phase in light–dark (LD) cycle, respectively. Yellow triangles indicate time points when the light pulse was given (30 min, 300 lux). Oblique red lines are the regression lines fitted to the consecutive activity onsets before and after the light pulse. (C) Circadian periods of the behaviour rhythms under constant darkness (DD) condition in Jnk3-deficient (n=22, black columns) and wild-type mice (n=16, open columns). (D) Phase response to brief light pulses (300 lux, 30 min) given at six different circadian times (CT)s. Phase-advance and phase-delay shifts are expressed in positive and negative values, respectively. (E) Changes of circadian periods after the brief light pulses. Lengthening and shortening of the circadian period are expressed in negative and positive values, respectively. (F) Changes in circadian periods under constant light (LL) conditions in Jnk3-deficient (n=5) and wild-type mice (n=5). (C–F) Data are means with s.d. Single and double asterisks indicate P<0.05 and P<0.01, respectively (post hoc Tukey Krammer test, versus wild type). JNK, c-Jun N-terminal kinase.
Another striking effect of Jnk3 deficiency was observed in constant light condition (LL), where the free-running period of WT mice became longer than that in DD (23.6±0.2) depending on the light intensity (Fig 4F, supplementary Fig S5B online; 24.7±0.2 and 26.3±0.6 h in 10 and 300 lux, respectively). This property is widely known as ‘Aschoff's rule’; the higher the light intensity in LL is, the longer the circadian period becomes in the nocturnal animals, and vice versa in the diurnal species [22]. In spite of the evolutionary importance, Aschoff's rule has not been explained at the molecular level. Surprisingly, Jnk3-deficient mice showed almost a constant period in LL at various light intensities (Fig 4F, supplementary Fig S5C online; 24.9 and 25.1 h in 10 and 300 lux, respectively). As a result, the free-running period of Jnk3-deficient mice became even shorter than that of WT mice in LL of 300 lux (Fig 4F). A recent report [23] predicted that a change in the shape of phase-response curve causes different period responses to LL in Per mutant mice. On the other hand, reduced responsiveness of Jnk3-deficient mice to light intensities is unlikely due to changes in responses to brief light pulses, because the reduction that was caused by the mutation in the phase-advance shift (1.97 h in total) was almost equivalent to that in the phase-delay shift (1.94 h in total). The present results together pinpoint a key role of JNK3-mediated posttranslational process not only in the phase-dependent phase-shift by a light pulse, but also in regulation of the oscillation speed (period) in response to environmental light intensities.
Light-activation of JNK in the mouse SCN
We investigated E-box-mediated transcription in the SCN of the Jnk3 mutant mice, because it affects the circadian period of the behavioural rhythms. We found that expression levels of Per1 and Per2 were significantly elevated in the mutant SCN (supplementary Fig S6A–D online), suggesting Jnk3 deficiency upregulates E-box-mediated transcription. Intriguingly, although the behavioural rhythms of the mutant mice were far less sensitive to the light pulse at CT20 or CT22 (Fig 4D,E), Per1 and Per2 in the mutant SCN were induced by the light normally to levels that were observed in WT SCN (supplementary Fig S6E,F online). These observations indicate that Per induction-independent phase-shift signalling is sensitive to Jnk3 deficiency, while Jnk3 seems to have a marginal role in CRE-mediated transcription for Per induction.
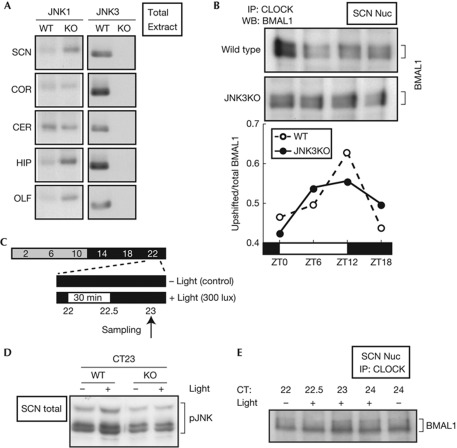
We unexpectedly found remarkable elevation of cytosolic and nuclear JNK1 protein at any time of the day (supplementary Fig S7E,F online) albeit with a minor effect on the mRNA level (supplementary Fig S7C online). The increase of JNK1 was evident in not only the SCN but also hippocampus and olfactory bulb, while it was mostly unaffected in the cortex and cerebellum (Fig 5A) or in whole brain extracts (supplementary Fig S7G online). Even with the increase of JNK1 protein, the overall activation level of JNK in the mutant SCN was lower than that in WT (Fig 5D, lanes 1 and 3). In fact, the BMAL1 phosphorylation levels at the peak (ZT12) and trough (ZT0) were slightly lower in the mutant SCN than WT (Fig 5B). Importantly, 30-min light at CT22 stimulated JNK phosphorylation in the WT SCN [11] (Fig 5C,D), whereas the light-dependent activation of JNK was no more evident in the mutant SCN (Fig 5D). Furthermore, the light pulse at CT22 also stimulated BMAL1 phosphorylation in the SCN (Fig 5E).
Figure 5.
BMAL1 phosphorylation in the mouse suprachiasmatic nucleus (SCN). (A) The total extracts of indicated brain regions were prepared at ZT6 from wild-type mice (WT) or Jnk3-deficient mice (KO). Equal protein amounts of the samples were subjected to immunoblot analysis. CER, cerebellum; COR, cortex; HIP, hippocampus; OLF, olfactory bulb. (B) The SCN nuclear extracts were prepared at ZT0, 6, 12 and 18 from 15 mice (for each time point) of WT and KO mice. They were immunoprecipitated (IP) with anti-CLOCK antibody and subjected to immunoblot analysis with anti-BMAL1 antibody. The relative level of the upshifted band was plotted at the bottom. (C) Time schedules for SCN sampling on the first day of constant darkness (DD). (D) Light-induced change of JNK phosphorylation level in the SCN of WT and KO mice was investigated after exposure to 30-min light pulse at circadian time (CT)22. Total extract was prepared from the SCN punch of WT and KO mice, and subjected to immunoblot analysis with anti-phospho-JNK antibody. (E) Light-induced change of BMAL1 phosphorylation level in the SCN of WT mice was investigated after exposure to 30-min light pulse at CT22. JNK, c-Jun N-terminal kinase; WB, western blot.
All these observations highlight essential roles of JNK not only in the normal oscillation of the mammalian clock but also in its photic regulation, that is, (i) the phase-dependent phase-shift, (ii) the aftereffect of light and (iii) light intensity-dependent period-response (Aschoff's rule).
Methods
Cell culture and transfection. NIH3T3 and HEK293T cells were maintained at 37°C under 5% CO2, 95% air in Dulbecco’s modified Eagle’s medium (Nissui) containing 1.8 mg/ml NaHCO3 and 4.5 mg/ml glucose supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum. For transient transfection, the cells were plated in 12-well plates 24 h before the experiments, and transiently transfected by using Lipofect AMINE PLUS Reagent (Invitrogen) according to manufacturer’s directions. Total amount of transfected DNA was kept constant in an experiment by adding the empty plasmid. For activation of JNKs, the cells were treated for 30 min with sorbitol just before collecting the cells.
Plasmid. The mammalian expression vectors used were Myc–CLOCK/pSG5 (a kind gift of Dr Paolo Sassone-Corsi), BMAL1/pcDNA3.1 (a kind gift of Dr Steven M. Reppert) and Flag–JNK1/pSRα (a kind gift of Dr Mutsuhiro Takekawa). Ala mutations were introduced into BMAL1 by using a site-directed PCR mutagenesis method. Full-length mouse Jnk1α1, Jnk2α1 and Jnk3α1 complementary DNA (cDNA) were cloned from the total cDNA of the mouse SCN (C57BL/6J) by reverse transcription–PCR analysis with gene-specific primers. Mammalian expression vectors for expression Flag epitope-tagged JNK isoforms were generated by inserting the Jnk cDNAs into the pSG5 vector, with a slight modification to create NotI sites, a Kozak sequence, and a Flag epitope. Full-length human Mkk7, Jnk1α1 and Jnk3α1 cDNAs were cloned and inserted into the pCI vector for Flag–MKK7–JNK1/pCI and Flag–MKK7–JNK3/pCI. Lys93 in JNK3 was mutated to Ala as a kinase-dead mutant. For knockdown of JNK1 and JNK2, shRNA were designed using siDirect (http://sidirect2.rnai.jp/), a web-based software, and the following sequences were used: shJNK1; 5′-GAGAACUAGUUCUUAUGAAGU-3′, shJNK2; 5′-GUAUAUUACUGUUUGGUAUGA-3′, and shJNK1/2; 5′-GGAAUAAAGUUAUUGAACAGC-3′. The oligonucleotides to express the shRNA were inserted into the pBS-mU6 vector.
Antibodies, immunoprecipitation and immunoblot analysis. The nuclear and cytoplasmic fractions were prepared as described [6]. Anti-CLOCK monoclonal antibody (mAb), CLNT1, was used for immunoprecipitation as described in our previous paper [6]. In immunoblot analysis, antibodies used were CLSP3 anti-CLOCK mAb [6], B1BH2 anti-BMAL1 mAb [6], anti-JNK1/3 (Santa Cruz Biotechnology, C17), anti-JNK2 (Millipore), anti-JNK3 (Millipore, C05T), anti-phospho-JNKs (Cell Signaling) and anti-β-actin (Sigma-Aldrich). For specific detection of JNK1, anti-JNK1 (Santa Cruz Biotechnology, F3) was used, as shown in Figure 5A.
Real-time monitoring assay. Real-time monitoring assay was performed as described [24] with modifications. NIH3T3 cells plated on 35-mm dishes were transiently transfected by shRNA vectors with Bmal1-luc/pGL4, a firefly luciferase reporter under regulation of a 0.3-kb Bmal1 promoter. The cells were treated with 0.1 μM dexamethasone for 2 h, and then the medium was replaced by a recording medium. Bioluminescence was recorded with Dish Type Luminescencer, Kronos (ATTO) or LumiCycle (Actimetrics).
Animals and behavioural rhythm measurement. Spontaneous locomotor activities of male homozygote mice of Jnk3-deficient [20] (Jackson Laboratory, Mapk10tm1Flv) and WT control mice were measured every minute by an infrared thermal sensor. Behavioural activity rhythms were analysed by Clock Lab (Actimetrics), and circadian periods were determined by a χ2-periodogram.
Additional methods are available as supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Dr Mutsuhiro Takekawa (Nagoya University) for JNK1 expression plasmids and helpful discussion and Yohko Suzuki for animal care. This work was supported in part by Grants-in-Aid for Scientific Research (no. 21390064 for SH and no. 19107002 for YF) and by Global COE program (Integrative Life Science Based on the Study of Biosignaling Mechanisms) from MEXT, Japan.
Author contributions: H.Y., S.H., H.N., K.-I.H., and Y.F. wrote the manuscript; H.Y., S.H., K.I., H.N., K.-I.H. and Y.F. designed the experiments; H.Y., S.H., K.I., H.N., S.-Y.N., D.O., H.K., N.S., H.M., N.W., H.D. and T.H. performed the experiments.
The authors declare that they have no conflict of interest.
05/01/2012
Since advance online publication, the legend to Fig 2 has been amended to remove an incorrect explanation for the KD abbreviation.
References
- Hastings M, Reddy A, Maywood E (2003) A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4: 649–661 [DOI] [PubMed] [Google Scholar]
- Hirota T, Fukada Y (2004) Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 21: 359–368 [DOI] [PubMed] [Google Scholar]
- Dunlap J (1999) Molecular bases for circadian clocks. Cell 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup D (2007) Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8: 139–148 [DOI] [PubMed] [Google Scholar]
- Johnson C, Mori T, Xu Y (2008) A cyanobacterial circadian clockwork. Curr Biol 18: R816–R825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitane H, Takao T, Satomi Y, Du N, Okano T, Fukada Y (2009) Roles of CLOCK phosphorylation in suppression of E-box-dependent transcription. Mol Cell Biol 29: 3675–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E, Vielhaber E, Hinz W, Virshup D (2002) The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem 277: 17248–17254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru T, Hirayama J, Isojima Y, Nagai K, Norioka S, Takamatsu K, Sassone-Corsi P (2009) CK2alpha phosphorylates BMAL1 to regulate the mammalian clock. Nat Struct Mol Biol 16: 446–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P (2010) Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One 5: e8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Okano T, Fukada Y (2002) Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J Biol Chem 277: 267–271 [DOI] [PubMed] [Google Scholar]
- Pizzio G, Hainich E, Ferreyra G, Coso O, Golombek D (2003) Circadian and photic regulation of ERK, JNK and p38 in the hamster SCN. Neuroreport 14: 1417–1419 [DOI] [PubMed] [Google Scholar]
- Davis R (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Chansard M, Molyneux P, Nomura K, Harrington M, Fukuhara C (2007) c-Jun N-terminal kinase inhibitor SP600125 modulates the period of mammalian circadian rhythms. Neuroscience 145: 812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B et al. (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P (2003) The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isojima Y et al. (2009) CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci USA 106: 15744–15749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ et al. (2010) Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA 107: 15240–15245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C, Yang D, Samanta Roy D, Davis R, Rakic P, Flavell R (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22: 667–676 [DOI] [PubMed] [Google Scholar]
- Martin J, Mohit A, Miller C (1996) Developmental expression in the mouse nervous system of the p493F12 SAP kinase. Brain Res Mol Brain Res 35: 47–57 [DOI] [PubMed] [Google Scholar]
- Yang D, Kuan C, Whitmarsh A, Rincón M, Zheng T, Davis R, Rakic P, Flavell R (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389: 865–870 [DOI] [PubMed] [Google Scholar]
- Pittendrigh C, Daan S (1976) A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol 106: 223–252 [Google Scholar]
- Aschoff J (1960) Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25: 11–28 [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Friday RC, Yamazaki S (2010) Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci 30: 12179–12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N, Hirota T, Kawamoto T, Kato Y, Tsubota T, Fukada Y (2008) Activation of TGF-beta/activin signalling resets the circadian clock through rapid induction of Dec1 transcripts. Nat Cell Biol 10: 1463–1469 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.