Abstract
Several bone marrow-derived cells have been shown to promote tumour growth and progression. These cells can home to the primary tumour and become active components of the tumour microenvironment. Recent studies have also identified bone marrow-derived cells—such as mesenchymal stem cells and regulatory T cells—as contributors to cancer metastasis. The innate versatility of these cells provides diverse functional aid to promote malignancy, ranging from structural support to signal-mediated suppression of the host immune response. Here, we review the role of mesenchymal stem cells and regulatory T cells in cancer metastasis. A better understanding of the bipolar nature of these bone marrow-derived cells in physiological and malignant contexts could pave the way for new therapeutics against metastatic disease.
Keywords: bone marrow-derived cells, cancer metastasis, mesenchymal stem cells, regulatory T cells
See the Glossary for abbreviations used in this article.
Glossary.
- Ang1
angiopoietin 1
- Ap1
activator protein 1
- β-GBP
β-galactoside-binding protein
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CTL
cytotoxic T lymphocyte
- CTLA4
cytotoxic T-lymphocyte antigen 4
- DFAT
dedifferentiated fat
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ErbB2
v-erb-b2 erythroblastic leukaemia viral oncogene homologue 2
- FOXP3
forkhead box P3
- FSP1
fibroblast-specific protein 1
- HGF
hepatocyte growth factor
- HLA
human leukocyte antigen
- HLA-G5
human leukocyte antigen-G5
- IFN-γ
interferon-γ
- IL
interleukin
- IL-17BR
IL-17B receptor
- MCA
3-methylcholanthrene
- M-CSF
macrophage colony-stimulating factor
- MMP
matrix metalloproteinases
- NK
natural killer
- NOD/SCID
nonobese diabetic/severe combined immunodeficient
- PGE2
prostaglandin E2
- Rag
recombination-activation gene
- RANK(L)
receptor activator of nuclear factor-κB (ligand)
- Sca1
stem cell antigen 1
- SDF1
stromal-derived factor 1
- shRNA
short hairpin RNA
- SMA
smooth muscle actin
- TGF-β
transforming growth factor-β
- TH2 cell
T helper 2 cell
- TNF-α
tumour necrosis factor- α
- TRAIL
tumour necrosis factor-related apoptosis-inducing ligand
- VEGF(R)
vascular endothelial growth factor (receptor)
- VLA4
very late antigen 4
Introduction
Cancer metastasis accounts for over 90% of mortality from solid malignancies and is a multi-step process that allows primary tumour cells to escape from the site of origin and colonize distant organs [1,2]. The metastatic process has traditionally been classified into several distinct stages—local invasion, intravasation, survival in the circulation, extravasation and colonization—each of which is regulated intrinsically by the tumour cell and extrinsically by the surrounding stroma [3,4,5]. The complexity of this multi-stage process has enraptured many generations of cancer researchers, starting with Stephen Paget's century-old ‘seed and soil' hypothesis that compared tumour cells to ‘seeds' that are systematically distributed, but only inhabit particular environments, or ‘soils,' which are supportive of their sustained growth [6]. Although distinct genetic profiles of tumour cells with particular proclivities to colonize specific distant organs have begun to be elucidated, how such metastasis genes exert their functions in the context of tumour–stroma interactions remains a major topic of ongoing research [3,7,8,9].
Throughout the expansion of the primary tumour, a vast array of host cells—ranging from macrophages to fibroblasts—create and sustain a favourable microenvironment for malignant growth [4,10]. Bone marrow-derived cells, including mesenchymal stem cells (MSCs) [11,12,13,14] and immunosuppressive cells, such as regulatory T cells (Tregs) [15,16,17], have been identified as major components of the primary tumour microenvironment. More recently, new evidence corroborates the contribution of these populations in the metastatic process [18,19,20], largely by providing cell motility-inducing factors and promoting a protective microenvironment for tumour cells throughout their journey to distant organs. These studies therefore reinforce the idea that the stromal components of the tumour microenvironment can play an active role in promoting cancer metastasis. However, given the complexity and multi-faceted progression of tumour metastasis, an in-depth analysis of these metastasis-promoting interactions is still incomplete. In addition, the function of bone marrow-derived cells in modulating the immune system to promote metastasis must be viewed in the context of the general controversy surrounding immunosurveillance of tumour cells [21,22,23], both in primary tumour growth and in cancer metastasis. Despite such challenges, numerous studies have implicated several immune cells, such as immature myeloid cells [24,25,26], mast cells [27,28], macrophages [29,30], platelets [31,32], neutrophils [33], and haematopoietic and endothelial progenitor cells [34,35,36] to promote cancer metastasis, and have already been addressed in other reviews [37,38,39,40]. Here, we review our understanding of the contribution of MSCs and Tregs to cancer metastasis and discuss their potential as therapeutic targets in the treatment of metastatic disease.
Tumour–MSC crosstalk in metastatic progression
The bone marrow stroma, which is the master haematopoietic compartment, is composed of various cell types and crucial regulators required for creating an ideal niche for the maintenance of haematopoietic stem and progenitor cells [41,42,43]. A dominant subpopulation in the bone marrow stroma is believed to be mesenchymal in nature [44,45,46], with a primitive population of MSCs able to differentiate into osteoblasts, adipocytes and chondrocytes [47,48]. MSCs have also been reported to differentiate into fibroblasts [49,50,51] and pericytes [52,53], although studies showing similar differentiation potential of dermal pericytes [54], retinal pericytes [55] and primary fibroblast-like populations [56] suggest that both fibroblasts and pericytes might not be terminally differentiated progenies.
Despite numerous studies characterizing the versatile differentiation potential of MSCs [57,58,59], a general consensus is lacking on the immunophenotypic markers used to isolate this multi-potential population [49,60]. A broad array of MSC markers have been used in various studies in both human and mouse models, including CD73, CD90, CD105, CD140, CD146 and Sca1, and the clonogenic purity of the MSC population has been proposed to vary depending on which combinations of these markers are used for isolation [52,53,61,62,63]. Given that a multipotent cell able to differentiate into osteoblasts, adipocytes and chondrocytes meets the criteria of an MSC [61] and given the extremely heterogeneous nature of this population, it is essential to evaluate studies on MSCs in a context-dependent manner. It is also important to note that the bone marrow, although providing a significant proportion, is not the only source of MSCs. Adipose tissue [64,65] and the umbilical cord [66,67] have been shown to create a niche for MSCs, which can also be recruited to sites of wound healing and the primary tumour. These tissue-specific MSCs meet the tri-lineage criterion mentioned above, but multiple studies show varying differentiation propensity toward specific lineages and plasticity depending on the source of MSC isolation [68,69,70,71]. For example, adipose tissue-derived MSCs, otherwise referred to as adipose-derived stem cells (ADSCs), can differentiate into osteoblasts [72], adipocytes, chondrocytes [73,74], myoblasts [75] and even endothelial cells [76,77], but they have also been shown to arise from mature human adipocytes through a process known as dedifferentiation [78,79]. These reprogrammed multipotential cells, commonly referred to as DFAT cells, acquire the surface markers of ADSCs and have the capacity to differentiate into osteoblasts, chondrocytes and adipocytes in vitro [79,80]. However, these findings are still contested, as conflicting results can be obtained depending on the culture conditions and immunophenotypic markers used for isolation, emphasizing the need for in vivo lineage tracing studies of these versatile stem cells. The plasticity of MSCs to transdifferentiate in various settings is crucial for their normal physiological functions, but also allows them to have a more ominous role in cancer. For example, one of the major functions of MSCs is their differentiation into connective tissue during wound healing [81,82,83] and secretion of a battery of growth-modulating factors, such as IL-6 [11,84] and Ang1 [53], to promote accelerated regeneration of injured tissue. In cancer, this process can create a growth-promoting tumour microenvironment in which MSCs differentiate into cancer-associated fibroblasts (CAFs) [14,85,86], which secrete IL-6 [87] and VEGF [88] to promote tumour angiogenesis. In this context, MSCs act in sharp contrast to their known endogenous functionality by exacerbating what has been described as “a wound that never heals” [89].
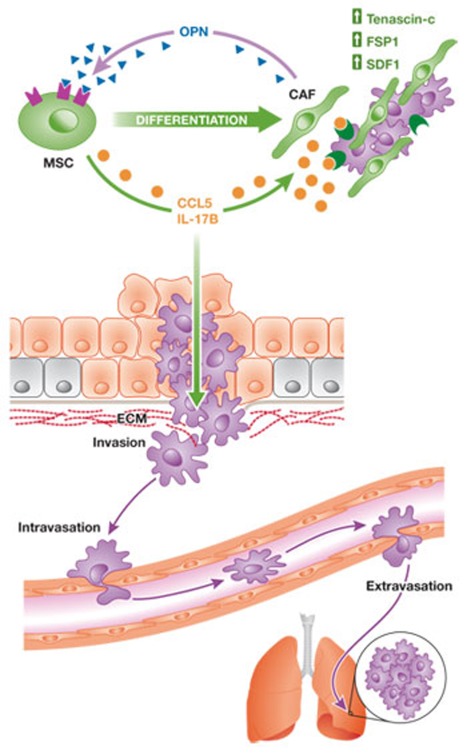
Although the contribution of MSCs to primary tumour growth has been studied extensively in a variety of cancers [11], including breast cancer [13], colon cancer [90] and lymphoma [91], studies demonstrating their potential to promote tumour metastasis are relatively rare in comparison. Nevertheless, MSCs have been recently shown to facilitate tumour metastasis by secreting inflammatory cytokines to promote cell motility (Fig 1). Subcutaneous co-injection of the human breast cancer cell line MDA-MB-231 and human bone marrow-derived cells into immunodeficient mice significantly enhances lung and liver metastasis [13,18]. These studies identify CCL5 as an MSC-derived metastasis-promoting factor, the expression of which increases after the interaction of MSCs with cancer cells (Fig 1). CCL5-induced Akt activation allows tumour cells to extravasate from the circulation to colonize distal organs, and thereby significantly increases metastatic potential [13]. Tumour-derived osteopontin (OPN) was also recently found to promote CCL5 secretion from MSCs by stimulating the binding of c-Jun homodimers to the CCL5 promoter and, thus, stimulating its transactivation (Fig 1; [18]). Aptamer-mediated neutralization of circulating OPN in MDA-MB-231 xenograft models reduces serum CCL5 levels, as well as lung and liver metastasis. In this model, the contribution of CCL5 to metastasis does not seem to depend on its effect on the primary tumour stroma, as there is no difference in infiltrating macrophages, angiogenesis or SMA-positive stromal cells between tumours initiated by CCL5 overexpressing MDA-MB-231 cells and those by control MDA-MB-231 cells [13].
Figure 1. Mesenchymal stem cells promote cancer metastasis.
MSCs can secrete inflammatory cytokines—such as CCL5 and IL-17B—to facilitate cell motility, which is necessary for tumour cell invasion through the surrounding ECM, intravasation into blood vessels and extravasation from the circulation at target sites. In addition, MSCs can differentiate into CAFs, which express tenascin-C, FSP1 and SDF1 to support tumour progression and create a pro-metastatic microenvironment. Feedback mechanisms sustain these pro-metastatic effects of MSCs on tumour cells, as is the case of tumour-secreted OPN promoting both CCL5 transcription and CAF marker expression. Such MSC–tumour cell interactions have been observed both in the primary tumour as well as at the sites of metastases. CAF, cancer-associated fibroblast; CCL, CC chemokine ligand; ECM, extracellular matrix; FSP1, fibroblast-specific protein 1; IL, interleukin; MSC, mesenchymal stem cell; OPN, osteopontin; SDF1, stromal-derived factor 1.
In addition to promoting tumour-intrinsic metastatic properties, the tumour–MSC interaction can modulate the stromal microenvironment to promote metastasis [18]. Tumour cell-derived OPN promotes the expression of cancer-associated fibroblast markers—such as αSMA, tenascin-C, SDF1 and FSP1—in human MSCs (Fig 1). Interestingly, the expression of these markers is enhanced in MSCs isolated from the metastatic site, suggesting another mechanism whereby OPN contributes to cancer metastasis. Human and mouse MSCs have been shown to transdifferentiate into CAFs when co-injected with Skov3 ovarian cancer cells [14] or with MKN45 gastric cancer cells [87]. In addition to secreting tumour growth-promoting factors such as EGF and IL-6, these MSC-derived CAFs also express extracellular matrix- and angiogenesis-regulating proteins to create a metastasis-promoting stromal microenvironment for the primary tumour. Human MSC-derived CAFs are also found at the sites of metastasis, have similar characteristics as in the primary tumour [18,92,93] and probably create a fostering environment for tumour cells—‘the metastatic niche'. In this regard, it is of particular interest that tenascin-C, which is derived from cancer cells and myofibroblasts, has been recently shown to generate a metastatic niche to facilitate lung metastasis [94]. As noted above, tenascin-C expression is also increased in OPN-stimulated human MSCs.
CAFs are thought to be one of the most important and aggressive supporters of tumour growth and invasion [95,96,97,98,99,100], but they have also been recently implicated in facilitating metastasis after the cancer cells have entered the circulation [101,102]. Periostin—a crucial extracellular component in bone and heart formation—is upregulated in αSMA+ vimentin+ myofibroblasts in metastatic lungs [103]. Periostin deficiency in spontaneous mammary tumour-bearing mice significantly reduces pulmonary metastasis and is thought to disrupt the putative cancer stem cell niche at the metastatic site. Despite such similarities in the characteristics between the MSC-derived CAFs that have been recently identified and CAFs of various sources, it remains to be determined whether MSCs actively promote metastasis as a differentiated CAF at the metastatic site (Sidebar A).
Sidebar A | In need of answers.
How are tumour-associated MSCs, Tregs and other bone marrow-derived cells different from those present in normal tissues?
Do bone marrow-derived cells have a role in a particular stage of metastasis, distinct from other stages?
What is the nature of tumour metastatic niches and how do they differ from normal adult stem cell niches, such as the haematopoietic stem cell niche? What are the roles of the various bone marrow-derived cells in such niches?
How can we interfere with the interaction of bone marrow-derived cells with tumour cells to inhibit tumour metastasis?
The emerging theory of the pre-metastatic niche proposes that target organs can be primed by secreted factors from primary tumours to create a more accommodating microenvironment before the arrival of metastatic tumour cells [25,104,105]. For example, the accumulation of VEGFR1-positive myeloid progenitors in pre-metastatic lungs creates favourable docking sites for lung carcinoma tumour cells [25]. Tumour-secreted factors, such as placental growth factor (PlGF), are transmitted from the primary tumour to the metastatic organ, causing resident fibroblasts to produce fibronectin at future sites of metastasis. The secreted factors also stimulate the recruitment of bone marrow-derived progenitor cells that express VEGFR1 and integrin VLA4, which is a fibronectin receptor [25]. Although this study showed that VEGFR1 activity is crucial for metastasis, another report claims that VEGFR1 deficiency in a genetic model that lacks the tyrosine kinase domain of VEGFR1 does not affect spontaneous metastasis of Lewis lung carcinoma and melanoma [106]. This aspect of CAF targeting clearly requires further study, as does whether the resident fibroblasts of the pre-metastatic niche arise from local MSCs or from the bone marrow. In addition, although MSCs isolated from sites of metastasis seem to have stronger CAF-associated marker expression than those in the primary tumour, whether and how metastatic MSCs differentially contribute to primary and secondary tumorigenesis and whether MSC-derived CAFs precede tumour seeding to facilitate the colonization process remain open questions (Sidebar A).
Another molecule recently implicated in promoting metastasis through an MSC-dependent mechanism is the pro-inflammatory cytokine IL-17B [107], which is secreted by human MSCs that migrate to the primary tumour in a TGF-β-dependent manner [93]. The IL-17BR is a prognostic indicator associated with invasive tumour progression [108,109], and its ectopic expression in MDA-MB-231 and SUM1315 breast cancer cells leads to increased migration in vitro, as well as increased frequency of lung and liver metastases in vivo (Fig 1). IL-17BR expression is significantly higher in metastatic bone lesions of SUM1315 cells and is thought to promote organ-specific metastasis to the bone, although ectopic IL-17BR alone is not sufficient to promote metastasis [93]. The IL-17 family consists of six structurally related members, which are produced by and act on various immune cells [110,111] to regulate immune function in inflammation, autoimmunity, host defence against bacterial and fungal infections and tumorigenesis [107,112]. IL-17A+ immune cells in the tumour microenvironment have been shown to promote tumour growth by increasing the production of pro-angiogenic factors—such as VEGF and prostaglandins [113,114]—and recruiting neutrophils to the primary tumour through the production of IL-8 [115,116]. Interestingly, IL-17A reduces TNF-induced CCL5 expression in mouse lung fibroblasts [117], whereas IL-17E, which binds to IL-17BR, has the opposite effect [118]. Although the roles of IL-17A and E in tumorigenesis have been characterized in numerous studies [119,120,121], the function of IL-17B in both tumorigenesis and immunity remains largely unknown. Both IL-17B and C cause neutrophil infiltration by the induction of TNF and IL-1β expression in monocytes [118,119]. In the light of the physiological role of IL-17B, IL-17B-producing MSCs could facilitate the metastatic process by promoting tumour angiogenesis and recruiting neutrophils to the metastatic site. This would support β2-integrin-mediated docking of the tumour cells to the endothelial wall [122].
Notably, there are significant variations in how tumour cells use MSCs to enhance their metastatic potential. Although MDA-MB-231 and SUM1315 are both innately metastatic to the bone and can be induced to metastasize to the liver and lungs upon ectopic expression of IL-17BR, only SUM1315 cells express significantly higher levels of IL-17BR in bone metastasis. shRNA-mediated knockdown of CCR5 in MCF7/Ras and HMLER cells does not affect metastatic potential, whereas lung metastasis is seriously compromised in MDA-MB-231 and MDA-MB-435 cell lines under these conditions. In addition, the metastasis-promoting potential of MSCs can be independent of their effect on primary tumour growth, as is the case in the enhancement of MCF7/Ras metastasis by MSC admixture [13]. By contrast, MSCs apparently inhibit hepatocellular carcinoma metastasis by suppressing TGF-β and MMP2, although they promote primary tumour growth in vivo [123]. Therefore, further investigation into how various cancer subtypes alter their metastatic potential in response to MSCs and whether these interactions are tumour type-dependent and context-dependent will be crucial for developing new therapeutic strategies to block the metastasis-promoting function of MSCs (Sidebar A).
MSC-mediated immunomodulation in metastasis
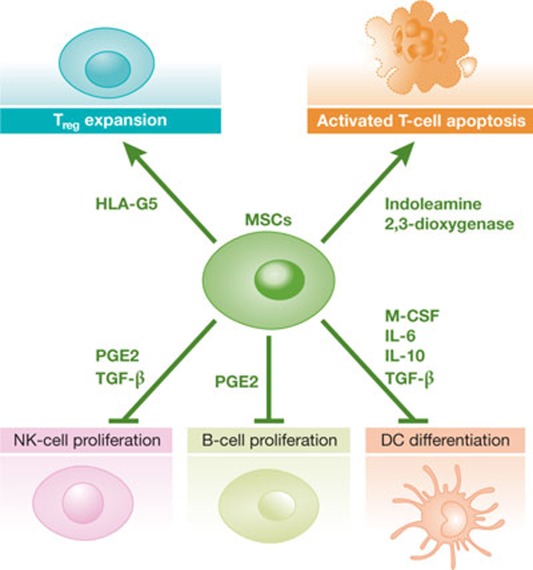
The contribution of MSCs to tumour growth and progression is similar to that in wound healing; both processes benefit from physical support of the stroma provided by MSCs with their versatile plasticity. However, one major difference between the endogenous role of MSCs healing a wound and their tumorigenic role at the ‘never-healing’ wound site is their influence on immune cells—the former is immunostimulatory whereas the latter is generally immunosuppressive [124]. Human MSCs suppress the proliferation of T cells through mediators such as TGF-β and HGF [125], and induce apoptosis of activated T cells through indoleamine 2,3-dioxygenase secretion [126]. MSCs also interfere with dendritic cell differentiation 127–129], which is crucial for the production of pro-inflammatory cytokines—including TNF-α, IFN-γ and IL-12—as well as B- and NK-cell proliferation [130,131]. This markedly hampers the normal inflammatory function of immune cells (Fig 2). The MSC-driven promotion of Treg cell expansion and immunosuppressive activity is of particular interest, as many reports suggest that Tregs are a prognostic factor in a variety of cancers (discussed later). These FoxP3+ CD25Hi CD4+ cells expand after stimulation by MSC-derived HLA-G5 [132] and can maintain their immunosuppressive activity for an extended period of time when cultured with MSCs in vitro [133]. Most of the aforementioned studies have led to promising results in the use of MSCs to treat graft-versus-host disease, allogeneic bone marrow transplantations and skin transplantation [124,134].
Figure 2. Immunomodulatory properties of mesenchymal stem cells.
MSCs can secrete a variety of immunomodulatory factors to promote an immunosuppressive environment. They induce apoptosis of activated T cells and the expansion of immunosuppressive Tregs through the secretion of indoleamine 2,3-dioxygenase and HLA-G5, respectively. MSCs can also produce PGE2 and TGF-β to inhibit NK-cell proliferation, as well as secrete PGE2 to inhibit B-cell proliferation. MSC secretion of M-CSF, IL-6, IL-10 and TGF-β also suppresses DC differentiation. DC, dendritic cell; HLA-G5, human leukocyte antigen G5; IL, interleukin; M-CSF, macrophage colony-stimulating factor; MSC, mesenchymal stem cell; NK, natural killer; PGE2, prostaglandin E2; Treg cell, regulatory T cell; TGF-β, transforming growth factor-β.
The immunomodulatory properties of MSCs have also been proposed to have an influential role in tumour growth, although evidence for this is limited. Injection of MSCs with B16 melanoma cells into syngeneic recipients allows tumour growth, whereas the tumour cells are rejected in the absence of MSCs or if their numbers are reduced [135]. More recently, MSC-secreted TGF-β1 has been shown to promote Treg cell expansion, and blocking TGF-β1 or depleting Tregs has been shown to increase CTL- and NK-mediated lysis of T47D breast cancer cells in vitro [136]. Finally, CAFs polarize the tumour microenvironment to an immunosuppressive TH2 cytokine profile, which can stimulate Treg expansion and promote 4T1 tumour growth and metastasis to the lungs [137]. This suggests that differentiated progenies of MSCs might also contribute to immunosuppression. Although these studies confirm that tumour-associated MSCs can exert immunosuppression, they do not provide unequivocal evidence that MSC-mediated immunosuppression can promote metastatic tumour growth independently of other MSC-dependent mechanisms discussed above.
The prospect of targeting MSCs to render the tumour microenvironment more immunoreactive has been discussed but must be considered in the light of the controversy surrounding tumour immunosurveillance. Burnet and Thomas proposed in 1957 that tumour cells with their “new antigenic potentialities” can induce an immune response against the primary tumour; successful tumorigenesis occurs if the tumour cells are able to escape recognition by the innate and adaptive immune systems [138,139,140]. Deficiency of Rag [141], the depletion of CD4+ and CD8+ T cells, and neutralization of IFN-γ in MCA-treated mice [142] have all been shown to significantly increase the incidence of tumorigenesis, supporting the immunosurveillance hypothesis. However, other studies have shown that immunoediting—a process by which tumour cells can lose their immunogenicity through loss of the major histocompatibility complex [138]—is not observed in other strains of spontaneous cancer-prone mice. For example, tumours spontaneously arising from simian virus 40 T antigen (SV40 Tag) can elicit a Tag-specific B-cell and T-cell response, but this response cannot suppress tumorigenesis [23]. In fact, some key studies have demonstrated a pro-tumorigenic role for adaptive immune cells [143,144]. CD4+ effector T cells, which secrete cytokines to support CTL proliferation, also secrete cytokines such as IL-4 that stimulate EGF production from tumour-associated macrophages (TAMs) in a spontaneous MMTV-PyMT murine mammary adenocarcinoma model. This, in turn, promotes EGF receptor-mediated invasion and subsequent metastasis to the lungs [144]. In line with these reports, CCL5 and IL-17B—which have been discussed above as mediators of cancer metastasis—are inflammatory cytokines known to recruit and activate adaptive immune cells. Therefore, further investigation is needed regarding the immunomodulatory properties of MSCs in the context of tumorigenesis and metastasis.
Tregs and metastasis
There is rapidly growing evidence that Tregs infiltrate tumours and positively correlate with poor prognosis in cancer patients[15,145,146,147], with the exception of renal cell carcinoma and certain haematological malignancies [148]. Tregs maintain immune tolerance and prevent inflammatory disease by suppressing cytotoxic T-cell activity and the proliferation of effector T cells [149,150,151]. Various subsets of Tregs of different immunophenotypes are found in both lymphoid and non-lymphoid organs [152,153,154]. As in the case of MSCs, there are many markers—such as CD25, FOXP3 and CTLA4—used to identify Tregs, the immunosuppressive role of which has been well-characterized [15]. Impairment of Treg function induces a range of autoimmune and inflammatory diseases, such as type I diabetes [155], rheumatoid arthritis [156] and systemic lupus erythematosus [157]. Hypomorphic alleles of the FoxP3 gene and the loss of CTLA4 expression—which are both required for Treg maintenance and function [158,159,160]—lead to severe systemic autoimmunity and lymphoproliferative disease in humans and animal models [15,161,162]. This suggests that Treg infiltration into the tumour mass could render the tumour microenvironment immunosuppressive and thus prevent the inflammatory response to tumours.
Indeed, depletion of Tregs suppresses tumour progression in models of breast cancer [163], leukaemia, myeloma, fibrosarcoma [164], colon adenocarcinoma [165] and lung cancer [166]. Furthermore, the infiltration of Tregs into the primary tumour has been recently proposed to promote metastatic potential (Fig 3; [19,163,167]), although some studies disagree with these findings due to the differences in animal models and cell lines [168,169]. Administration of autologous CD25 antibody—which depletes the Treg pool—alone or with concomitant stimulation of NKT cell activity can suppress pulmonary metastasis of 4T1 murine mammary tumour cells [163]. This suggests additive effects on the suppression of tumour metastasis when Tregs are inactivated and NKT cells are relieved from Treg-mediated suppression (Fig 3).
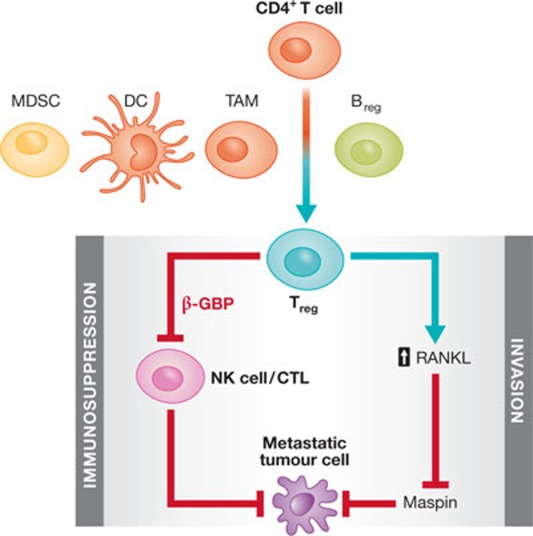
Figure 3. Tregs promote cancer metastasis through immunosuppression and by stimulating invasive behaviour.
Tumour-associated Bregs enhance the conversion of CD4+ T cells into immunosuppressive Tregs, which further promote cancer metastasis through immunosuppression and stimulating cell motility. Other immune cells, including MDSCs, DCs and TAMs, secrete various cytokines to recruit and activate Tregs. Tregs, in turn, inhibit the proliferation of tumour-reactive NK cells and CTLs to create a metastasis-permissive immune environment. Tregs can also produce abundant RANKL, which has been shown to promote tumour cell invasion through maspin suppression. B/Treg, regulatory B/T cell; CTL, cytotoxic T lymphocyte; DC, dendritic cell; MDSC, myeloid-derived suppressor cell; NK, natural killer; RANKL, receptor activator of nuclear factor-κB (ligand); TAM, tumour-associated macrophage.
Another study supporting the role of Tregs in promoting metastasis showed suppression of 4T1 lung metastasis after administration of CD25 antibody, and that the secretion of CCR4 ligand, CCL17 and CCL22 in the lungs can recruit CCR4+ 4T1 and CCR4+ Tregs [167]. In addition, the loss of lung metastasis in NOD/SCID mice can be restored by transferring CD25+ CD4+Tregs from BALB/c mice [166]. Most importantly, this study identified a molecule responsible for Treg-mediated apoptosis of NK cells, β-GBP, which is secreted by Tregs and specifically affects NK cells [167]. The same research group also identified a population of immunosuppressive B cells that express one of the Treg markers, CD25, as well as B-cell markers B220 and CD19 [170]. This immunosuppressive population can also promote 4T1 lung metastasis, as shown by its reduction after B220 antibody treatment. Culturing CD25+ B220+ CD19+ regulatory B cells (Breg) with non-regulatory CD4+ T cells leads to increased expression of FOXP3 in the latter population and the acquisition of immunosuppressive properties in vitro, suggesting that Breg cells might promote Treg conversion (Fig 3). Furthermore, only transfer of both regulatory B cells and non-Tregs—and not of Breg cells alone—can restore lung metastasis in NOD/SCID mice, demonstrating that Bregs have an indirect, supporting role in Treg-mediated lung metastasis. These studies provide fairly convincing evidence that Tregs have a crucial role in lung metastasis, but they also strongly suggest that there could be many other subpopulations involved in this process. For example, a specific subset of CD4+ CD25+ Tregs that express CCR6 is more frequently found in breast and colorectal tumours than its CCR6− counterpart, and relies on TGF-β signalling for propagation in situ [171]. CD11c+ dendritic cells, which secrete TGF-β and stabilize FOXP3 and CCR6 expression, and TAMs, which recruit CCR6+ Tregs through CCL20 secretion, mediate this subtype-specific immunosuppressive response [171]. Other immune cells, such as myeloid-derived suppressor cells (MDSCs), secrete various cytokines—such as TGF-β—to recruit and activate Tregs in tumorigenic settings (Fig 3; [172,173]).
It has also been shown, however, that Tregs might promote metastasis independently of their immunosuppressive activity. RANK activity can repress the expression of maspin, which regulates cell adhesion and has been characterized as a metastasis inhibitor in breast cancer [174,175,176]. Tregs in murine mammary carcinoma, which are induced by the overexpression of ErbB2, are a major source of RANKL and promote metastasis by suppressing maspin expression (Fig 3; [19]). In this case, Treg cell-mediated metastasis does not occur through immunomodulation, as pulmonary metastasis can be enhanced with exogenous RANKL in the complete absence of T cells.
Therapeutic potential
Effective treatment for metastatic disease remains elusive, as the dissemination of tumour cells severely hinders the ability to deliver effectively therapeutic agents to numerous metastatic colonies [2]. Furthermore, various components of the metastatic microenvironment offer tumour cells protection and resilience against therapy [177]. Therefore, ideal candidates for the treatment of cancer metastasis should be able to efficiently home to metastatic sites and disrupt the protective environment of the metastatic lesions.
Application of MSCs. The tumour-targeting affinity of MSCs has been characterized, although some controversy remains, and proposed to be a potentially effective mode of drug delivery for cancer treatment [178,179,180]. Furthermore, MSCs might incorporate into the tumour stroma as CAFs [11], thereby effectively infiltrating the tumour microenvironment. Many therapeutic agents, ranging from oncolytic adenoviruses in ovarian cancer [181] to cytokines in melanoma [182] and various adenocarcinomas [183], have been delivered by tumour-tropic MSCs and shown to suppress tumour growth in experimental models. MSCs engineered to produce and deliver TRAIL in metastatic breast cancer [184] and pancreatic carcinoma [185] have been recently shown to suppress lung metastasis. The administration of TRAIL holds high therapeutic potential, as it induces apoptosis in transformed cells with minimal toxicity towards normal cells [186,187]. The application of a delivery platform is crucial, however, as the unstable pharmacokinetics of TRAIL in the circulation hinder efficient delivery to the tumour [188]. Studies demonstrating the targeting of MSCs to metastatic organs [18,92,93] suggest that TRAIL could be delivered to these metastatic sites effectively by using this strategy. Indeed, TRAIL-expressing MSCs have been shown to target metastatic lungs in an MDA-MB-231 experimental metastasis model [184].
Despite the potential advantage of using MSCs for drug delivery, their pro-tumorigenic and pro-metastatic effects described above are a cause for concern and could possibly outweigh the benefits of efficient delivery to tumour metastasis. Nevertheless, MSC drug delivery studies so far show that this is not the case [179,185,189,190] and it remains unknown whether such engineered MSCs lose their innate potential to promote tumour progression from genetic manipulation, or the pro-tumorigenic effect of such engineered MSCs has minimal impact in the context of such experimental settings.
However, a major caveat of the aforementioned MSC-based therapeutics is that the preclinical studies began MSC-mediated drug delivery relatively early in tumour progression—10 days after subcutaneous injection of pancreatic carcinoma cells [185] and 7 days after intravenous injection of breast adenocarcinoma cells [184]—whereas patients with metastatic disease would require treatment at much later stages of progression. Therefore, whether MSC drug delivery would elicit anti-metastatic effects in a clinical setting still needs to be explored.
The greatest challenge in searching for therapeutic targets to disrupt tumour–MSC interactions lies in the fact that certain molecular mechanisms defined under pathological conditions are also crucial components of physiologically normal conditions. For example, CCL5 is secreted by tumour-associated MSCs to promote primary tumour growth and metastasis in a malignant setting, but its endogenous function is to recruit and activate inflammation-inducing immune cells [191]. Targeting of CCL5 as a therapeutic option, therefore, might be problematic and result in severely adverse effects unrelated to the intended therapeutic impact on cancer. Alternatively, extensive characterization of cancer-associated MSCs as compared with normal MSCs might yield unique cancer-associated MSC-specific properties as potential therapeutic targets. This is especially important when devising a therapeutic strategy to inhibit MSC activity, as normal MSCs are crucial in wound healing and tissue regeneration [54]. In fact, normal MSCs and their derivatives are being considered in numerous clinical trials for the treatment of bone fractures, stroke, multiple sclerosis and leukaemia [192,193,194]. For example, osteoprogenitors expanded in vitro on hydroxyapatite scaffolds have been used to successfully treat 4 patients with diaphyseal segmental defects in long bones [195,196]. Therefore, a detailed molecular characterization of cancer-associated MSCs in various metastasis organ sites might provide new therapeutic approaches to target organ-specific metastasis.
Application of regulatory T cells. There is a strong clinical correlation between the presence of Tregs and poor prognosis of cancer patients [16,17,197]. The amount of Tregs in the primary tumours and sentinel lymph nodes has been proposed as a strong prognostic indicator for metastasis-free survival in breast cancer [198,199] and papillary thyroid cancer [200]. For example, the probability of 10-year survival of lymph node FOXP3-negative and FOXP3-positive subgroups of breast cancer patients is 41% and 18%, respectively [198]. Therefore, a great effort to suppress Treg cell expansion and the immunosuppressive activity of this population has been made but has resulted in limited success.
Selective Treg depletion by expressing FOXP3-driven diphtheria toxin receptor improves therapeutic vaccination against ovalbumin-expressing B16 melanoma cells in mice by allowing the accumulation of activated CD8+ CTLs [201]. However, Treg cell depletion in the peripheral blood of melanoma patients with the CD25 neutralizing antibody Daclizumab failed to enhance the efficacy of dendritic cell vaccination [202]. By contrast, administration of the recombinant IL-2 diphtheria toxin conjugate denileukin diftitox to renal cell carcinoma patients, followed by RNA-transfected dendritic cell vaccination, significantly increased tumour cell-reactive T-cell responses [203]. This suggests that different cancer types might have different sensitivity to immunosurveillance, as well as different evasion mechanisms [204].
The suppression of cancer metastasis in patients receiving Treg-cell-depleting agents has not yet been reported, although their benefits in the context of tumour progression and metastasis are being assessed in clinical trials (NCT01307618).
Conclusion and future directions
It has become increasingly evident that the distinct stages of tumour progression cannot be viewed as a linear cascade, but rather must be placed in the context of an intricate network of tumour–stroma interactions with multiple signalling and cellular feedback loops. Bone marrow-derived cells are crucial mediators in fulfilling the potential of tumour cells for distant metastasis, by using their innate versatility to perform a wide range of supportive functions. MSCs can put on many faces as accomplices in tumour progression and metastasis. Similarly, Tregs contribute to these complex processes not only with their immunosuppressive properties but also by inducing cell motility. Both MSCs and Tregs demonstrate how a beneficial endogenous function can be rendered harmful in the context of cancer progression. However, non-discriminatory targeting of MSCs and Tregs could lead to dysfunctional wound healing and autoimmune diseases, respectively. Therefore, how various bone marrow-derived populations in the metastatic context can be distinguished from those with normal endogenous function is a crucial goal that requires further research. Not only the MSCs, Tregs and other bone marrow-derived cells present at the primary tumour should receive attention, but also the bone marrow-derived cells recruited to the metastatic niche to promote organ-specific metastasis should become a major topic for further investigation. Given the challenges of targeting tumour-specific pathways, investigating the specificity of metastasis-promoting bone marrow-derived cells could provide a new targeted strategy for cancer treatment.

Yibin Kang & Bong Ihn Koh
Acknowledgments
The authors thank B. Ell and B. Yeung for critical reading of the manuscript. The authors' research is funded by the Brewster Foundation, Champalimaud Foundation, American Cancer Society, Komen for the Cure, New Jersey Commission on Cancer Research, the US Department of Defense and the National Institutes of Health (R01CA134519 and R01CA141062).
Footnotes
The authors declare that they have no conflict of interest.
References
- Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127: 679–695 [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147: 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J (2009) Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9: 274–284 [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9: 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Coussens LM (2011) Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization. Int J Cancer 128: 2536–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133: 571–573 [PubMed] [Google Scholar]
- Bos PD et al. (2009) Genes that mediate breast cancer metastasis to the brain. Nature 459: 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3: 537–549 [DOI] [PubMed] [Google Scholar]
- Minn AJ et al. (2005) Genes that mediate breast cancer metastasis to lung. Nature 436: 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd (2011) Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld SA, DeClerck YA (2010) Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 29: 249–261 [DOI] [PubMed] [Google Scholar]
- Karnoub AE et al. (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563 [DOI] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE 4: e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Koch MA (2011) Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol 11: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ et al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949 [DOI] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24: 5373–5380 [DOI] [PubMed] [Google Scholar]
- Mi Z, Bhattacharya SD, Kim VM, Guo H, Talbot LJ, Kuo PC (2011) Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 32: 477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 470: 548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ (2011) CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71: 2892–2900 [DOI] [PubMed] [Google Scholar]
- Manjili MH (2011) Revisiting cancer immunoediting by understanding cancer immune complexity. J Pathol 224: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Blankenstein T (2004) A cancer immunosurveillance controversy. Nat Immunol 5: 35. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T (2005) Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 437: 141–146 [DOI] [PubMed] [Google Scholar]
- Kitamura T et al. (2007) SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet 39: 467–475 [DOI] [PubMed] [Google Scholar]
- Kaplan RN et al. (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8: 1369–1375 [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang Y, Zhao J, Yang Z, Li D, Katirai F, Huang B (2011) Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev 30: 177–184 [DOI] [PubMed] [Google Scholar]
- Strouch MJ et al. (2010) Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 16: 2257–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA (2010) IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 24: 241–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475: 222–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO (2011) Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Liang S, Sharma A, Dong C, Robertson GP (2010) Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70: 6071–6082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergun S, Hohn HP, Kilic N, Singer BB, Tilki D (2008) Endothelial and hematopoietic progenitor cells (EPCs and HPCs): hand in hand fate determining partners for cancer cells. Stem Cell Rev 4: 169–177 [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319: 195–198 [DOI] [PubMed] [Google Scholar]
- Kerbel RS et al. (2008) Endothelial progenitor cells are cellular hubs essential for neoangiogenesis of certain aggressive adenocarcinomas and metastatic transition but not adenomas. Proc Natl Acad Sci USA 105: E54–E55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE (2011) Immune cell infiltration of primary and metastatic lesions: mechanisms and clinical impact. Semin Cancer Biol 21: 131–138 [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140: 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Johansson M, Coussens LM (2008) Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev 27: 11–18 [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Scadden DT (2006) The hematopoietic stem cell in its place. Nat Immunol 7: 333–337 [DOI] [PubMed] [Google Scholar]
- Calvi LM et al. (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121 [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Hauser AE, Nakayama T, Radbruch A (2010) Organization of immunological memory by bone marrow stroma. Nat Rev Immunol 10: 193–200 [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T (2004) Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20: 707–718 [DOI] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A (2009) Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30: 721–730 [DOI] [PubMed] [Google Scholar]
- Pittenger MF et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147 [DOI] [PubMed] [Google Scholar]
- Jiang Y et al. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41–49 [DOI] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2: 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini A, Mattoli S (2007) The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87: 858–870 [DOI] [PubMed] [Google Scholar]
- Lee CH, Shah B, Moioli EK, Mao JJ (2010) CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120: 3340–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S et al. (2009) Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med 206: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B et al. (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131: 324–336 [DOI] [PubMed] [Google Scholar]
- Paquet-Fifield S et al. (2009) A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest 119: 2795–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas DT et al. (2008) Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 36: 642–654 [DOI] [PubMed] [Google Scholar]
- Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y (2007) Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 25: 1610–1617 [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI (1995) Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5azacytidine. Muscle Nerve 18: 1417–1426 [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 96: 10711–10716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y et al. (2005) Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106: 756–763 [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Levin S, Zipori D (2011) The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev 7: 560–568 [DOI] [PubMed] [Google Scholar]
- Dominici M et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317 [DOI] [PubMed] [Google Scholar]
- Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL (2003) Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci USA 100: 5840–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada T, Reis RL, Gomes ME (2011) Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev 7: 64–76 [DOI] [PubMed] [Google Scholar]
- Dubois SG, Floyd EZ, Zvonic S, Kilroy G, Wu X, Carling S, Halvorsen YD, Ravussin E, Gimble JM (2008) Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol 449: 69–79 [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228 [DOI] [PubMed] [Google Scholar]
- Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21: 105–110 [DOI] [PubMed] [Google Scholar]
- Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 22: 1330–1337 [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119: 2204–2213 [DOI] [PubMed] [Google Scholar]
- Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA (2004) Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells 22: 1263–1278 [DOI] [PubMed] [Google Scholar]
- Kaltz N, Ringe J, Holzwarth C, Charbord P, Niemeyer M, Jacobs VR, Peschel C, Haupl T, Oostendorp RA (2010) Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp Cell Res 316: 2609–2617 [DOI] [PubMed] [Google Scholar]
- Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W (2003) Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum 48: 418–429 [DOI] [PubMed] [Google Scholar]
- Cowan CM et al. (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22: 560–567 [DOI] [PubMed] [Google Scholar]
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F (2004) Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25: 3211–3222 [DOI] [PubMed] [Google Scholar]
- Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F (2002) Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290: 763–769 [DOI] [PubMed] [Google Scholar]
- Rodriguez AM et al. (2005) Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med 201: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A (2004) Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349–355 [DOI] [PubMed] [Google Scholar]
- Planat-Benard V et al. (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109: 656–663 [DOI] [PubMed] [Google Scholar]
- Yagi K, Kondo D, Okazaki Y, Kano K (2004) A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun 321: 967–974 [DOI] [PubMed] [Google Scholar]
- Matsumoto T et al. (2008) Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 215: 210–222 [DOI] [PubMed] [Google Scholar]
- Oki Y, Watanabe S, Endo T, Kano K (2008) Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct 33: 211–222 [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Miyoshi H (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669 [DOI] [PubMed] [Google Scholar]
- Morigi M et al. (2008) Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 26: 2075–2082 [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 3: e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH (2008) The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 26: 1047–1055 [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Glod JW, Banerjee D (2009) Mesenchymal stem cells: flip side of the coin. Cancer Res 69: 1255–1258 [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68: 4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quante M et al. (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19: 257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckermann BM et al. (2008) VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 99: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659 [DOI] [PubMed] [Google Scholar]
- Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80: 267–274 [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noel D, Jorgensen C (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102: 3837–3844 [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W, Chayama K (2010) Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer 127: 2323–2333 [DOI] [PubMed] [Google Scholar]
- Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M (2010) Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res 70: 10044–10050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T et al. (2011) Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17: 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6: 392–401 [DOI] [PubMed] [Google Scholar]
- Micke P, Ostman A (2005) Exploring the tumour environment: cancer-associated fibroblasts as targets in cancer therapy. Expert Opin Ther Targets 9: 1217–1233 [DOI] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120: 303–313 [DOI] [PubMed] [Google Scholar]
- Eck SM, Cote AL, Winkelman WD, Brinckerhoff CE (2009) CXCR4 and matrix metalloproteinase-1 are elevated in breast carcinoma-associated fibroblasts and in normal mammary fibroblasts exposed to factors secreted by breast cancer cells. Mol Cancer Res 7: 1033–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Dazin P, Tlsty TD (1998) A novel coculture technique demonstrates that normal human prostatic fibroblasts contribute to tumor formation of LNCaP cells by retarding cell death. Cancer Res 58: 4525–4530 [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR (1999) Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK (2010) Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 107: 21677–21682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SY et al. (2008) Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res 68: 9996–10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2012) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481: 85–89 [DOI] [PubMed] [Google Scholar]
- Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M (2002) MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2: 289–300 [DOI] [PubMed] [Google Scholar]
- Dawson MR, Duda DG, Chae SS, Fukumura D, Jain RK (2009) VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS ONE 4: e6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S (2011) Functional specialization of interleukin-17 family members. Immunity 34: 149–162 [DOI] [PubMed] [Google Scholar]
- Dunn L, Demichele A (2009) Genomic predictors of outcome and treatment response in breast cancer. Mol Diagn Ther 13: 7390. [DOI] [PubMed] [Google Scholar]
- Jung MY, Kim SH, Cho D, Kim TS (2009) Analysis of the expression profiles of cytokines and cytokine-related genes during the progression of breast cancer growth in mice. Oncol Rep 22: 1141–1147 [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21: 467–476 [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852 [DOI] [PubMed] [Google Scholar]
- Hirota K, Ahlfors H, Duarte JH, Stockinger B (2012) Regulation and function of innate and adaptive interleukin-17-producing cells. EMBO Rep 13: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T,Robbins PD, Tahara H, Lotze MT (2003) Interleukin-17 promotes angiogenesis and tumor growth. Blood 101: 2620–2627 [DOI] [PubMed] [Google Scholar]
- Tartour E et al. (1999) Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res 59: 3698–3704 [PubMed] [Google Scholar]
- Pelletier M et al. (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115: 335–343 [DOI] [PubMed] [Google Scholar]
- Iida T et al. (2011) Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep 25: 1271–1277 [DOI] [PubMed] [Google Scholar]
- Schnyder B, Schnyder-Candrian S, Pansky A, Schmitz ML, Heim M, Ryffel B, Moser R (2005) IL-17 reduces TNF-induced Rantes and VCAM-1 expression. Cytokine 31: 191–202 [DOI] [PubMed] [Google Scholar]
- Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q (2006) IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J Allergy Clin Immunol 117: 590–596 [DOI] [PubMed] [Google Scholar]
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H (2009) IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 206: 1457–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL (2010) Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA 107: 5540–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatar T et al. (2010) IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol Immunother 59: 805–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Ozdemir T, Chung CY, Robertson GP, Dong C (2011) Sequential binding of alphaVbeta3 and ICAM-1 determines fibrin-mediated melanoma capture and stable adhesion to CD11b/CD18 on neutrophils. J Immunol 186: 242–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC et al. (2010) Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sci 101: 2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V (2006) Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 36: 2566–2573 [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843 [DOI] [PubMed] [Google Scholar]
- Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC (2005) Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia 19: 1597–1604 [DOI] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N (2005) Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105: 4120–4126 [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE (2006) Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 177: 2080–2087 [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC (2004) Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 13: 263–271 [DOI] [PubMed] [Google Scholar]
- Corcione A et al. (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107: 367–372 [DOI] [PubMed] [Google Scholar]
- Rutella S, Danese S, Leone G (2006) Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108: 1435–1440 [DOI] [PubMed] [Google Scholar]
- Selmani Z et al. (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26: 212–222 [DOI] [PubMed] [Google Scholar]
- Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A (2008) Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol 36: 309–318 [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822 [DOI] [PubMed] [Google Scholar]
- Djouad F, Bony C, Apparailly F, Louis-Plence P, Jorgensen C,Noel D (2006) Earlier onset of syngeneic tumors in the presence of mesenchymal stem cells. Transplantation 82: 1060–1066 [DOI] [PubMed] [Google Scholar]
- Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P (2010) Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol 184: 5885–5894 [DOI] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One 4: e7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331: 1565–1570 [DOI] [PubMed] [Google Scholar]
- Dighe AS, Richards E, Old LJ, Schreiber RD (1994) Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1: 447–456 [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95: 7556–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111 [DOI] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450: 903–907 [DOI] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM (2005) De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7: 411–423 [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM (2009) CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16: 91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F et al. (2011) CD8(+) cytotoxic Tcell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat, ; DOI: [DOI] [PubMed] [Google Scholar]
- Barnett JC et al. (2010) Ovarian cancer tumor infiltrating T-regulatory (T(reg)) cells are associated with a metastatic phenotype. Gynecol Oncol 116: 556–562 [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Yang ZY, Zhang LD, Ping B, Wang SG, Ma KS, Li XW, Dong JH (2010) Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol 71: 1180–1186 [DOI] [PubMed] [Google Scholar]
- Curiel TJ (2008) Regulatory T cells and treatment of cancer. Curr Opin Immunol 20: 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH (2006) Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25: 129–141 [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3: 135–142 [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Shevach EM (2001) Cutting edge: control of CD8+ Tcell activation by CD4+CD25+ immunoregulatory cells. J Immunol 167: 1137–1140 [DOI] [PubMed] [Google Scholar]
- Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD,Rudensky AY, Campbell DJ (2007) Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med 204: 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133: 775–787 [DOI] [PubMed] [Google Scholar]
- Feuerer M, Hill JA, Mathis D, Benoist C (2009) Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol 10: 689–695 [DOI] [PubMed] [Google Scholar]
- Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI (2005) Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes 54: 92–99 [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 200: 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz DA (2008) Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther 10: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Rudensky A (2009) Control of regulatory Tcell lineage commitment and maintenance. Immunity 30: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336 [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory Tcell development by the transcription factor Foxp3. Science 299: 1057–1061 [DOI] [PubMed] [Google Scholar]
- Bennett CL et al. (2001) The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21 [DOI] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM (2000) JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 106: R75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Gu Y, Zhang H, Simon AK, Chen X, Wu C, Xu XN, Jiang S (2010) Depletion of CD4+CD25+ regulatory T cells enhances natural killer T cell-mediated anti-tumour immunity in a murine mammary breast cancer model. Clin Exp Immunol 159: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E (1999) Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 59: 3128–3133 [PubMed] [Google Scholar]
- Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ (2010) Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 70: 7800–7809 [DOI] [PubMed] [Google Scholar]
- Granville CA et al. (2009) A central role for Foxp3+ regulatory T cells in K-Ras-driven lung tumorigenesis. PLoS One 4: e5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud PB et al. (2009) Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 69: 5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M et al. (2005) A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 202: 1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK (2002) Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol 169: 5796–5804 [DOI] [PubMed] [Google Scholar]
- Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A (2011) Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 T cells to T-regulatory cells. Cancer Res 71: 3505–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Xu W, Wen Z, Xiong S (2011) In situ prior proliferation of CD4+ CCR6+ regulatory T cells facilitated by TGF-beta secreting DCs is crucial for their enrichment and suppression in tumor immunity. PLoS ONE 6: e20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res 66: 6807–6815 [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66: 1123–1131 [DOI] [PubMed] [Google Scholar]
- Khalkhali-Ellis Z (2006) Maspin: the new frontier. Clin Cancer Res 12: 7279–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HY, Zhang W, Liang R, Abraham S, Kittrell FS, Medina D, Zhang M (2001) Blocking tumor growth, invasion, and metastasis by maspin in a syngeneic breast cancer model. Cancer Res 61: 6945–6951 [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R (1994) Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263: 526–529 [DOI] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT (2010) DNA damage-mediated induction of a chemoresistant niche. Cell 143: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S et al. (2009) Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27: 2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamizo A et al. (2005) Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65: 3307–3318 [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M (2004) Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96: 1593–1603 [DOI] [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L (2006) Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther 5: 755–766 [DOI] [PubMed] [Google Scholar]
- Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S (2008) Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells 26: 2332–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X et al. (2008) A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther 16: 749–756 [DOI] [PubMed] [Google Scholar]
- Loebinger MR, Eddaoudi A, Davies D, Janes SM (2009) Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 69: 4134–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr A, Albarenque SM, Deedigan L, Yu R, Reidy M, Fulda S, Zwacka RM (2010) Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells 28: 2109–2120 [DOI] [PubMed] [Google Scholar]
- Wiley SR et al. (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3: 673–682 [DOI] [PubMed] [Google Scholar]
- Walczak H et al. (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5: 157–163 [DOI] [PubMed] [Google Scholar]
- Ashkenazi A et al. (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasportas LS et al. (2009) Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA 106: 4822–4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M (2002) Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 62: 3603–3608 [PubMed] [Google Scholar]
- Baggiolini M (1998) Chemokines and leukocyte traffic. Nature 392: 565–568 [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28: 1099–1106 [DOI] [PubMed] [Google Scholar]
- Karussis D et al. (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67: 1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan ML, Blazar BR, DeFor TE, Wagner JE (2009) Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant 43: 447–454 [DOI] [PubMed] [Google Scholar]
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M (2001) Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344: 385–386 [DOI] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M, Cancedda R (2007) Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13: 947–955 [DOI] [PubMed] [Google Scholar]
- Perrone G et al. (2008) Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 44: 1875–1882 [DOI] [PubMed] [Google Scholar]
- Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, Tagliabue E, Balsari A (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27: 1746–1752 [DOI] [PubMed] [Google Scholar]
- Nakamura R et al. (2009) Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur J Cancer 45: 2123–2131 [DOI] [PubMed] [Google Scholar]
- French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR (2010) Tumor-associated lymphocytes and increased FoxP3+ regulatory Tcell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab 95: 2325–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages K et al. (2010) Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res 70: 7788–7799 [DOI] [PubMed] [Google Scholar]
- Jacobs JF et al. (2010) Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res 16: 5067–5078 [DOI] [PubMed] [Google Scholar]
- Dannull J et al. (2005) Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115: 3623–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TJ, Smyth MJ (2011) Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev 30: 125–140 [DOI] [PubMed] [Google Scholar]